Abstract
Background
Cortactin and focal adhesion kinase (FAK) are two important components among actin cross-linking proteins that play a central role in cell migration.
Methods
The aims of this study were to evaluate the expression of cortactin and FAK in normal colorectal mucosa and colorectal adenocarcinoma (CRC) using tissue microarray of 2 mm cores to correlate their expression with other clinicopathological factors and, investigate their prognostic significance.
Results
Twenty (9%) and 24 cases (11%) of normal colorectal mucosa were immunoreactive for cortactin and FAK. In addition, 184 (84%) and 133 cases (61%) of CRCs were immunoreactive for cortactin and FAK, respectively. Cortactin expression was associated with histologic differentiation and FAK expression. Cortactin, but not FAK expression was also correlated with poor overall and relapse-free survival and served well as an independent prognostic factor for poor survival.
Conclusions
Cortactin expression, in association with FAK expression, may plays an important role in tumor progression. Furthermore, it may also be a satisfactory biomarker to predict tumor progression and survival in CRC patients.
Keywords: Colorectal neoplasms, Cortactin, Focal adhesion protein-tyrosine kinases, Immunohistochemistry
Cortactin is a p80/p85 multi-domain protein that was first identified as a major substrate of the Src oncogene.1 Later it was confirmed to be phosphorylated on tyrosine in response to stimuli such as fibroblast growth factors, epidermal growth factors and integrins that induce remodeling of the cortical actin cytoskeleton.2 Cortactin consists of an N-terminal acidic domain that binds to and activates the actin-related protein-2/3 (Arp2/3) complex, and the six-and-a half tandem repeats (cortactin repeats) at its N-terminus, followed by an α-helix, a proline-rich region and an Src Homology-3 domain at its C-terminus.3 Cortactin functions in an actin assembly via interaction with the Arp2/3 complex, which is dependent on Src-mediated phosphorylation of cortactin. While Src connects cortactin to growth factor receptors and mitogen-activated protein kinase signaling, Rho family GTPases, such as Rac and Cdc42, as well as focal adhesion kinase (FAK)/Arp3 interaction render cortactin function possible in stress fiber assembly and the formation of lamellipodia and filopodia.4,5 Remodeling of the actin cytoskeleton has effects on cell migration, motility, and adhesion, as well as on tumor invasion and metastasis.6 Recent reports have revealed that cortactin is overexpressed in many types of human cancers, including head and neck, colorectal, gastric, hepatocellular, breast and ovarian cancers.7-12 Interestingly, human cortactin was identified as one of the overexpressed genes within the amplified chromosome 11q13 region13 and is also frequently amplified in human malignancies such as breast carcinoma, head and neck carcinoma, and gastric adenocarcinoma.8,9,14 In some studies, the amplification of 11q13 and overexpression of cortactin correlate with poor prognosis for patients with lymph node metastasis.9,14 However, overexpression has also been reported in tumors without that amplification.12
FAK is a 125 kDa protein tyrosine kinase which is a critical mediator of signaling events between cells and their extracellular matrix, and plays a central role in cell migration.15 FAK is typically located at structures known as focal adhesions that are multi-protein structures linking the extracellular matrix to the cytoplasmic cytoskeleton.16 Additional components of focal adhesions include actin, filamin, cinculin, talin, paxillin, and tensin.17 In tumor cells, FAK is thought to have a dual function of promoting tumor cell adhesion and inhibiting apoptosis.15 Increased expression of FAK has been detected in tumors of the breast, prostate, colon, and brain.16,18
Colorectal carcinoma is one of the most common carcinomas in the world. Despite the recent development of diagnostic and therapeutic procedures, the death rate has not changed during the past fifty years.19 Recent studies indicate that enhancement of cell motility and loss of cell-cell adhesion is essential to tumor progression. Cortactin and FAK are two important components among these actin cross-linking proteins. It has been reported that FAK-Src complex modulates the tyrosine phosphorylation of cortactin which promotes cell motility.20 In addition, it has been reported that both cortactin and FAK are involved in the malignant transformation of laryngeal premalignancies.11 However, the relationship between the expressions of cortactin and/or FAK and clinicopathological parameters of colorectal adenocarcinoma (CRC) remains unknown. In this study, we performed immunohistochemical staining to examine the expression of cortactin and FAK in CRCs, and to correlate this with the clinicopathologic factors and prognosis of CRCs.
MATERIALS AND METHODS
Case selection and specimens
In this study, we only included CRC and excluded other histologic variants. A total of 220 CRC samples were obtained from patients who had undergone surgery between 2003 and 2004 at Chonbuk National University Hospital. Patients who received preoperative chemotherapy or radiotherapy were excluded from this study. We obtained clinical data from the patients' records, and survival data were collected in August 2011. This study received a local ethics committee approval from the Institutional Review Board of Chonbuk National University Hospital. Of the 220 patients, 136 patients were male and 84 patients were female. The mean age of the patients at the time of surgery was 62.2 years (range, 28 to 84 years). Hematoxylin and eosin (H&E) stained slides were reviewed and were graded according to the World Health Organization classification.21 Pathologic staging was reviewed based on the tumor, node, metastasis staging system described by the American Joint Committee on Cancer.22 The patients were grouped according to their age, gender, location, tumor size, differentiation, lymphovascular invasion, pathologic stage (I and II vs III and IV), and preoperative serum carcinoembryonic antigen (CEA) level. Formalin-fixed paraffin-embedded tissue blocks of 220 cases were obtained, and three cores were taken from every case. Each case was represented by one normal core and two tumor cores with a cross-sectioned diameter of 2 mm. The tissue microarray slides from original paraffin-embedded specimens were stained uniformly with H&E. Each pathological diagnosis was reviewed by two experienced pathologists (H.S.Park and Y.N.Kim) who were blind to each other's evaluation. Paraffin-embedded tissue samples for immunohistochemistry were provided by the Chonbuk National University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health, Welfare and Family Affairs.
Immunohistochemistry and interpretation
Tissue microarray sections were cut into 4-µm thick tissue sections, and endogenous activity was quenched by incubation with 3% hydrogen peroxidase for 30 minutes after deparaffinization and hydration. Tissue sections were treated with a microwave antigen retrieval procedure in 0.01 M sodium citrate buffer (pH 6.0) for cortactin and with a high pressure cooker antigen retrieval procedure in 0.01 M sodium citrate buffer (pH 9.0) for FAK. After blocking endogenous peroxidase, sections were incubated with Protein Block Serum-Free (Dako, Carpinteria, CA, USA) at room temperature for 10 minutes to block non-specific staining. The sections were then incubated at 4℃ overnight with monoclonal anti-cortactin (1:100, clone 30, BD Bioscience, San Jose, CA, USA) and polyclonal anti-FAK (1:50, Cell Signaling Technology, Danvers, MA, USA). After incubation with the appropriate biotin-conjugated secondary antibody and subsequently with a streptavidin solution, color development was done using 3-amino-9-ethylcarbazole as a chromogen and the tissues were counterstained with hematoxylin. An immunohistochemical analysis was done by four authors (Y.N.Kim, J.E.Choi, K.Y.Jang, and H.S.Park) without knowledge of the clinicopathologic information. Each case was evaluated by multiplying score of staining intensity and stained area of cortactin and FAK. Staining intensity was determined as negative, 0; weak, 1; moderate, 2; and strong, 3. Stained area was divided into four groups: negative, 0; ≤25% of cells, 1; >25% and ≤50% of cells, 2; >50% of cells, 3. In addition, immunoreactivity was divided into two groups on the basis of the multiplying score; 0-2 was defined as negative, ≥3 was defined as positive immunoreactivity.7 Of the 220 cases, 2 cases for cortactin and 3 cases for FAK were missed during immunostaining. Eventually, 218 cases for cortactin and 217 cases for FAK were evaluated for immunohistochemical analysis.
Statistical analysis
The points of interest were the relapse-free survival and the overall survival of patients. The point that required follow-up was the last date of contact or the patient's date of death until August 2011. Overall survival was calculated as the time from diagnosis to the date of death or last contact. Patients who were alive at last contact were treated as censored for overall survival analysis. Relapse-free survival was calculated from the time of diagnosis to the date of recurrence, distant metastasis, death, or last contact. Patients who were alive at the last contact and who had not experienced recurrence were treated as censored for relapse-free survival analysis. The associations between the expression of cortactin or FAK and other clinicopathologic factors were analyzed using Pearson's chi-square test. A univariate and multivariate Cox proportional hazard regression analysis was done to estimate the impact of clinicopathologic factors and the expression of each marker on a relapse-free survival and overall survival. Kaplan-Meier survival curves were constructed to further illustrate the impact of overall survival when indicated. SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and a p-value of <0.05 was considered statistically significant.
RESULTS
Expression of cortactin and FAK and their association with clinicopathologic variables in CRCs
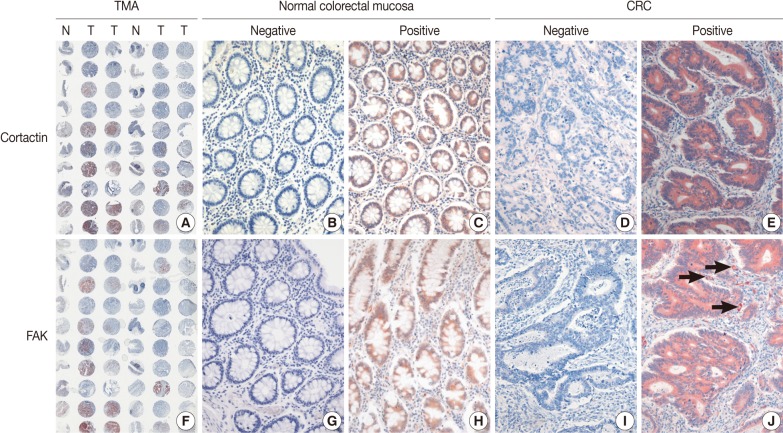
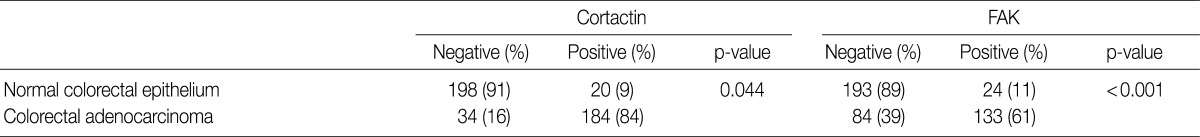
The immunohistochemical staining of cortactin and FAK in microarray tissue of normal colorectal mucosa and CRC are shown in Fig. 1. Expression of cortactin and FAK was found in epithelial cells, endothelial cells and smooth muscle cells, but not in other stromal cells. The intensity of cytoplasmic expression of cortactin and FAK were only weak to moderate in normal colorectal mucosal epithelium, and were moderate to strong in CRCs. In CRCs, there was no difference in staining intensity of cortactin and FAK whether or not the tumor cells were located in the center of tumor nests. As shown in Table 1, cortactin expression was seen in 84% (184 of 218 cases) of CRCs and 9% (20 of 218 cases) of normal colorectal mucosa, respectively, while FAK expression was seen in 61% (133 of 217 cases) of CRCs and 11% (24 of 217 cases) of normal colorectal mucosa, respectively. The positive expression rate of cortactin and FAK was significantly higher in CRCs than in normal colorectal mucosa.
Fig. 1.
Immunohistochemical staining of cortactin (A-E) and focal adhesion kinase (FAK) (F-J) in microarray tissues of normal colorectal mucosa and colorectal adenocarcinoma (CRC). Tissue microarray (TMA) slide (A, F) is represented by one core of normal colorectal mucosa (N) and two cores of CRC (T). Most normal colorectal mucosas are negative for cortactin (A, B) and FAK (F, G). Some normal colorectal mucosas are positive for cortactin (C) and FAK (H), however, the intensity of cytoplasmic expression is weak to moderate. Some CRCs are negative for cortactin (A, D) and FAK (F, I), whereas most are positive for cortactin (E) and FAK (J) with more intense staining than those of normal colorectal mucosas. Note the expression of FAK on peritumoral blood vessels (J, arrows).
Table 1.
Expressions of cortactin and focal adhesion kinase (FAK) in normal colorectal epithelium and colorectal adenocarcinoma
Values are presented as number (%).
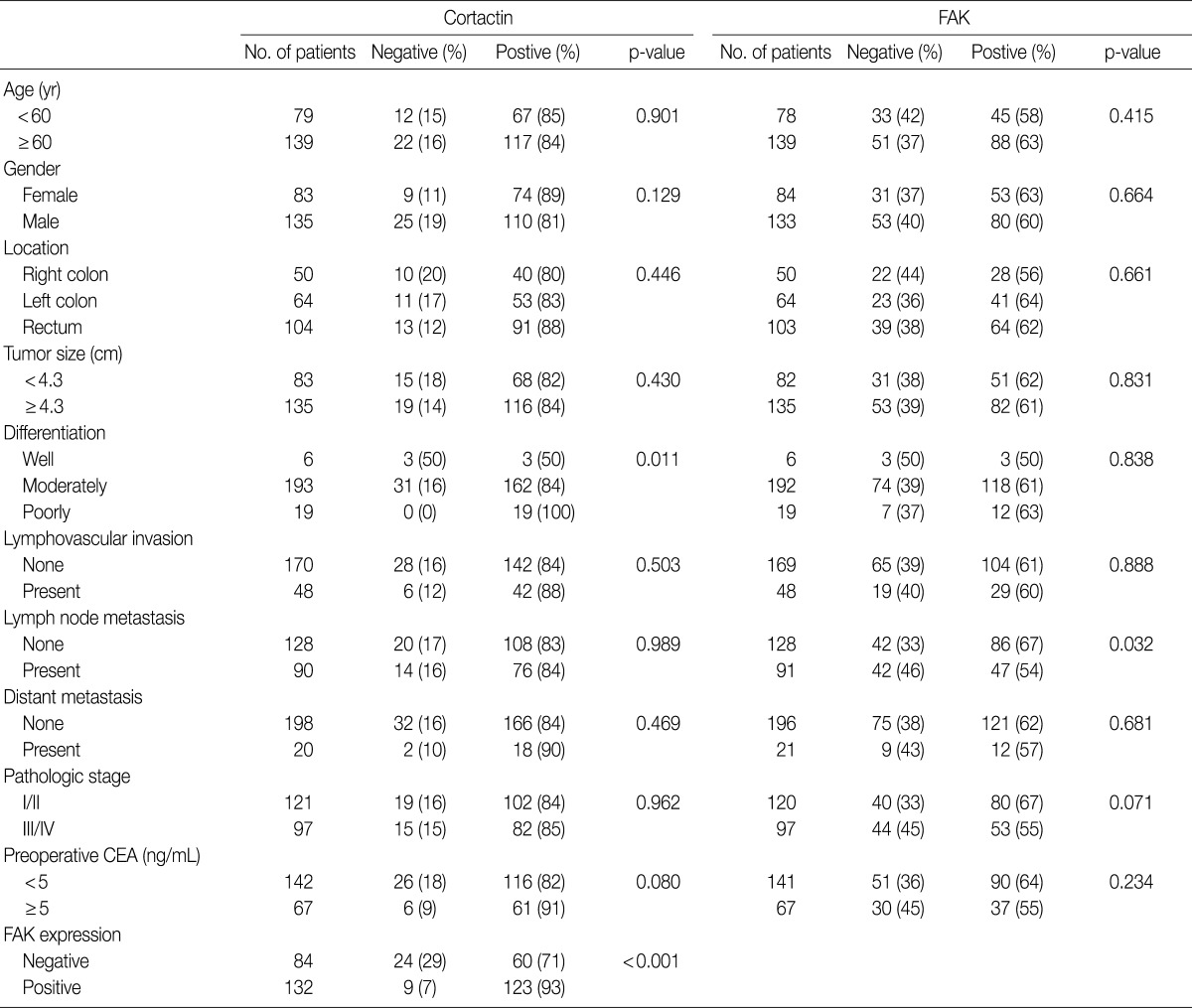
The clinicopathologic features are summarized in Table 2. Among the 218 cases of CRCs, cortactin expression was correlated with poor differentiation (p=0.011) and positive expression of FAK (p<0.001). Other clinicopathologic variables including age, gender, location, tumor size, lymphovascular invasion, pathologic stage and preoperative CEA had no association with cortactin expression. However, among the 217 cases of CRCs, FAK expression was correlated with lymph node metastasis (p=0.032) but not with other clinicopathological variables such as age, gender, location, tumor size, differentiation, lymphovascular invasion, pathologic stage and preoperative CEA.
Table 2.
Correlation of cortactin and FAK expression and clinicopathologic variables
Values are presented as number (%).
FAK, focal adhesion kinase; CEA, carcinoembryonic antigen.
Correlation of the expression of cortactin and FAK with overall survival and relapse-free survival in CRCs
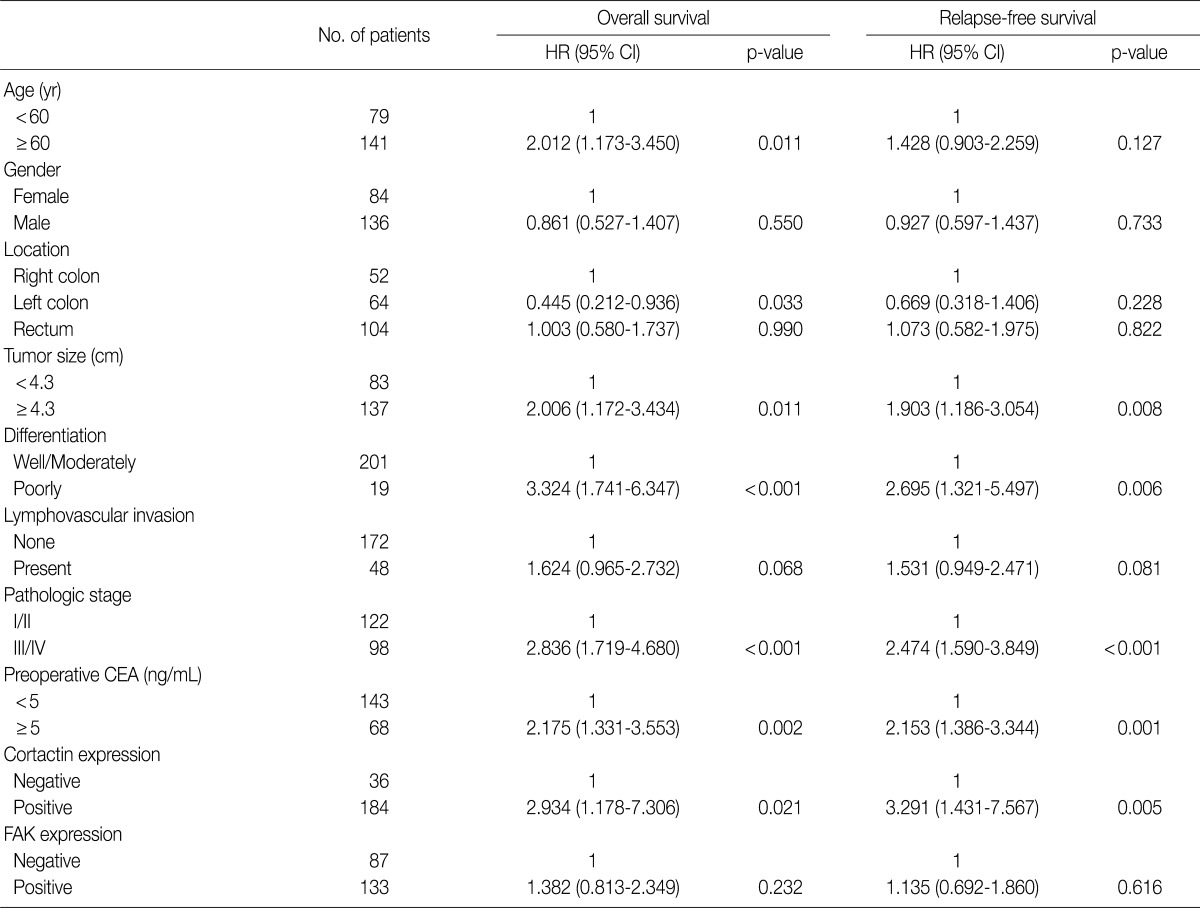
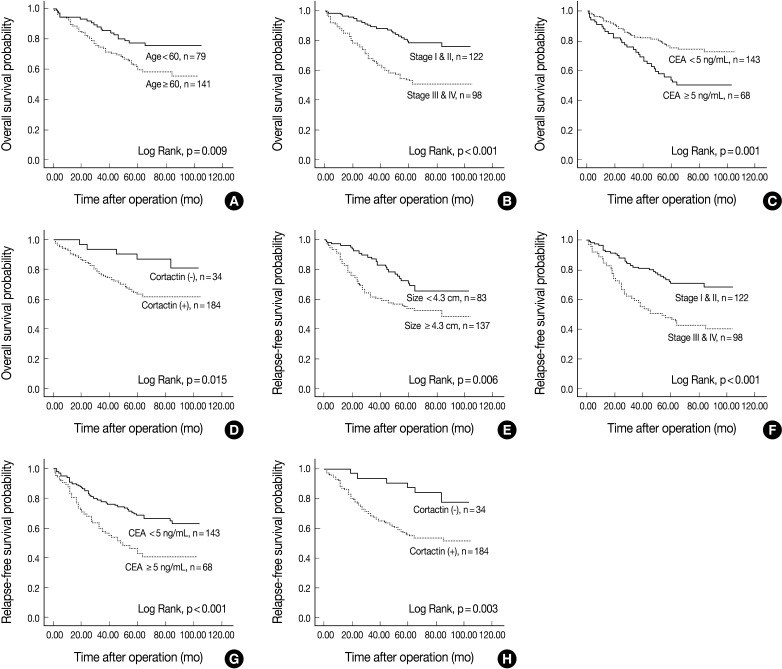
The univariate Cox proportional hazard analysis of the expression of cortactin and FAK and their association with overall survival and relapse-free survival are shown in Table 3. Patient's age over 60 years (p=0.011), tumor size larger than 4.3 cm (p=0.011), poor differentiation (p<0.001), advanced pathologic stage (p<0.001), preoperative CEA level over 5.0 ng/mL (p=0.002) and cortactin expression (p=0.021) predicted shorter overall survival. Tumors located in the left colon (p=0.033) predicted longer overall survival than those in the right colon. On the other hand, a tumor size larger than 4.3 cm (p=0.008), a tumor with poor differentiation (p=0.006), an advanced pathologic stage (p<0.001), a preoperative CEA level over 5.0 ng/mL (p=0.001) and a cortactin expression (p=0.005) predicted shorter relapse-free survival. However, FAK expression in CRCs predicted neither shorter overall nor shorter relapse-free survival. Kaplan-Meier survival curves for overall survival in relation to the patient's age, pathologic stage, preoperative CEA level, and cortactin expression in CRCs are presented in Fig. 2A-D. In addition, Kaplan-Meier survival curves for relapse-free survival in relation to tumor size, pathologic stage, preoperative CEA level and cortactin expression in CRCs are also presented in Fig. 2E-H.
Table 3.
Univariate cox regression analysis of overall and relapse-free survival according to the clinicopathologic parameters and the expression of cortactin or FAK
FAK, focal adhesion kinase; HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen.
Fig. 2.
Kaplan-Meier survival curves for overall survival in colorectal adenocarcinomas (CRCs) (A-D). Patients' age over 60 yr (A), advanced pathologic stage (B), preoperative carcinoembryonic antigen (CEA) level over 5.0 ng/mL (C) and cortactin expression (D) predict poor overall survival. Kaplan-Meier survival curves for relapse-free survival in CRCs (E-H). Tumor size larger than 4.3 cm (E), advanced pathologic stage (F), preoperative CEA level over 5.0 ng/mL (G) and cortactin expression (H) predict poor relapse-free survival.
Expression of cortactin as an independent prognostic factor for worse survival outcome in CRCs
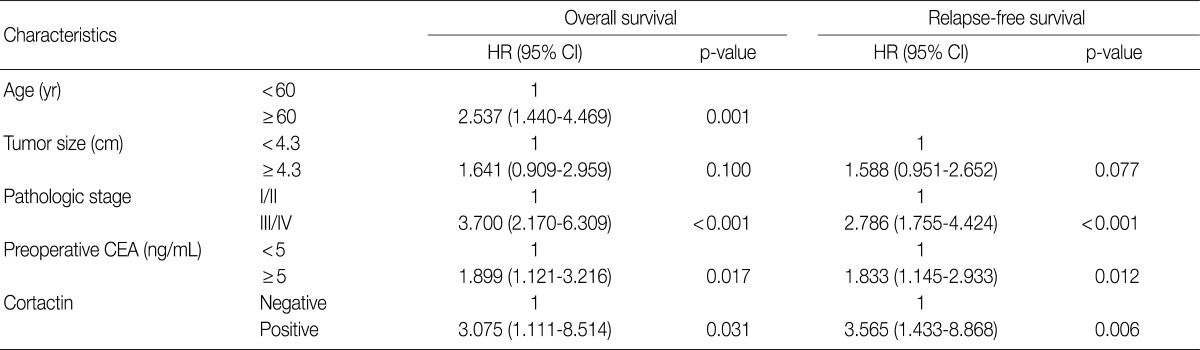
A multivariate analysis was done in 209 patients with complete information for clinicopathologic factors including age, tumor size, pathologic stage, preoperative CEA and cortactin expression. From the multivariate analysis, pathologic stage, preoperative CEA and cortactin expression were independent prognostic factors that were significantly associated with both overall and relapse-free survival (Table 4). An advanced stage (III/IV) was an independent prognostic factor which was significantly associated with poor overall (adjusted hazard ratio [HR], 3.700; 95% confidence interval [CI], 2.170 to 6.309; p<0.001) and relapse-free survival (adjusted HR, 2.786; 95% CI, 1.755 to 4.424; p<0.001). A preoperative CEA over 5 ng/mL was an independent prognostic factor significantly associated with poor overall (adjusted HR, 1.899; 95% CI, 1.121 to 3.216; p=0.017) and relapse-free survival (adjusted HR, 1.833; 95% CI, 1.145 to 2.933; p=0.012). Cortactin expression was an independent prognostic factor significantly associated with poor overall (adjusted HR, 3.075; 95% CI, 1.111 to 8.514; p=0.031) and relapse-free survival (adjusted HR, 3.565; 95% CI, 1.433 to 8.868; p=0.006). In addition, an age of over 60 years was also an independent prognostic factors significantly associated with poor overall (adjusted HR, 2.537; 95% CI, 1.440 to 4.469; p=0.001), but not relapse-free survival.
Table 4.
Multivariate cox regression analysis of overall and relapse-free survival according to the clinicopathologic parameters and cortactin expression
HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen.
DISCUSSION
In the present study, we examined the immunohistochemical expressions of cortactin and FAK in human CRCs and their prognostic significance. We have demonstrated that the positive expression rates of cortactin and FAK were significantly higher in CRCs than in normal colorectal mucosa. Cortactin expression correlated with poor differentiation and positive expression of FAK. It also served an independent prognostic factor and predicted shorter overall and relapse-free survival. Additionally, cortactin expression showed slight correlation with a high preoperative CEA level of more than 5 ng/mL. In contrast, FAK expression did not predict overall and relapse-free survival. However, it correlated with lymph node metastasis and albeit slightly, with an advanced pathologic stage.
That the expression of cortactin was higher and more intense in CRCs than in normal colorectal epithelia, this finding is consistent with other study results. For instance, Lee et al.10 found higher expression of cortactin in the CRC and tubular adenoma than in the normal colorectal epithelia. Thus, cortactin expression may be a critical event in the development of CRCs. On the other hand, Cai et al.7 reported that cortactin expression is a significant prognostic factor for overall survival in rectal cancer patients. They also mentioned that the expression of cortactin was associated with tumor invasion, histologic grade and preoperative CEA level in stage II & III CRC patients.7 Additionally, Hirakawa et al.23 demonstrated that overexpression of cortactin and its localization at the cell periphery were critical factors for the progression of cancer. They also suggested that the association of ZO-1 and cortactin plays an important role in regulating cell adhesion and spreading.23 In contrast, other investigators argued that cortactin expression was not a predictor for overall survival, although it was associated with more advanced cancer stages.10 Moreover, Zhang et al.24 reported that cortactin expression was negatively correlated with tumor-node-metastasis staging and lymphatic invasion status in CRC.
FAK expression was found to be higher in invasive tumors and metastatic tumors of the colon rather than in adenomatous tissues.25 This suggests that FAK expression may result in changes in the signaling pathways involved in tumor cell invasion. Cance et al.16 also showed up-regulation of FAK in tumor and relative lack of expression in normal colon tissue. Furthermore, Murata et al.26 suggested that high expression of FAK predicts the recurrence of colorectal cancer. Contrastingly, studies found that FAK expression is not a prognostic predictor in certain carcinomas such as colonic adenocarcinoma, small cell lung cancer, pancreatic cancer and squamous cell carcinoma of head and neck.27-30 Theocharis et al.27 reported that FAK expression had no association with any clinicopathologic parameters including age, gender, location of tumor, stage and grade in the colonic adenocarcinoma. The discordant findings on the prognostic role of FAK expression may be attributed to differences in tissue types or tumor progression stages. Furthermore, it may also be caused by differences in methodologies (e.g., different antibodies used to evaluate FAK expression) and to the fact that FAK expression does not reflect its enzymatic activity. We demonstrated that FAK expression was higher and more intense in CRCs than in normal colorectal epithelia indicating its potential role in the development of CRCs. In addition, FAK expression was significantly correlated with cortactin expression and showed a tendency to be associated with the pathologic stage. Together, FAK expression may also mediate the progression of CRCs.
Recent studies revealed that cortactin acts as a bridging molecule between actin filaments and focal adhesions and is recruited by FAK into focal adhesions where it is phosphorylated by the FAK-Src complex.15,20 Although cortactin and FAK have been reported to be important factors in tumor migration, invasion, and metastasis, the relationship between expression of these proteins and tumor progression has not been clearly established. In this study, we observed lower expression of cortactin and FAK in normal colorectal epithelia than in CRCs. This indicates ongoing migratory events of normal colorectal epithelial cells, and moreover, the possibility that normal colorectal epithelia adjacent to the invasive cancers contain genetic abnormalities that could have an influence on the expression of cortactin and FAK. Further studies are needed to clarify this observation.
In conclusion, our results demonstrated that the cortactin expression was associated with differentiation, and was an independent factor for prognosis in CRC. Although multiple factors contribute to tumor progression, our findings suggest that cortactin expression is associated with FAK expression and may play an important role in tumor progression. Moreover, expression of cortactin is a satisfactory biomarker to predict tumor progression and survival in CRC patients.
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (No. 2011-0028223).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Wu H, Reynolds AB, Kanner SB, Vines RR, Parsons JT. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol Cell Biol. 1991;11:5113–5124. doi: 10.1128/mcb.11.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vuori K, Ruoslahti E. Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J Biol Chem. 1995;270:22259–22262. doi: 10.1074/jbc.270.38.22259. [DOI] [PubMed] [Google Scholar]
- 3.Buday L, Downward J. Roles of cortactin in tumor pathogenesis. Biochim Biophys Acta. 2007;1775:263–273. doi: 10.1016/j.bbcan.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 5.Serrels B, Serrels A, Brunton VG, et al. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat Cell Biol. 2007;9:1046–1056. doi: 10.1038/ncb1626. [DOI] [PubMed] [Google Scholar]
- 6.van Rossum AG, Moolenaar WH, Schuuring E. Cortactin affects cell migration by regulating intercellular adhesion and cell spreading. Exp Cell Res. 2006;312:1658–1670. doi: 10.1016/j.yexcr.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Cai JH, Zhao R, Zhu JW, et al. Expression of cortactin correlates with a poor prognosis in patients with stages II-III colorectal adenocarcinoma. J Gastrointest Surg. 2010;14:1248–1257. doi: 10.1007/s11605-010-1247-2. [DOI] [PubMed] [Google Scholar]
- 8.Tsai WC, Jin JS, Chang WK, et al. Association of cortactin and fascin-1 expression in gastric adenocarcinoma: correlation with clinicopathological parameters. J Histochem Cytochem. 2007;55:955–962. doi: 10.1369/jhc.7A7235.2007. [DOI] [PubMed] [Google Scholar]
- 9.Ormandy CJ, Musgrove EA, Hui R, Daly RJ, Sutherland RL. Cyclin D1, EMS1 and 11q13 amplification in breast cancer. Breast Cancer Res Treat. 2003;78:323–335. doi: 10.1023/a:1023033708204. [DOI] [PubMed] [Google Scholar]
- 10.Lee YY, Yu CP, Lin CK, et al. Expression of survivin and cortactin in colorectal adenocarcinoma: association with clinicopathological parameters. Dis Markers. 2009;26:9–18. doi: 10.3233/DMA-2009-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigo JP, Álvarez-Alija G, Menéndez ST, et al. Cortactin and focal adhesion kinase as predictors of cancer risk in patients with laryngeal premalignancy. Cancer Prev Res (Phila) 2011;4:1333–1341. doi: 10.1158/1940-6207.CAPR-10-0338. [DOI] [PubMed] [Google Scholar]
- 12.Yuan BZ, Zhou X, Zimonjic DB, Durkin ME, Popescu NC. Amplification and overexpression of the EMS 1 oncogene, a possible prognostic marker, in human hepatocellular carcinoma. J Mol Diagn. 2003;5:48–53. doi: 10.1016/S1525-1578(10)60451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuuring E, Verhoeven E, Mooi WJ, Michalides RJ. Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene. 1992;7:355–361. [PubMed] [Google Scholar]
- 14.Schuuring E. The involvement of the chromosome 11q13 region in human malignancies: cyclin D1 and EMS1 are two new candidate oncogenes: a review. Gene. 1995;159:83–96. doi: 10.1016/0378-1119(94)00562-7. [DOI] [PubMed] [Google Scholar]
- 15.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 16.Cance WG, Harris JE, Iacocca MV, et al. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res. 2000;6:2417–2423. [PubMed] [Google Scholar]
- 17.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabarra-Niecko V, Schaller MD, Dunty JM. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev. 2003;22:359–374. doi: 10.1023/a:1023725029589. [DOI] [PubMed] [Google Scholar]
- 19.Luo C, Pan H, Mines M, Watson K, Zhang J, Fan GH. CXCL12 induces tyrosine phosphorylation of cortactin, which plays a role in CXC chemokine receptor 4-mediated extracellular signal-regulated kinase activation and chemotaxis. J Biol Chem. 2006;281:30081–30093. doi: 10.1074/jbc.M605837200. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Liu Y, Liao K. Tyrosine phosphorylation of cortactin by the FAK-Src complex at focal adhesions regulates cell motility. BMC Cell Biol. 2011;12:49. doi: 10.1186/1471-2121-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton SR, Nakamura SI, Bosman FT, et al. Tumours of the colon and rectum. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC Press; 2010. pp. 131–182. [Google Scholar]
- 22.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 23.Hirakawa H, Shibata K, Nakayama T. Localization of cortactin is associated with colorectal cancer development. Int J Oncol. 2009;35:1271–1276. doi: 10.3892/ijo_00000444. [DOI] [PubMed] [Google Scholar]
- 24.Zhang LH, Tian B, Diao LR, et al. Dominant expression of 85-kDa form of cortactin in colorectal cancer. J Cancer Res Clin Oncol. 2006;132:113–120. doi: 10.1007/s00432-005-0046-8. [DOI] [PubMed] [Google Scholar]
- 25.Chen XM, Huang BQ, Splinter PL, et al. Cryptosporidium parvum invasion of biliary epithelia requires host cell tyrosine phosphorylation of cortactin via c-Src. Gastroenterology. 2003;125:216–228. doi: 10.1016/s0016-5085(03)00662-0. [DOI] [PubMed] [Google Scholar]
- 26.Murata T, Naomoto Y, Yamatsuji T, et al. Localization of FAK is related with colorectal carcinogenesis. Int J Oncol. 2008;32:791–796. [PubMed] [Google Scholar]
- 27.Theocharis SE, Kouraklis GP, Kakisis JD, et al. Focal adhesion kinase expression is not a prognostic predictor in colon adenocarcinoma patients. Eur J Surg Oncol. 2003;29:571–574. doi: 10.1016/s0748-7983(03)00120-3. [DOI] [PubMed] [Google Scholar]
- 28.Ocak S, Chen H, Callison C, Gonzalez AL, Massion PP. Expression of focal adhesion kinase in small-cell lung carcinoma. Cancer. 2012;118:1293–1301. doi: 10.1002/cncr.26382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canel M, Secades P, Rodrigo JP, et al. Overexpression of focal adhesion kinase in head and neck squamous cell carcinoma is independent of fak gene copy number. Clin Cancer Res. 2006;12(11 Pt 1):3272–3279. doi: 10.1158/1078-0432.CCR-05-1583. [DOI] [PubMed] [Google Scholar]
- 30.Furuyama K, Doi R, Mori T, et al. Clinical significance of focal adhesion kinase in resectable pancreatic cancer. World J Surg. 2006;30:219–226. doi: 10.1007/s00268-005-0165-z. [DOI] [PubMed] [Google Scholar]