Abstract
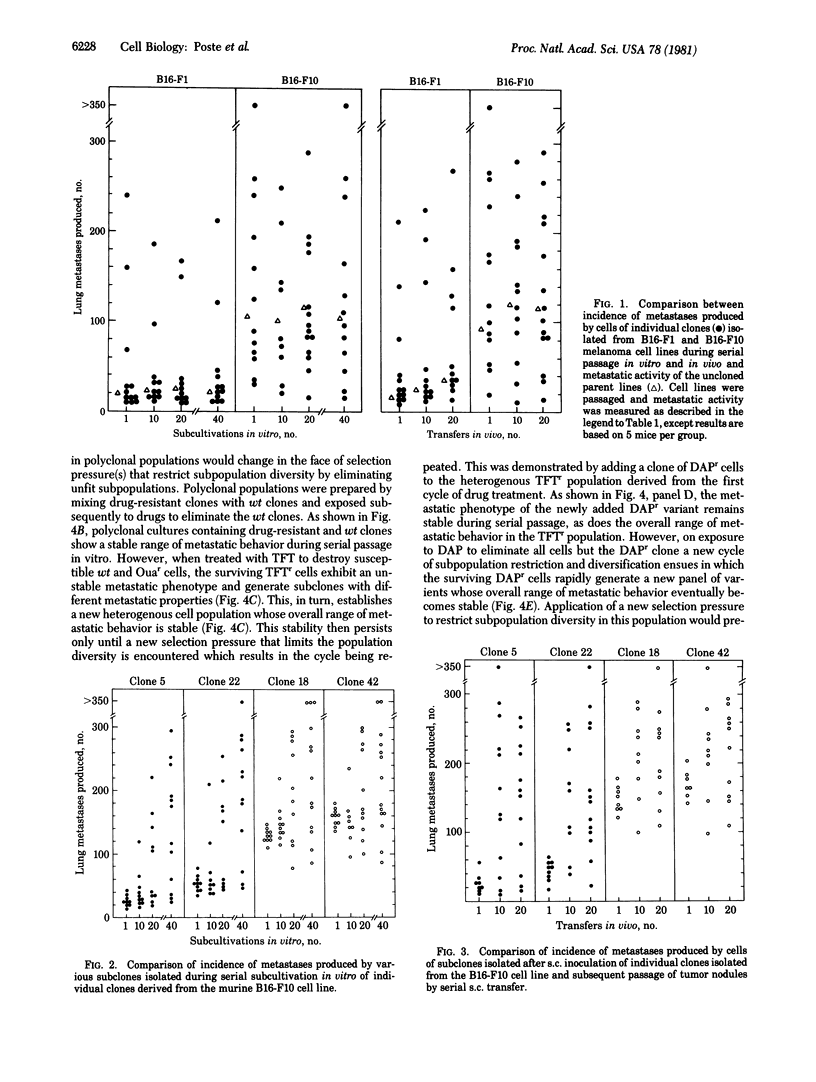
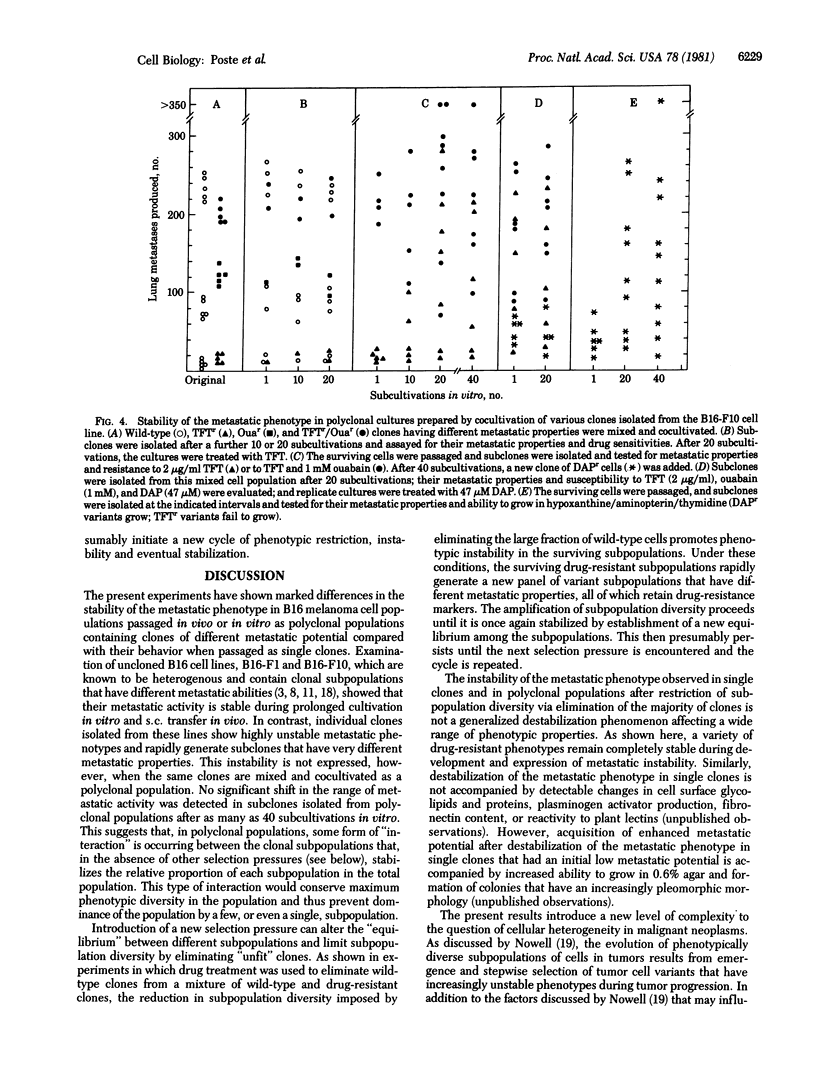
Analysis of the metastatic properties of clones isolated from mouse B16 melanoma cell lines (B16-F1 and F10) shows extensive cellular heterogeneity and the presence of subpopulations that have widely differing metastatic abilities. This pattern of metastatic heterogeneity is maintained during serial passage in vitro and in vivo. In contrast, even a short serial passage of individual clones isolated from these heterogeneous parent lines results in rapid emergence of variant subclones that have different metastatic properties. If several clones are mixed and cocultivated, this instability is not expressed. These data suggest that, in polyclonal populations, the various clonal subpopulations somehow interact with one another to "stabilize" their relative proportions within the population. Restriction of clonal diversity by selective killing of the majority of clones in a polyclonal population eliminates the stabilizing restraints and stimulates rapid emergence of new subpopulations to create heterogeneous populations containing a new panel of phenotypically diverse subpopulations that then reach stable proportions until the next selection pressure(s) is encountered.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cifone M. A., Kripke M. L., Fidler I. J. Growth rate and chromosome number of tumor cell lines with different metastatic potential. J Supramol Struct. 1979;11(4):467–476. doi: 10.1002/jss.400110405. [DOI] [PubMed] [Google Scholar]
- DeWys W. D. Studies correlating the growth rate of a tumor and its metastases and providing evidence for tumor-related systemic growth-retarding factors. Cancer Res. 1972 Feb;32(2):374–379. [PubMed] [Google Scholar]
- Enders J. F., Diamandopoulos G. T. A study of variation and progression in oncongenicity in an SV 40-transformed hamster heart cell line and its clones. Proc R Soc Lond B Biol Sci. 1969 Feb 25;171(1025):431–443. doi: 10.1098/rspb.1969.0004. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Gersten D. M., Budmen M. B. Characterization in vivo and in vitro of tumor cells selected for resistance to syngeneic lymphocyte-mediated cytotoxicity. Cancer Res. 1976 Sep;36(9 PT1):3160–3165. [PubMed] [Google Scholar]
- Fidler I. J., Kripke M. L. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977 Aug 26;197(4306):893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Selection of successive tumour lines for metastasis. Nat New Biol. 1973 Apr 4;242(118):148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- GREENE H. S., HARVEY E. K. The inhibitory influence of a transplanted hamster lymphoma on metastasis. Cancer Res. 1960 Aug;20:1094–1100. [PubMed] [Google Scholar]
- Gorelik E., Segal S., Feldman M. Growth of a local tumor exerts a specific inhibitory effect on progression of lung metastases. Int J Cancer. 1978 May 15;21(5):617–625. doi: 10.1002/ijc.2910210512. [DOI] [PubMed] [Google Scholar]
- Hart I. R. The selection and characterization of an invasive variant of the B16 melanoma. Am J Pathol. 1979 Dec;97(3):587–600. [PMC free article] [PubMed] [Google Scholar]
- KETCHAM A. S., KINSEY D. L., WEXLER H., MANTEL N. The development of spontaneous metastases after the removal of a "primary" tumor. II. Standardization protocol of 5 animal tumors. Cancer. 1961 Jul-Aug;14:875–882. doi: 10.1002/1097-0142(199007/08)14:4<875::aid-cncr2820140425>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S., Twiddy R. R., Robertson D. M. Induction of a tumor with greatly increased metastatic growth potential by injection of cells from a low-metastatic H-2 heterozygous tumor cell line into an H-2 incompatible parental strain. Int J Cancer. 1978 Nov 15;22(5):583–594. doi: 10.1002/ijc.2910220513. [DOI] [PubMed] [Google Scholar]
- Kripke M. L., Gruys E., Fidler I. J. Metastatic heterogeneity of cells from an ultraviolet light-induced murine fibrosarcoma of recent origin. Cancer Res. 1978 Sep;38(9):2962–2967. [PubMed] [Google Scholar]
- Liotta L. A., Vembu D., Saini R. K., Boone C. In vivo monitoring of the death rate of artificial murine pulmonary micrometastases. Cancer Res. 1978 May;38(5):1231–1236. [PubMed] [Google Scholar]
- Miller B. E., Miller F. R., Leith J., Heppner G. H. Growth interaction in vivo between tumor subpopulations derived from a single mouse mammary tumor. Cancer Res. 1980 Nov;40(11):3977–3981. [PubMed] [Google Scholar]
- Miller F. R., Heppner G. H. Immunologic heterogeneity of tumor cell subpopulations from a single mouse mammary tumor. J Natl Cancer Inst. 1979 Dec;63(6):1457–1463. [PubMed] [Google Scholar]
- Newcomb E. W., Silverstein S. C., Silagi S. Malignant mouse melanoma cells do not form tumors when mixed with cells of a non-malignant subclone: relationships between plasminogen activator expression by the tumor cells and the host's immune response. J Cell Physiol. 1978 May;95(2):169–177. doi: 10.1002/jcp.1040950206. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Poste G., Doll J., Hart I. R., Fidler I. J. In vitro selection of murine B16 melanoma variants with enhanced tissue-invasive properties. Cancer Res. 1980 May;40(5):1636–1644. [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Poste G., Flood M. K. Cells transformed by temperature-sensitive mutants of avian sarcoma virus cause tumors in vivo at permissive and nonpermissive temperatures. Cell. 1979 Aug;17(4):789–800. doi: 10.1016/0092-8674(79)90319-2. [DOI] [PubMed] [Google Scholar]
- SCHATTEN W. E. An experimental study of postoperative tumor metastases. I. Growth of pulmonary metastases following total removal of primary leg tumor. Cancer. 1958 May-Jun;11(3):455–459. doi: 10.1002/1097-0142(195805/06)11:3<455::aid-cncr2820110303>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V., Shantz G., Clauer K., Komitowski D., Zimmermann H. P., Lohmann-Matthes M. L. Tumor metastases and cell-mediated immunity in a model system in DBA/2 mice. I. Tumor invasiveness in vitro and metastasis formation in vivo. Int J Cancer. 1979 Feb;23(2):233–244. doi: 10.1002/ijc.2910230215. [DOI] [PubMed] [Google Scholar]
- Shearman P. J., Longenecker B. M. Selection for virulence and organ-specific metastasis of herpesvirus-transformed lymphoma cells. Int J Cancer. 1980 Mar 15;25(3):363–369. doi: 10.1002/ijc.2910250310. [DOI] [PubMed] [Google Scholar]
- Sheldon P. W., Fowler J. F. The effect of irradiating a transplanted murine lymphosarcoma on the subsequent development of metastases. Br J Cancer. 1973 Dec;28(6):508–514. doi: 10.1038/bjc.1973.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Withers H. R., Koehler M. W. Heterogeneity and variability of artificial lung colony-forming ability among clones from mouse fibrosarcoma. Cancer Res. 1978 Oct;38(10):3349–3351. [PubMed] [Google Scholar]
- Talmadge J. E., Starkey J. R., Davis W. C., Cohen A. L. Introduction of metastatic heterogeneity by short-term in vivo passage of a cloned transformed cell line. J Supramol Struct. 1979;12(2):227–243. doi: 10.1002/jss.400120208. [DOI] [PubMed] [Google Scholar]
- Yuhas J. M., Pazmiño N. H. Inhibition of subcutaneously growing line 1 carcinomas due to metastatic spread. Cancer Res. 1974 Aug;34(8):2005–2010. [PubMed] [Google Scholar]



