Abstract
Background
Transmembrane protease serine 2-ETS related gene (TMPRSS2-ERG) gene fusion, the most common genetic alternation in prostate cancer, is associated with protein expression of the oncogene ERG. Recently, an immunohistochemical staining method using an anti-ERG antibody was shown to have a strong correlation with altered ERG protein expression.
Methods
We analyzed a total of 303 radical prostatectomy specimens (obtained from Korean prostate cancer cases) using a constructed tissue microarray and ERG immunohistochemical staining. Thereafter, we evaluated the association between ERG expression and clinicopathological factors.
Results
The ERG-positive rate was 24.4% (74/303) and significantly higher ERG expression was observed in the subgroup with a lower Gleason score (p=0.004). Analysis of the histologic pattern of prostate adenocarcinomas revealed that tumors with discrete glandular units (Gleason pattern 3) displayed higher frequency of ERG expression (p=0.016). The ERG-positive rate was lower than that found (approximately 50%) in studies involving western populations. Other factors including age, tumor volume, initial protein-specific antigen level, a pathological stage and margin status were not significantly related with the ERG expression.
Conclusions
ERG immunohistochemical staining is significantly higher in tumors with well-formed glands and is associated with a lower Gleason score.
Keywords: Prostate neoplasms, ERG, Immunohistochemistry
The fusion between the ETS related gene (ERG), and the promoter of the highly-expressed transmembrane protease serine 2 (TMPRSS2) gene, is the most common recurrent genetic rearrangement in prostate adenocarcinoma.1 It occurs in 15.3-72%2,3 of prostate cancers in the western population, but only in about 20.9 to 28% in Korean and Japanese populations.4-6 Studies on the relationship between the TMPRSS2-ERG fusion and clinical outcome in prostate adenocarcinoma yielded conflicting results. For instance, while Attard et al.7 reported that the TMPRSS2-ERG fusion is associated with poor overall survival, Saramäki et al.8 identified longer progression-free survival.
Fluorescence in situ hybridization (FISH) and quantitative polymerase chain reaction (QPCR) have been used to detect the fusion of ERG-TMPRSS2.9 Recently, Park et al.10 described a substantive detection method using the ERG-specific antibody EPR3864 in paraffin-embedded tissue. Another group also reported excellent correlation of ERG rearrangement using FISH.11 Moreover, using semi-quantitative methods in both prostate needle biopsy and radical prostatectomy specimens,12 the intensity of immunohistochemical staining has been associated with high ERG transcript levels. Falzarano et al.13 reported that the overall specificity and sensitivity of ERG immunohistochemical staining were 99% and 96%, respectively. Immunohistochemistry (IHC) is a routine laboratory procedure and a relatively simple, low-cost method compared to other molecular techniques such as FISH and QPCR. Therefore, given its cost-efficiency, immunohistochemical staining is a novel candidate approach for detecting ERG expression.
In this study, we analyzed ERG protein expression in large series of radical prostatectomy specimens using immunohistochemical staining. We also investigated the relationship between IHC results and clinical and pathological parameters.
MATERIALS AND METHODS
Patient information
We collected paraffin-embedded prostate tissue samples from a total of 303 patients who underwent radical prostatectomy between 1999 and 2006. Radical prostatectomy specimens were routinely cut in 4 mm-thick transverse slices with perpendicular sections from the apex to the base (bladder neck) and totally embedded in paraffin. The Gleason score, tumor volume, extraprostatic invasion, surgical margin status, seminal vesicle invasion, a pT and a pN stage were evaluated using pathologic records and slide review. Age and preoperative, initial plasma levels of protein-specific antigen (PSA) were collected from patient medical records. The Gleason pattern was assessed in accordance with the criteria outlined at the 2005 International Society of Urological Pathology (ISUP) Consensus Conference.14 A pathologic stage was assessed in accordance with the 7th Edition Cancer Staging Guidelines established by the American Joint Committee on Cancer in 2010.
Tissue microarray (TMA) construction
To construct the TMA, one representative core tumor tissue section (2 mm in diameter) was taken from an individual paraffin block and arranged in a new TMA block using a trephine apparatus (SuperBioChips Laboratories, Seoul, Korea).15
Immunohistochemistry (IHC)
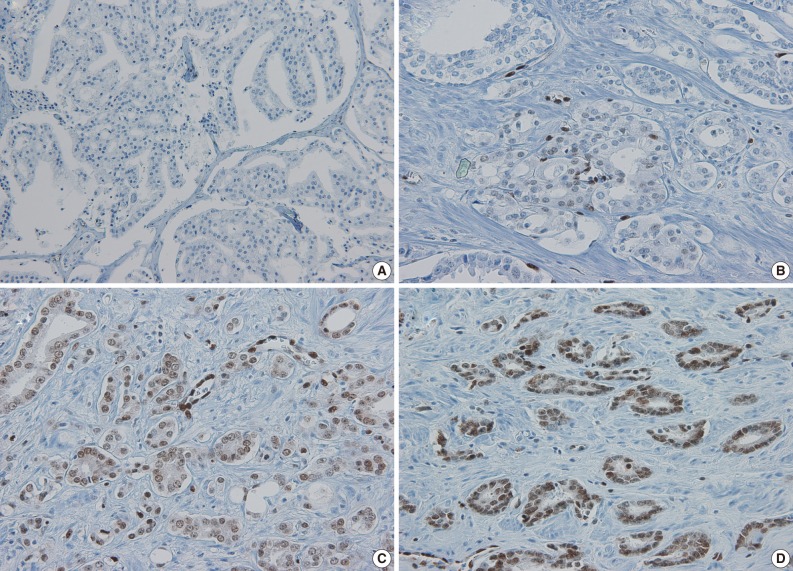
Sections mounted on superfrost slides were deparaffinized. IHC was conducted using Ventana Benchmark XT automated staining system (Ventana Medical Systems, Tucson, AZ, USA) and reagents, and an anti-ERG rabbit monoclonal antibody (RTU, clone EPR3864, Ventana Medical Systems).10 Heat-induced epitope retrieval was performed for anti-ERG antibody. Primary antibody was incubated for 16 minutes at room temperature. For IHC using the anti-ERG rabbit monoclonal antibody (rabbit ERG-MAb), a secondary antibody (Ultraview anti-Rabbit HRP, Ventana Medical Systems) was applied for 16 minutes at room temperature. Secondary antibody detection was performed using the Ultraview DAB detection kit (Ventana Medical Systems). Slides were counterstained with Hematoxylin II for 4 minutes followed by the addition of Bluing Reagent (Ventana Medical Systems) for 4 minutes at 37.1℃. The nuclei of endothelial cells were used as intrinsic positive control for ERG protein expression, as previously described.10 To interpret ERG immunohistochemical staining, the intensity of ERG expression was scored as negative (0, no staining), weak (1+, only visible at high magnification), moderate (2+, visible at low magnification) or strong (3+, striking at low magnification) (Fig. 1). Repeated immunohistochemical staining was performed using full section of a matched radical prostatectomy specimen in cases where equivocal results were found in immunohistochemical staining using TMA.
Fig. 1.
Immunohistochemical staining for ETS related gene (ERG). Representative examples showing (A) negative, (B) weak positive (1+), (C) moderately positive (2+), and (D) strongly positive (3+) staining for ERG.
Statistics
The chi-square test was used to compare the results of ERG immunostaining with clinicopathologic variables and the histologic pattern. All reported p-values were two-sided, and significance was set at p<0.05. All statistical analyses were conducted using SPSS ver. 19.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinicopathological factors
The mean age of prostatectomy patients was 65.7 years (median, 66 years; standard deviation [SD], 6.4 years; range, 44 to 85 years). The mean initial PSA level was 11.0 ng/mL (median, 8.7 ng/mL; SD, 11.2 ng/mL; range, 1.7 to 98 ng/mL). The mean percentage of the tumor volume of the prostatectomy specimens was 18.6% (median, 10%; SD, 18.3%; range, 1 to 95%).
Correlation between ERG immunoreactivity and clinicopathological factors
Of the 303 cases, only 74 (24.4%) showed positive ERG immunohistochemical staining. Among them, 31.1% (26/74) were scored as 1+, 51.3% (38/74) as 2+, and 17.6% (13/74) as 3+. To perform subgroup analysis, the primary Gleason pattern was categorized into the group with grade 3 and the group with grade 4 or 5. The Gleason score was also categorized as below 7 and above 8. The pathologic staging was simply classified using pT2 (including pT2a, pT2b, and pT2c) and pT3 (including pT3a, pT3b, and pT3c).
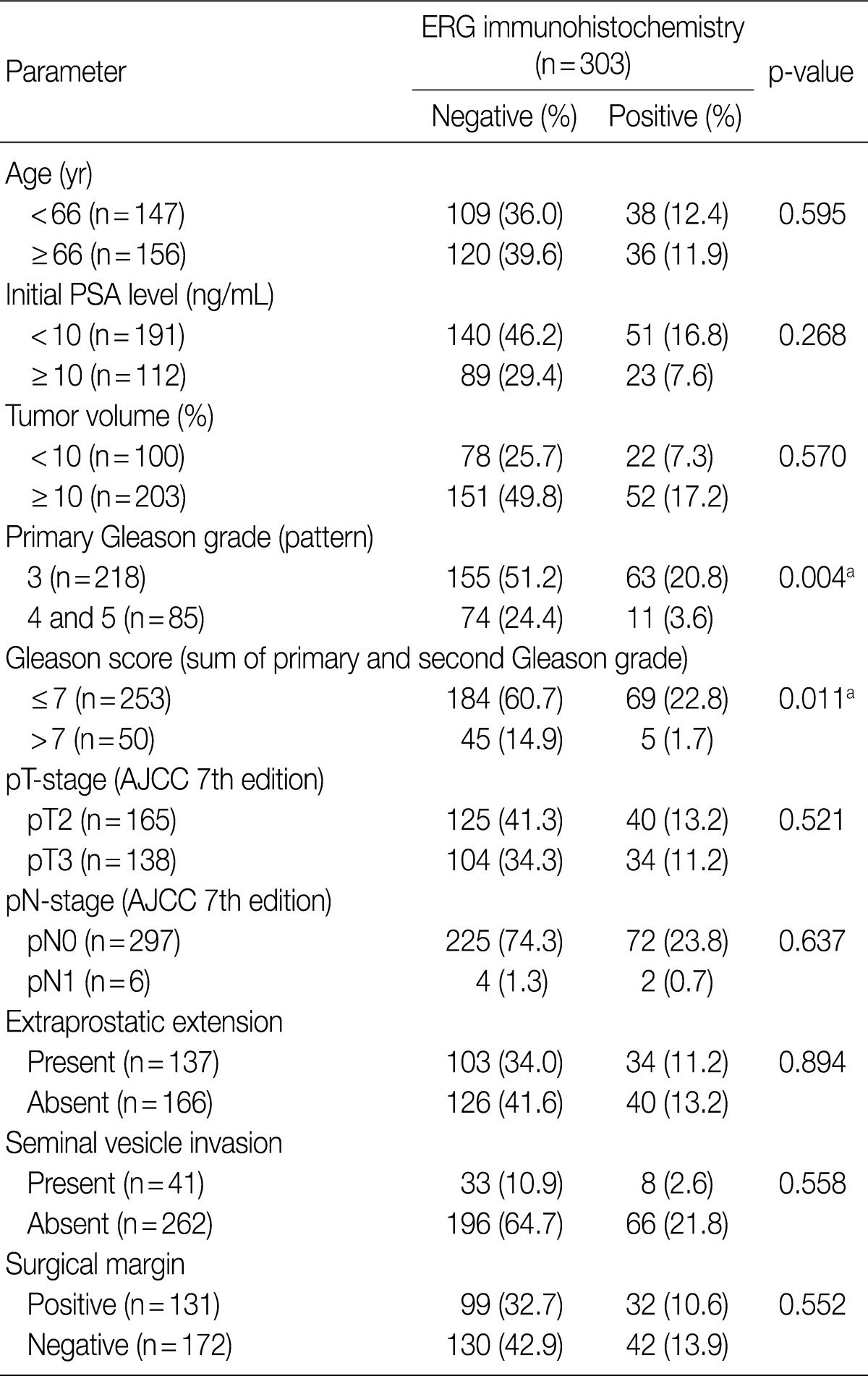
The correlation between clinicopathological factors and ERG immunostaining is summarized in Table 1. As shown in the Table, ERG expression was more frequently detected in the subgroup with a lower primary Gleason grade than in the subgroup with a higher grade (p=0.004). Additionally, the frequency of ERG expression was significantly higher in the subgroup with the lower Gleason score (≤7) than in the subgroup with the higher score (p=0.011). No other clinicopathological factors correlated with ERG expression.
Table 1.
Correlation between clinicopathological factors and results of ERG immunostaining
AJCC, American Joint Committee on Cancer; ERG, ETS related gene; PSA, prostate specific antigen.
ap<0.05.
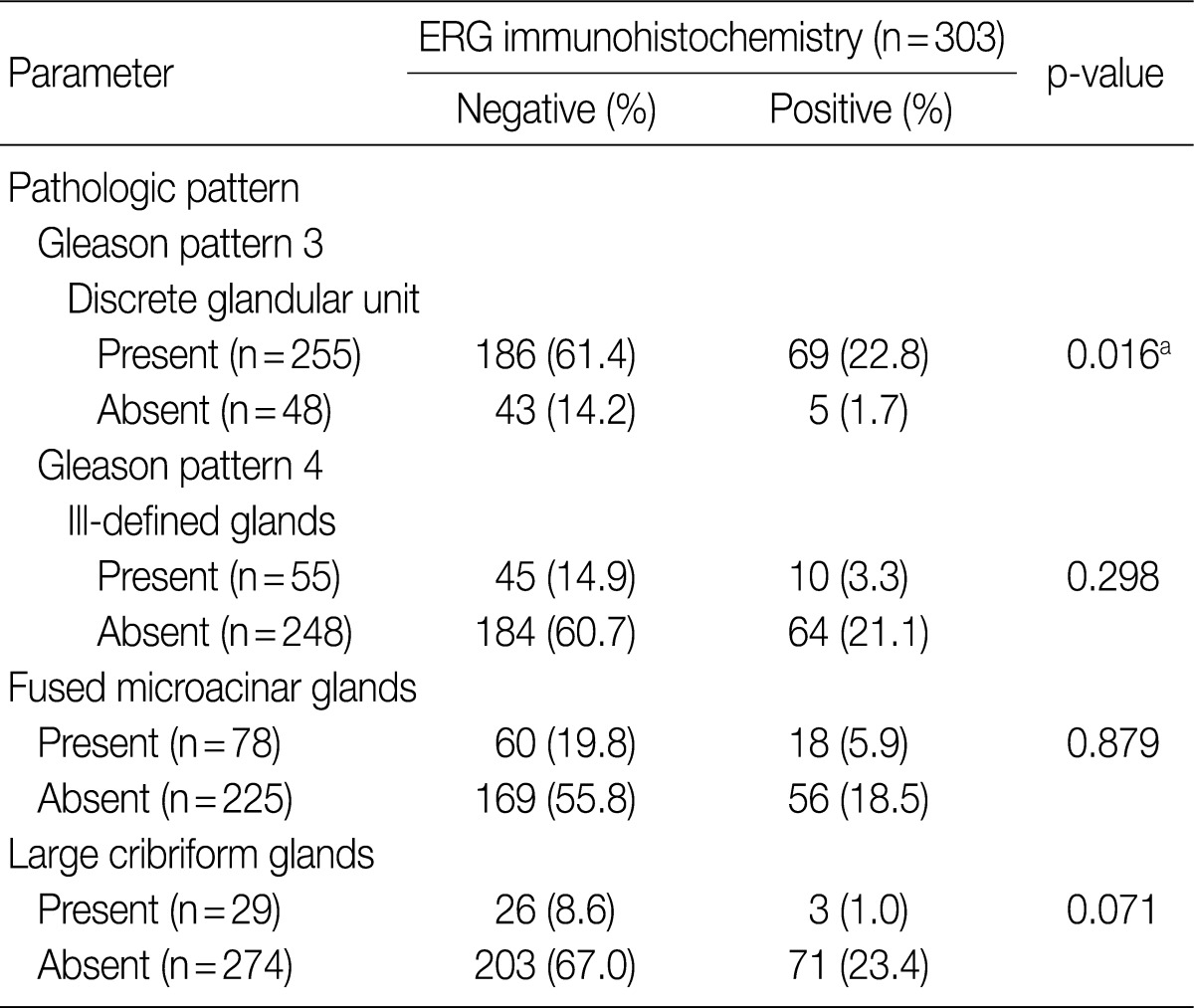
To evaluate the relationship between ERG protein expression detected by immunohistochemical staining and the histologic pattern of prostate adenocarcinoma subgroups were defined using the histologic patterns described in the 2005 ISUP Modified Gleason System (see Materials and Methods). Table 2 shows that ERG staining differed depending on the histologic pattern. There was a significantly higher incidence of ERG-positive staining in tumors with discrete glandular units (Gleason pattern 3; 69/225 cases, 27.1%) than in tumors without them (5/48 cases, 10.4%) (p=0.016). Although statistical significance was not achieved due to the small sample size, the cases with large cribriform glands showed lower incidence of ERG-positive staining (3/29 cases, 10.3%) than those without them (71/274 cases, 25.9%) (Table 2, Fig. 2). Moreover, 25 (33.8%) of the 74 ERG positive cases showed heterogeneous staining.
Table 2.
Correlation between prostate adenocarcinoma histologic pattern and results of ETS related gene (ERG) immunostaining
ap<0.05.
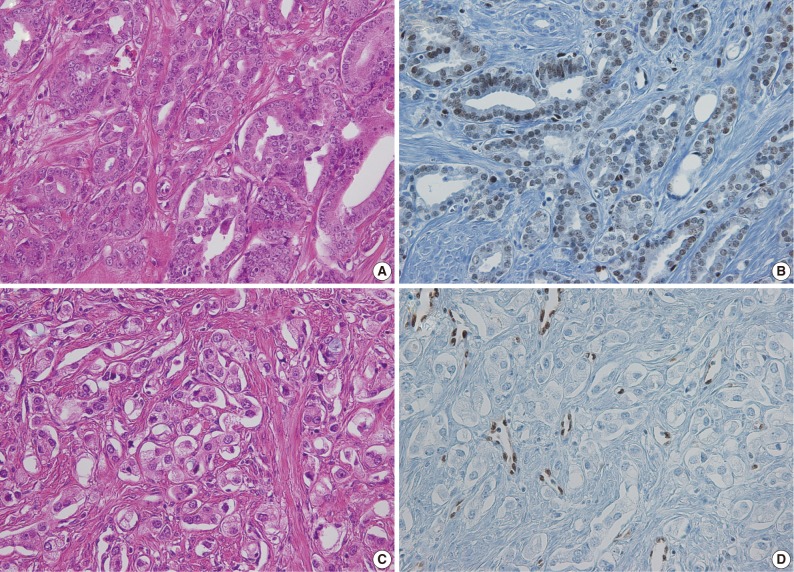
Fig. 2.
Prostate cancer with lower Gleason grade and well-formed glands on hematoxylin and eosin (H&E) slides showing (A) positive ETS related gene (ERG) immunostaining. (B) Tumor with high Gleason grade and ill-defined glands on H&E slide (C) showing negative ERG immunostaining (D). (B, D) Endothelial cells showing positive ERG immunoreactivity.
DISCUSSION
Tomlins et al.1 first reported the recurrent genetic fusion of TMPRSS2 and ERG. ERG, a member of the ETS family of transcription factors, has been associated with normal developmental processes, such as mesoderm formation, among others.16 In contrast to mesenchymal tumors, the recurrent genetic fusion that occurs during tumorigenesis is very rare in carcinomas. Furthermore, the TMPRSS2-ERG fusion may serve to explain the mechanism underlying oncogenic protein overexpression, although this case is not yet fully investigated.17
Since the discovery of the TMPRSS2-ERG fusion, several groups have examined its clinical implications for the prognosis of prostate adenocarcinoma. However, because of the variability of study cohorts, clinical endpoints and the methods for gene fusion detection, the relationship between gene fusion and clinical outcome has been variably described. Barwick et al.18 reported that the TMPRSS2-ERG fusion is associated with the prognosis of biochemical recurrence in multiple cohorts. On the other hand, Hermans et al.19 concluded that a favorable prognosis is significantly related to the TMPRSS2-ERG gene fusion. Several articles demonstrated that TMPRSS2-ERG gene fusion expression is an independent predictor of poor outcome in prostate cancer patients.20,21 However, other studies have indicated that the ERG gene fusion has no significant clinical implications or prognostic value.22,23
In our study, the ERG positive rate was 24.4%, which is relatively lower than those reported in western population-based studies. Nevertheless, it has been observed that the frequency of the TMPRSS2-ERG gene fusion ranges widely from 15.3% to over 70% depending on the detection method and the study groups.2,3 In a study involving the Japanese population, a relatively lower rate of ERG positivity has been reported (20.1% and 28%).5,6 Thus, in view of the present as well as the previous findings, it can be suggested that East Asian prostate cancer patients have a lower ERG-positive rate.
The associations between TMPRSS2-ERG gene fusion status and clinicopathologic parameters have varied between studies. While Attard et al.7 found higher ERG gene fusion rate in tumor cells with a higher Gleason grade, some groups reported no significant association between the Gleason pattern and ERG gene status.24,25 More recent studies, however, have shown that a lower Gleason grade is associated with a higher ERG gene fusion rate.26,27 Notably, our results coincide with the findings of these recent studies.
Several previous reports also showed that a higher PSA level and a higher pT stage were positively associated with ERG fusion rate.3,23 However, more recent studies identified lack of significant association between a T stage and a PSA level.28,29 We conclude that only primary Gleason patterns and Gleason scores are associated with a ERG-positive rate. Previous studies have also reported varied relationship between ERG gene fusion status and the histologic tumor pattern. Mosquera et al.24 found that the ERG gene fusion is more frequently observed in higher grade features of prostate cancer. However, another study noted that the ERG-positive rate is higher in Gleason pattern 3 tumors than in Gleason pattern 4 and 5 tumors.28,29 Our ERG immunohistochemical results showed a higher ERG-positive rate in tumors with Gleason pattern 3 which is represented by discrete glandular units. Moreover, although not statistically significant (p=0.071), a lower ERG-positive rate was observed in Gleason pattern 4 tumors containing large cribriform glands than in tumors without them. Furthermore, a number of studies have reported that heterogeneous ERG immunostaining was observed in the same tumor.12,28 In this study, we also observed heterogeneous ERG staining in 25 cases.
We previously reported the relationship between ERG gene rearrangement status and FISH analysis in the same cohort.4 As such, we can compare the present immunohistochemical staining results to those of the FISH. Compared with the results on FISH, the sensitivity of ERG immunohistochemical staining is 89.8%, and the specificity is 96.4%.
In summary, using ERG immunohistochemical staining on TMA, we found the ERG-positive rate in Korean prostate cancer cases to be 24.4%. It is significantly higher in tumors with a lower Gleason grade represented by discrete glandular units, and in cases displaying a lower primary Gleason grade and a lower Gleason score. Nevertheless, this rate is relatively lower than those observed in studies involving western populations.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant number: 2010-0004550).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 2.Demichelis F, Fall K, Perner S, et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26:4596–4599. doi: 10.1038/sj.onc.1210237. [DOI] [PubMed] [Google Scholar]
- 3.Rouzier C, Haudebourg J, Carpentier X, et al. Detection of the TMPRSS2-ETS fusion gene in prostate carcinomas: retrospective analysis of 55 formalin-fixed and paraffin-embedded samples with clinical data. Cancer Genet Cytogenet. 2008;183:21–27. doi: 10.1016/j.cancergencyto.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Lee K, Chae JY, Kwak C, Ku JH, Moon KC. TMPRSS2-ERG gene fusion and clinicopathologic characteristics of Korean prostate cancer patients. Urology. 2010;76:1268.e7–1268.e13. doi: 10.1016/j.urology.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Furusato B, van Leenders GJ, Trapman J, et al. Immunohistochemical ETS-related gene detection in a Japanese prostate cancer cohort: diagnostic use in Japanese prostate cancer patients. Pathol Int. 2011;61:409–414. doi: 10.1111/j.1440-1827.2011.02675.x. [DOI] [PubMed] [Google Scholar]
- 6.Miyagi Y, Sasaki T, Fujinami K, et al. ETS family-associated gene fusions in Japanese prostate cancer: analysis of 194 radical prostatectomy samples. Mod Pathol. 2010;23:1492–1498. doi: 10.1038/modpathol.2010.149. [DOI] [PubMed] [Google Scholar]
- 7.Attard G, Clark J, Ambroisine L, et al. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene. 2008;27:253–263. doi: 10.1038/sj.onc.1210640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saramäki OR, Harjula AE, Martikainen PM, Vessella RL, Tammela TL, Visakorpi T. TMPRSS2:ERG fusion identifies a subgroup of prostate cancers with a favorable prognosis. Clin Cancer Res. 2008;14:3395–3400. doi: 10.1158/1078-0432.CCR-07-2051. [DOI] [PubMed] [Google Scholar]
- 9.Tu JJ, Rohan S, Kao J, Kitabayashi N, Mathew S, Chen YT. Gene fusions between TMPRSS2 and ETS family genes in prostate cancer: frequency and transcript variant analysis by RT-PCR and FISH on paraffin-embedded tissues. Mod Pathol. 2007;20:921–928. doi: 10.1038/modpathol.3800903. [DOI] [PubMed] [Google Scholar]
- 10.Park K, Tomlins SA, Mudaliar KM, et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia. 2010;12:590–598. doi: 10.1593/neo.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaux A, Albadine R, Toubaji A, et al. Immunohistochemistry for ERG expression as a surrogate for TMPRSS2-ERG fusion detection in prostatic adenocarcinomas. Am J Surg Pathol. 2011;35:1014–1020. doi: 10.1097/PAS.0b013e31821e8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Leenders GJ, Boormans JL, Vissers CJ, et al. Antibody EPR3864 is specific for ERG genomic fusions in prostate cancer: implications for pathological practice. Mod Pathol. 2011;24:1128–1138. doi: 10.1038/modpathol.2011.65. [DOI] [PubMed] [Google Scholar]
- 13.Falzarano SM, Zhou M, Carver P, et al. ERG gene rearrangement status in prostate cancer detected by immunohistochemistry. Virchows Arch. 2011;459:441–447. doi: 10.1007/s00428-011-1128-4. [DOI] [PubMed] [Google Scholar]
- 14.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Yu J. Interrogating genomic and epigenomic data to understand prostate cancer. Biochim Biophys Acta. 2012;1825:186–196. doi: 10.1016/j.bbcan.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwamoto M, Higuchi Y, Enomoto-Iwamoto M, et al. The role of ERG (ets related gene) in cartilage development. Osteoarthritis Cartilage. 2001;9(Suppl A):S41–S47. doi: 10.1053/joca.2001.0443. [DOI] [PubMed] [Google Scholar]
- 17.Narod SA, Seth A, Nam R. Fusion in the ETS gene family and prostate cancer. Br J Cancer. 2008;99:847–851. doi: 10.1038/sj.bjc.6604558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barwick BG, Abramovitz M, Kodani M, et al. Prostate cancer genes associated with TMPRSS2-ERG gene fusion and prognostic of biochemical recurrence in multiple cohorts. Br J Cancer. 2010;102:570–576. doi: 10.1038/sj.bjc.6605519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermans KG, Boormans JL, Gasi D, et al. Overexpression of prostate-specific TMPRSS2(exon 0)-ERG fusion transcripts corresponds with favorable prognosis of prostate cancer. Clin Cancer Res. 2009;15:6398–6403. doi: 10.1158/1078-0432.CCR-09-1176. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimoto M, Joshua AM, Cunha IW, et al. Absence of TMPRSS2:ERG fusions and PTEN losses in prostate cancer is associated with a favorable outcome. Mod Pathol. 2008;21:1451–1460. doi: 10.1038/modpathol.2008.96. [DOI] [PubMed] [Google Scholar]
- 21.Nam RK, Sugar L, Wang Z, et al. Expression of TMPRSS2:ERG gene fusion in prostate cancer cells is an important prognostic factor for cancer progression. Cancer Biol Ther. 2007;6:40–45. doi: 10.4161/cbt.6.1.3489. [DOI] [PubMed] [Google Scholar]
- 22.Minner S, Enodien M, Sirma H, et al. ERG status is unrelated to PSA recurrence in radically operated prostate cancer in the absence of antihormonal therapy. Clin Cancer Res. 2011;17:5878–5888. doi: 10.1158/1078-0432.CCR-11-1251. [DOI] [PubMed] [Google Scholar]
- 23.Mehra R, Tomlins SA, Shen R, et al. Comprehensive assessment of TMPRSS2 and ETS family gene aberrations in clinically localized prostate cancer. Mod Pathol. 2007;20:538–544. doi: 10.1038/modpathol.3800769. [DOI] [PubMed] [Google Scholar]
- 24.Mosquera JM, Perner S, Demichelis F, et al. Morphological features of TMPRSS2-ERG gene fusion prostate cancer. J Pathol. 2007;212:91–101. doi: 10.1002/path.2154. [DOI] [PubMed] [Google Scholar]
- 25.Perner S, Demichelis F, Beroukhim R, et al. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res. 2006;66:8337–8341. doi: 10.1158/0008-5472.CAN-06-1482. [DOI] [PubMed] [Google Scholar]
- 26.Bismar TA, Dolph M, Teng LH, Liu S, Donnelly B. ERG protein expression reflects hormonal treatment response and is associated with Gleason score and prostate cancer specific mortality. Eur J Cancer. 2012;48:538–546. doi: 10.1016/j.ejca.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Darnel AD, Lafargue CJ, Vollmer RT, Corcos J, Bismar TA. TMPRSS2-ERG fusion is frequently observed in Gleason pattern 3 prostate cancer in a Canadian cohort. Cancer Biol Ther. 2009;8:125–130. doi: 10.4161/cbt.8.2.7134. [DOI] [PubMed] [Google Scholar]
- 28.Hoogland AM, Jenster G, van Weerden WM, et al. ERG immunohistochemistry is not predictive for PSA recurrence, local recurrence or overall survival after radical prostatectomy for prostate cancer. Mod Pathol. 2012;25:471–479. doi: 10.1038/modpathol.2011.176. [DOI] [PubMed] [Google Scholar]
- 29.Gopalan A, Leversha MA, Satagopan JM, et al. TMPRSS2-ERG gene fusion is not associated with outcome in patients treated by prostatectomy. Cancer Res. 2009;69:1400–1406. doi: 10.1158/0008-5472.CAN-08-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]