Abstract
Background
Ezrin, a member of the ezrin-radixin-moesin family, is implicated in tumor progression, metastatic dissemination, and adverse outcomes, in several cancer types. In this study, we explored the clinicopathological significance of ezrin expression in non-small cell lung carcinomas (NSCLCs).
Methods
Immunohistochemical analysis of tissue microarray with 112 surgically resected NSCLC specimens, was performed to examine the ezrin expression. We also correlated ezrin expression with other clinicopathological features and prognosis.
Results
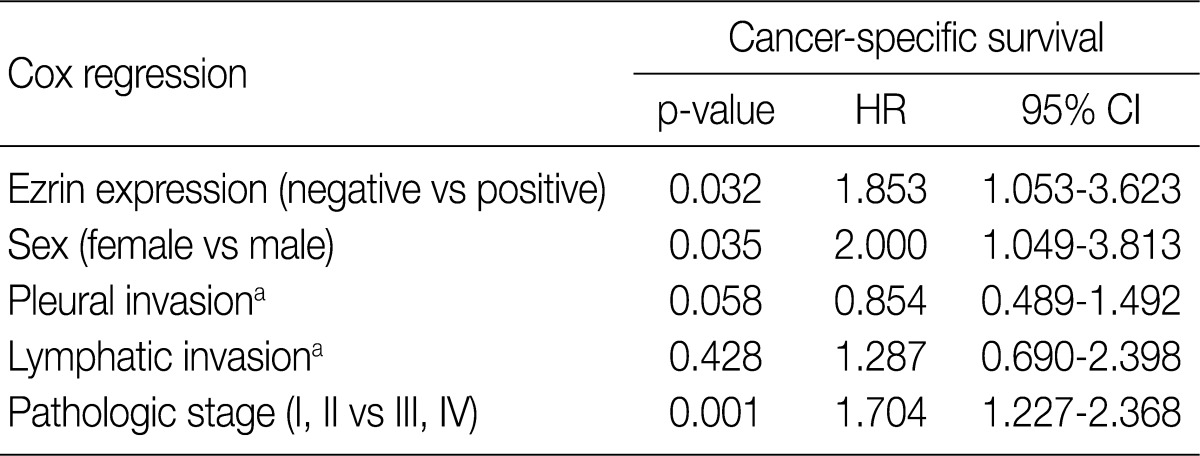
The ezrin-positive group revealed significantly higher correlation with pleural invasion (p=0.016) and pathologic stage (p=0.050). Univariate survival analysis showed that ezrin-positive group had a significantly shorter cancer-specific survival than ezrin-negative group (p=0.016). Meanwhile, female (p=0.030), no pleural invasion (p=0.023), no lymphatic invasion (p=0.026), and early pathologic stage (p=0.008) significantly correlated with longer survival. Multivariate survival analysis showed that variables such as ezrin positivity (p=0.032), female (p=0.035), and early pathologic stage (p=0.001) were independent prognostic factors for NSCLC.
Conclusions
Ezrin might be a molecular marker to predict poor prognosis of NSCLC.
Keywords: Carcinoma, non-small cell lung; Ezrin; Prognosis
Lung cancer is the leading cause of cancer-related deaths, worldwide, in both men and women, with over one million cases diagnosed yearly.1 Despite progress in the multimodality treatment of lung cancer, prognosis is still poor with 10 to 15% 5-year survival rates. Even in patients with resected stage IA tumors, the survival rate still ranges from 66 to 85%.2 Non-small cell lung cancer (NSCLC) accounts for >80% of all lung cancers. However, only a minority of NSCLC patients would be eligible for radical treatment as a curative care.
Ezrin belongs to the ezrin-radixin-moesin protein family, which acts as membrane organizers and linkers between the plasma membrane and cytoskeleton.3 Ezrin plays a positive role in maintaining the shape and the polarity of the cells in membrane-trafficking, cell migration, cell signaling, growth regulation, and differentiation.4 The cell signaling pathways associated with ezrin, include many oncogenic routes, such as protein kinase C, Rho-kinase, epidermal growth factor receptor, Src, mammalian target of rapamycin (mTOR), and PI3 kinase/Akt.5 Because of its unique functions, ezrin is actively involved in the biology of tumor development, especially in regulating the growth and metastatic capacity of cancer cells.6
Recently, many studies have suggested that increased ezrin expression was associated with certain outcome in various types of cancers.7-18 To date, however only a few studies have reported association between ezrin expression and clinicopathological parameters as well as its prognostic role in lung cancer.19-21 In this study, we examined ezrin expression in resected NSCLC specimens, explored the possible correlation between ezrin expression and clinicopathological variables, and determined its potential prognostic value.
MATERIALS AND METHODS
Patients and tissues
We retrospectively obtained formalin-fixed and paraffin-embedded tissues from 112 resected primary NSCLC cases, which were obtained from the Soonchunhyang University Cheonan Hospital, between January 1996 and October 2009. Tumors from patients who died from causes other than lung carcinoma and recurrent or metastatic tumors were excluded from this study. The patients with NSCLC consisted of 81 men and 31 women. The mean patient age was 63 years (standard deviation, 8.0; range, 42 to 82 years), and the mean tumor size was 3.8 cm (standard deviation 1.9; range, 1.0 to 8.6 cm).
Hematoxylin and eosin (H&E) stained slides were reviewed for each case to confirm the original diagnosis. Two pathologists (M.H.Oh and H.W.Lee) independently reviewed these slides based on the World Health Organization criteria,22 to select the most representative sections. Tumor specimens were histopathologically diagnosed as adenocarcinoma (ADC; n=38), squamous cell carcinoma (SCC; n=59), large cell carcinoma (n=1), large cell neuroendocrine carcinoma (n=6), and sarcomatoid carcinoma (n=8). The pathologic stage of each tumor was assigned, following the guidelines from the 7th edition of tumor-node-metastasis (TNM) classification.23 The total sample set comprised of 57 stage I, 38 stage II, 13 stage III, and 4 stage IV tumors. The patients' medical records were collected to obtain the following information: age, gender, and smoking status. Further data regarding the patients' survival status and cause of death were obtained from the patients' medical records and/or from an interview with the family. The median follow-up period for all patients was 23.0 months, with a range of 1 to 153 months.
Construction of tissue microarray (TMA)
The most representative tumor area was carefully marked on H&E stained slides of the sample tissue cores (2 mm in diameter) from formalin fixed, paraffin embedded tissue blocks. Representative cores were arranged in a trephine apparatus (Superbiochips Laboratories, Seoul, Korea). Core sample was obtained from each tumor and included in the TMA block. Control or normal parenchymal cores were also included. Some tumor cores contained non-neoplastic portion and they were used as internal control. One section from the block was stained with H&E for tissue confirmation.
Immunohistochemistry (IHC) for ezrin
IHC was performed to analyze ezrin expression. The materials and methods used in this study have been described previously.11,24,25 Sections (4-µm thick) from the TMA blocks were transferred to poly-L-lysine-coated glass slides and incubated in a dry oven, at 60℃ for 1 hour. Therefore, they were then dewaxed in xylene (three changes), rehydrated in a graded series of decreasing ethanol concentrations and rinsed in Tris-buffered saline (pH 7.4). Endogenous peroxidase activity was inactivated with 5% hydrogen peroxide in methanol for 15 minutes at 37℃. For ezrin staining, antigen retrieval was performed using a microwave treatment in the pH 6.0-epitope retrieval solution for 20 minutes. The tissue was incubated with a mouse monoclonal antibody against ezrin (1:100, clone 3C12, Abcam, Cambridge, UK) in a humidified chamber at 4℃ for 16 hours. Secondary antibody was applied, using a Bond polymer refine detection kit (Leica, Mount Waverley, Australia). Diaminobenzidine was used, as the chromogen, and the sections were counterstained with Mayer's hematoxylin. Positive controls, consisting of cases with known reactivity for the antibody, and negative controls, obtained by omitting the primary antibody, were also included.
Immunohistochemical assessment
Tumor cells were judged as positive if cytoplasmic and circumferential membranous staining were present. Immunohistochemical staining was separately evaluated by two investigators (M.H.Oh and H.W.Lee), and in the rare instance of a discrepancy between their judgment, they reviewed the slides together using a multi-head microscope. A semiquantitative immunohistochemical score was assigned, which included assessment of both intensity and extent of staining. Intensity of staining was given score of 0 to 3 (corresponding to negative, weak, moderate, and strong positivity). The extent of staining was scored as 0 (0%), 1 (1-10%), 2 (11-50%), and 3 (51-100%), according to the percentage of cells which stained positive for each protein. The product of intensity and extent score was used as the final score (0, 1, 2, 3, 4, 6, 9). Tissues having a final score of 9 were considered positive, while those having a final score of ≤6 were regarded as negative. Similar semiquantitative scoring systems have been successfully used for other TMA evaluations.25
Statistical analysis
Statistical anlaysis was conducted using SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA). Associations between the ezrin expression and the clinicopathological characteristics were analyzed, using the Pearson χ2 or Fisher's exact test. Kaplan-Meier analysis was performed for analysis of the survival curves and statistical significance was assessed using the log-rank test. Cancer-specific survival was defined as the time from the date of the surgery to the last follow-up visit or cancer-related death. Univariate analysis of cancer-specific survival was performed using the Kaplan-Meier method. Multivariate Cox proportional hazard regression analysis was used to assess the prognostic significance between ezrin expression, the other clinicopathological characteristics, and survival. Overall, 95% confidence intervals were used throughout the analyses. Statistical significance was defined as p<0.05.
RESULTS
Ezrin immunoreactivity
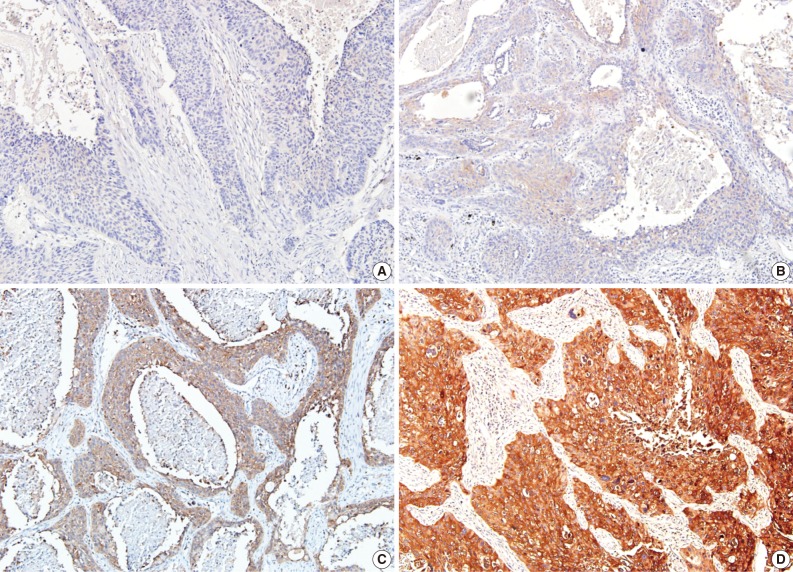
In the non-neoplastic pulmonary parenchyma, ezrin was expressed in the apical membrane of the bronchial epithelium and in the pneumocytes of the alveolar wall and inflammatory cells, including lymphocytes and plasma cells. Ezrin in NSCLC appeared mainly in the cytoplasm and circumferential membrane (Figs. 1, 2). Regarding intensity of staining, 8 cases (7.1%) were scored as 0, 27 cases (24.1%) as 1, 41 cases (36.6%) as 2, and 36 cases (32.2%) as 3. Regarding extent of staining, 8 cases (7.1%) were rated as 0, 0 case (0%) as 1, 3 cases (2.7%) as 2, and 101 cases (90.2%) as 3. As mentioned above, ezrin expression in NSCLC showed a diffuse staining pattern. After multiplying staining intensity and extent scores of the 112 immunostained NSCLC specimens, 8 cases (7.1%) were rated as 0, 3 cases (2.7%) as 2, 27 cases (24.1%) as 3, 0 case (0%) as 4, 41 cases (36.6%) as 6, and 33 (29.5%) as 9. The cases were subdivided into ezrin-negative groups (scores of 0, 2, 3, and 6; n=79 [70.5%]) and ezrin-positive group (scores of 9; n=33 [29.5%]) for statistical analysis.
Fig. 1.
Immunohistochemical staining of ezrin expression in adenocarcinoma: (A) negative staining in bronchioloalveolar portion of adenocarcinoma and internal positive control in interstitial lymphocytes, (B) weak (1+), (C) moderate (2+), (D) strong (3+) membranous and cytoplasmic staining intensity.
Fig. 2.
Immunohistochemical staining of ezrin expression in squamous cell carcinoma: (A) negative, (B) weak (1+), (C) moderate (2+), (D) strong (3+) membranous and cytoplasmic staining intensity.
Correlations between Ezrin expression and clinicopathologic parameters
The expression of ezrin was significantly higher in the tumors with pleural invasion and late pathologic stage. A weak correlation between ezrin expression and T status was also found. Other clinicopathologic variables, including sex, age, tumor size, histological type, smoking, lymphatic invasion, perineural invasion, nodal status, and metastasis, showed no correlation with ezrin expression (Table 1). Although we did not include adenocarcinoma in situ (formerly bronchioloalveolar carcinoma) for analysis in case of ADC, portions of adenocarcinoma in situ showed negativity (Fig. 1A).
Table 1.
Relationship of ezrin expression with clinicopathological parameters of 112 patients with NSCLC
NSCLC, non-small cell lung carcinoma; SCC, squamous cell carcinoma; ADC, adenocarcinoma; LCC, large cell carcinoma; SAC, sarcomatoid carcinoma.
aVisceral and/or parietal invasion.
Survival analysis
At the time of analysis, the number of cancer-specific deaths was 64 (57.1%). Ezrin expression, age, sex, histological type, smoking status, tumor size, pleural invasion, lymphatic invasion, perineural invasion, tumor status and nodal status of TNM, as well as pathologic stage were included in the univariate analysis to evaluate significance of each variable upon cancer-specific survival (Table 2). Ezrin positive groups (see above) displayed a significantly shorter cancer-specific survival than ezrin negative groups (p=0.016) (Fig. 3). Among the above-mentioned variables, female (p=0.030), no pleural invasion (p=0.023), no lymphatic invasion (p=0.026), and early pathologic stage (p=0.008) correlated significantly with longer survival (Table 2). To evaluate whether ezrin positivity in NSCLC is an independent predictor of cancer-specific survival, a multivariate analysis using the Cox proportional hazard model, was performed. This analysis included the variables ezrin expression, sex, pleural invasion, venous lymphatic invasion, and pathologic stage. All variables with a p<0.05 in a univariate analysis were included in a multivariate Cox model. Ezrin positivity (p=0.032), male (p=0.035), and late pathologic stage (p=0.001) were significantly poor prognostic factors for NSCLC. Multivariate analysis demonstrated that ezrin expression was an independent prognostic factor for cancer-specific survival (Table 3).
Table 2.
Univariate analysis of cancer-specific survival in 112 patients with NSCLC
NSCLC, non-small cell lung carinoma; ADC, adenocarcinoma; SCC, squamous cell carcinoma; LCC, large cell carcinoma; STC, sarcomatoid carcinoma.
aAbsent vs present.
Fig. 3.
Kaplan-Meier survival curve for 112 non-small cell lung carcinoma with regard to ezrin expression.
Table 3.
Multivariate analysis of cancer-specific survival in 112 patients with NSCLC
NSCLC, non-small cell lung carinoma; HR, hazard ratio; CI, confidence interval.
aAbsent vs present.
DISCUSSION
Following the purification of ezrin as a component of the intestinal microvilli that is tyrosine-phosphorylated, by epidermal growth factor,26 many studies have been focused on the relationship between malignant tumors and tumor invasiveness or related outcomes. Recently, many studies have suggested that increased ezrin expression was associated with poor outcome in various types of cancers, in various organs, including gastric carcinoma,7 colorectal carcinoma,8,9 nasopharyngeal carcinoma,10 esophageal SCC,11 breast carcinoma,12 osteosarcoma,13,14 various soft tissue sarcomas,15,16 cutaneous basal cell carcinoma and SCC,17 and melanoma.18 Some cohort studies19,21 suggested the distinct fuction of ezrin in tumor cell invasion or metastasis, however, no report to date has ever identified its favorable prognostic value. In this study, we analyzed the relationship between ezrin expression and clinicopathological parameters as well as survival. We observed that increased ezrin expression correlated with pleural invasion (p=0.016), tumor stage (p=0.050), and shorter survival (p=0.016). However, ezrin expression did not correlate with other clinicopathological parameters, such as sex, age, tumor size, histology, smoking, lymphatic invasion, perineural invasion, tumor status, nodal status, and metastasis.
Although the association of ezrin with tumor proliferation and invasiveness was confirmed in several types of cancers, the exact mechanism has not yet been defined. Hiscox and Jiang27 and McClatchey28 insisted that the metastatic process begins with a breakdown of the epithelial integrity causing the tumor cells to penetrate into the vascular system, where they finally obtains aggressive proliferation in appropriate target organs. Cell adhesion molecules and actin cytoskeleton play important roles during these processes. Khanna et al.13 and Fiévet et al.29 suggested that ezrin participated in invasiveness and metastasis processes believed to be predominantly late events in tumor progression. Interestingly, our study revealed that ezrin expression correlated to higher tumor stage (p=0.050), and bronchioloalveolar portion of ADC showed a trend of ezrin negativity.
Further elucidation of the molecular mechanisms underlying lung cancer progression is essential for the development of new effective therapeutic agents. Recent strategies include personalized treatment selecting those patients that are likely to respond to a particular chemotherapeutic regimen. Such approach may contribute to improved treatment efficacy while avoiding unnecessary side effects. With regard to the potential therapeutic use of ezrin, several possible approaches can be exploited such as the development of an antibody or an inhibitor of ezrin or its downstream molecules. Active researches are ongoing to identify such molecules. Previous studies have elucidated that the metastatic behavior of ezrin is related to the mTOR signaling pathway. Blocking this pathway has been shown to inhibit experimental lung metastasis. Currently, the mTOR inhibitor rapamycin and its analogues are being appraised in preclinical and clinical trials for the treatment of cancer.9,30 In this study, we used IHC to analyze the levels of ezrin expression in 112 clinicopathologically characterized NSCLC cases. In normal non-neoplastic tissue, ezrin was expressed in the apical portion of bronchial epithelium and pneumocytes. In malignant tumors, however, ezrin showed diffuse cytoplasm and circumferential membranous staining pattern. Wan et al.30 reported that in invasive breast tumors and cancer cell lines, switching the localization of ezrin from the apical membrane to either the complete membrane, or to the cytoplasm was correlated with dediffentiation and adverse features. In the present study, we found that ezrin expression in NSCLC showed a diffuse staining pattern, such that scores mainly correlated with the staining intensity but not staining extent. Abdou et al.17 have erstwhile suggested that intensity, rather than extent of ezrin expression, had a more probable impact on tumor behavior. At any rate, however, it is necessary to establish detailed and standardized immunohistochemical assessment of ezrin expression. Another important finding of our study is the consistently significant relationship between survival and ezrin expression in both univariate (p=0.016) and multivariate analyses (p=0.032). It indicates that ezrin might be a new molecular marker to predict the prognosis of NSCLC.
Despite providing new insights into the relationship between ezrin expression and clinical outcome in NSCLC, the present study has some limitations which restrict generalization of our results. For instance, we cannot exclude that selection bias could have influenced our findings because all of the enrolled patients were surgically resectable. Furthermore, our study is retrospective and included only one TMA core per case. It is of note, however, that several recent studies used the same methods we have applied herein such as scoring system and TMA block.7,11,15,16 Nevetheless, the present findings remain to be confirmed by further studies. Moreover, the value of ezrin as an independent predictor of NSCLC necessitates further multivariate analysis especially in a larger cohort of patients.
In summary, we have demonstrated that ezrin expression is associated with poor prognostic outcome in NSCLC and is higher in late stage tumors and those with pleural invasion. In view of these findings, it is suggested that ezrin might be a new molecular marker to predict the prognosis of NSCLC.
Acknowledgments
This work was supported in part by the Soonchunhyang University Research Fund.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Tsuchiya T, Akamine S, Muraoka M, et al. Stage IA non-small cell lung cancer: vessel invasion is a poor prognostic factor and a new target of adjuvant chemotherapy. Lung Cancer. 2007;56:341–348. doi: 10.1016/j.lungcan.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 4.Hunter KW. Ezrin, a key component in tumor metastasis. Trends Mol Med. 2004;10:201–204. doi: 10.1016/j.molmed.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Brambilla D, Fais S. The Janus-faced role of Ezrin in "linking" cells to either normal or metastatic phenotype. Int J Cancer. 2009;125:2239–2245. doi: 10.1002/ijc.24734. [DOI] [PubMed] [Google Scholar]
- 6.Bruce B, Khanna G, Ren L, et al. Expression of the cytoskeleton linker protein ezrin in human cancers. Clin Exp Metastasis. 2007;24:69–78. doi: 10.1007/s10585-006-9050-x. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Zhang X, Xin Y. Up-regulated expression of ezrin and c-Met proteins are related to the metastasis and prognosis of gastric carcinomas. Histol Histopathol. 2011;26:1111–1120. doi: 10.14670/HH-26.1111. [DOI] [PubMed] [Google Scholar]
- 8.Elzagheid A, Korkeila E, Bendardaf R, et al. Intense cytoplasmic ezrin immunoreactivity predicts poor survival in colorectal cancer. Hum Pathol. 2008;39:1737–1743. doi: 10.1016/j.humpath.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Korkeila EA, Syrjänen K, Bendardaf R, et al. Preoperative radiotherapy modulates ezrin expression and its value as a predictive marker in patients with rectal cancer. Hum Pathol. 2011;42:384–392. doi: 10.1016/j.humpath.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Lin GN, Jiang XL, Lu Y. Expression of ezrin correlates with poor prognosis of nasopharyngeal carcinoma. Tumour Biol. 2011;32:707–712. doi: 10.1007/s13277-011-0171-8. [DOI] [PubMed] [Google Scholar]
- 11.Xie JJ, Xu LY, Wu ZY, et al. Prognostic implication of ezrin expression in esophageal squamous cell carcinoma. J Surg Oncol. 2011;104:538–543. doi: 10.1002/jso.21909. [DOI] [PubMed] [Google Scholar]
- 12.Elliott BE, Meens JA, SenGupta SK, Louvard D, Arpin M. The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast Cancer Res. 2005;7:R365–R373. doi: 10.1186/bcr1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khanna C, Wan X, Bose S, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–186. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- 14.Kim C, Shin E, Hong S, et al. Clinical value of ezrin expression in primary osteosarcoma. Cancer Res Treat. 2009;41:138–144. doi: 10.4143/crt.2009.41.3.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JS, Jin MS, Lee JE, Kim MS, Jeon DG, Koh JS. Prognostic significance of ezrin expression in liposarcoma. Korean J Pathol. 2008;42:270–276. [Google Scholar]
- 16.Carneiro A, Bendahl PO, Åkerman M, et al. Ezrin expression predicts local recurrence and development of metastases in soft tissue sarcomas. J Clin Pathol. 2011;64:689–694. doi: 10.1136/jcp.2011.089805. [DOI] [PubMed] [Google Scholar]
- 17.Abdou AG, Maraee AH, El-Sayed EM, Elnaidany NF. Immunohistochemical expression of ezrin in cutaneous basal and squamous cell carcinomas. Ann Diagn Pathol. 2011;15:394–401. doi: 10.1016/j.anndiagpath.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Federici C, Brambilla D, Lozupone F, et al. Pleiotropic function of ezrin in human metastatic melanomas. Int J Cancer. 2009;124:2804–2812. doi: 10.1002/ijc.24255. [DOI] [PubMed] [Google Scholar]
- 19.Tokunou M, Niki T, Saitoh Y, Imamura H, Sakamoto M, Hirohashi S. Altered expression of the ERM proteins in lung adenocarcinoma. Lab Invest. 2000;80:1643–1650. doi: 10.1038/labinvest.3780174. [DOI] [PubMed] [Google Scholar]
- 20.Deng X, Tannehill-Gregg SH, Nadella MV, et al. Parathyroid hormone-related protein and ezrin are up-regulated in human lung cancer bone metastases. Clin Exp Metastasis. 2007;24:107–119. doi: 10.1007/s10585-007-9059-9. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Zhang M, Chen L, Huang W, Ma H. Expression and correlation of ezrin and survivin in non-small cell lung cancer. Chinese-German J Clin Oncol. 2010;9:381–384. [Google Scholar]
- 22.Travis WD, Brambilla E, Müller-Hermelink K, Harris CC. World Health Organization classification of tumours: pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: IARC Press; 2004. p. 10. [Google Scholar]
- 23.Travis WD IASLC Staging Committee. Reporting lung cancer pathology specimens. Impact of the anticipated 7th edition TNM classification based on recommendations of the IASLC Staging Committee. Histopathology. 2009;54:3–11. doi: 10.1111/j.1365-2559.2008.03179.x. [DOI] [PubMed] [Google Scholar]
- 24.Patara M, Santos EM, Coudry Rde A, Soares FA, Ferreira FO, Rossi BM. Ezrin expression as a prognostic marker in colorectal adenocarcinoma. Pathol Oncol Res. 2011;17:827–833. doi: 10.1007/s12253-011-9389-4. [DOI] [PubMed] [Google Scholar]
- 25.Jung EA, Cho HD, Lee JH, Oh MH. Clinicopathological significance of S100A4 expression in non-small cell lung carcinomas. Korean J Pathol. 2010;44:477–482. [Google Scholar]
- 26.Bretscher A. Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J Cell Biol. 1989;108:921–930. doi: 10.1083/jcb.108.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiscox S, Jiang WG. Ezrin regulates cell-cell and cell-matrix adhesion, a possible role with E-cadherin/beta-catenin. J Cell Sci. 1999;112(Pt 18):3081–3090. doi: 10.1242/jcs.112.18.3081. [DOI] [PubMed] [Google Scholar]
- 28.McClatchey AI. Merlin and ERM proteins: unappreciated roles in cancer development? Nat Rev Cancer. 2003;3:877–883. doi: 10.1038/nrc1213. [DOI] [PubMed] [Google Scholar]
- 29.Fiévet B, Louvard D, Arpin M. ERM proteins in epithelial cell organization and functions. Biochim Biophys Acta. 2007;1773:653–660. doi: 10.1016/j.bbamcr.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Wan X, Mendoza A, Khanna C, Helman LJ. Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res. 2005;65:2406–2411. doi: 10.1158/0008-5472.CAN-04-3135. [DOI] [PubMed] [Google Scholar]