Abstract
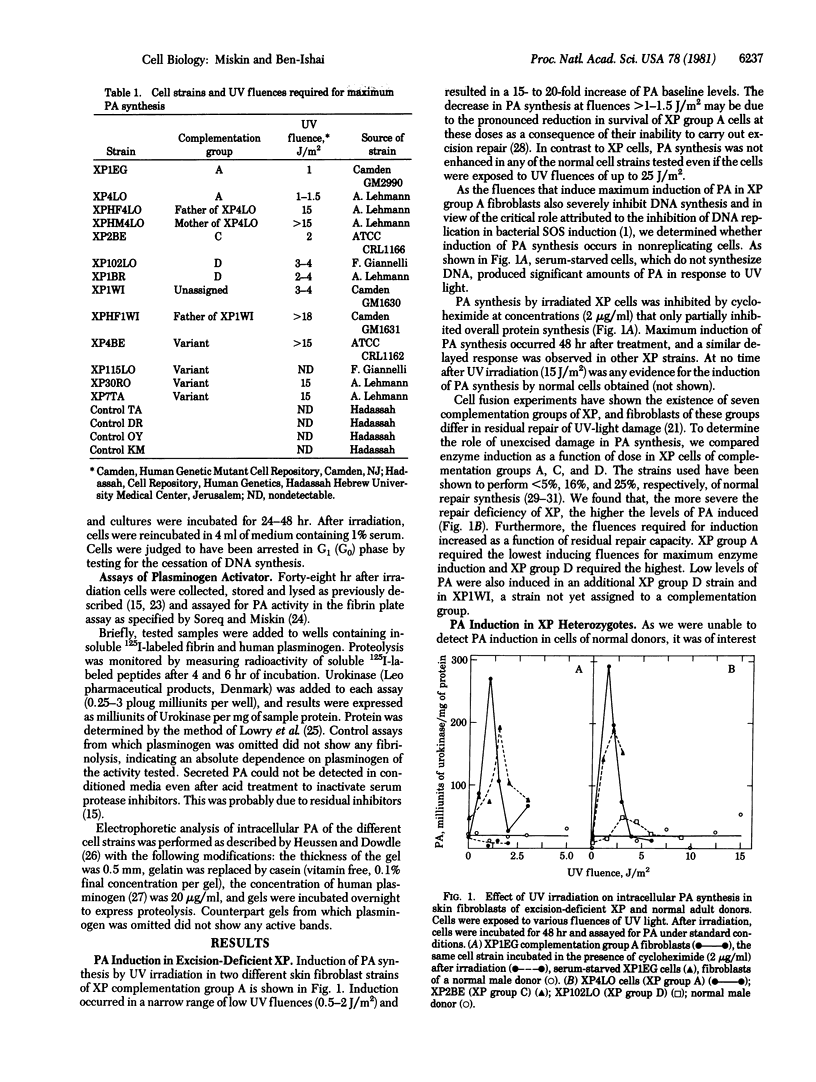
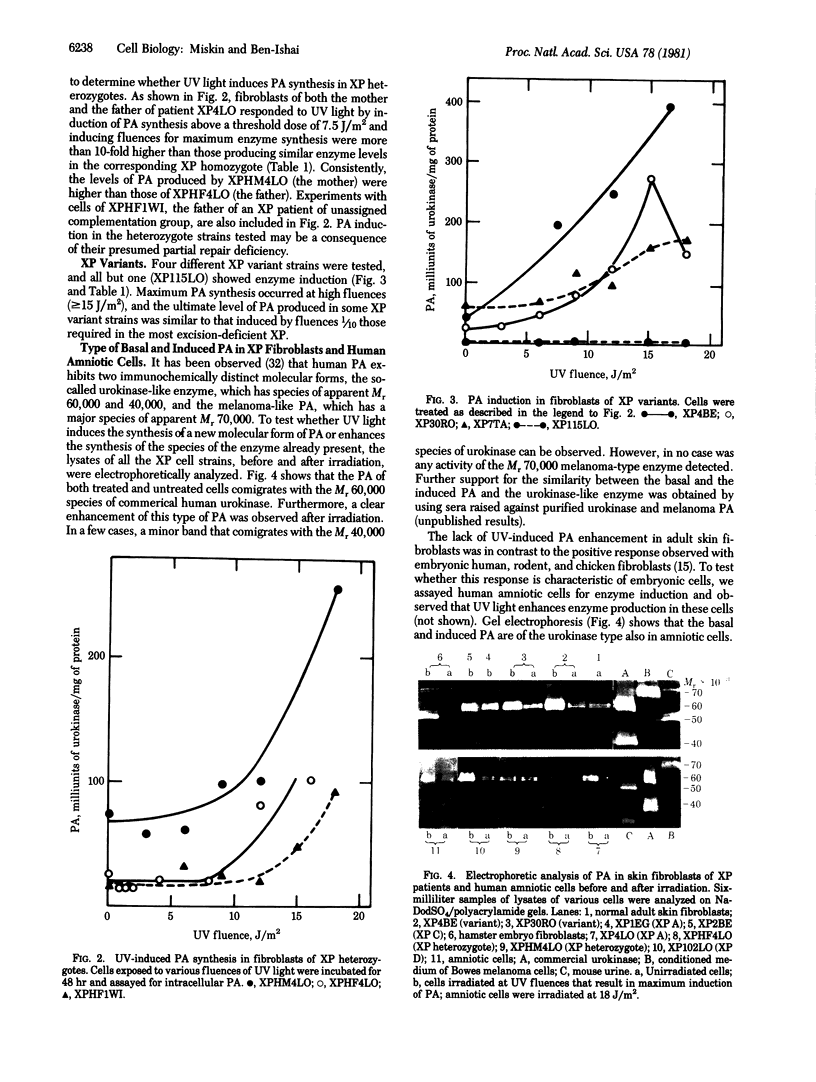
Normal and DNA repair-deficient human fibroblasts have been used to study induction of plasminogen activator (PA) by DNA damage. UV light induced the synthesis of PA in skin fibroblasts of all types of xeroderma pigmentosum (XP) in XP heterozygotes and in human amniotic cells. Enzyme induction was, however, not observed in fibroblasts of normal adults. In classical XP, which are deficient in excision repair, PA synthesis occurred in a narrow range of low-UV fluences. In such strains, the level of enzyme produced was correlated with the extent of repair deficiency. UV fluences required for PA induction in XP variants and XP heterozygotes were at least 10 times those inducing enzyme synthesis in excision-deficient XP. Maximum enzyme induction occurred 48 hr after irradiation, and the highest levels of enzyme produced were 15-20 times those of PA baseline levels. Electrophoretic analysis showed that UV irradiation enhances the synthesis of the Mr 60,000 human urokinase-type PA, which is present in low amounts in untreated cells. Our results suggest that PA induction in human cells is caused by unrepaired DNA damage and represents a eukaryotic SOS-like function. In addition, PA induction may provide a sensitive assay for detection of cellular DNA repair deficiencies and identification of XP heterozygotes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beers W. H., Strickland S., Reich E. Ovarian plasminogen activator: relationship to ovulation and hormonal regulation. Cell. 1975 Nov;6(3):387–394. doi: 10.1016/0092-8674(75)90188-9. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. The lexA gene product represses its own promoter. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1932–1936. doi: 10.1073/pnas.77.4.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E., Bootsma D. Xeroderma pigmentosum: biochemical and genetic characteristics. Annu Rev Genet. 1975;9:19–38. doi: 10.1146/annurev.ge.09.120175.000315. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Roberts J. W. E. coli recA protein-directed cleavage of phage lambda repressor requires polynucleotide. Nature. 1980 Jan 3;283(5742):26–30. doi: 10.1038/283026a0. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio S. M., Aebersold P. M., Setlow R. B. Enhancement of postreplication repair in ultraviolet-light-irradiated Chinese hamster cells by irradiation in G2 or S-phase. Biophys J. 1978 Jul;23(1):71–78. doi: 10.1016/S0006-3495(78)85433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio S. M., Setlow R. B. Defective and enhanced postreplication repair in classical and variant xeroderma pigmentosum cells treated with N-acetoxy-2-acetylaminofluorene. Cancer Res. 1978 Apr;38(4):1147–1153. [PubMed] [Google Scholar]
- D'Ambrosio S. M., Setlow R. B. Enhancement of postreplication repair in Chinese hamster cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2396–2400. doi: 10.1073/pnas.73.7.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta U. B., Summers W. C. Ultraviolet reactivation of herpes simplex virus is mutagenic and inducible in mammlian cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2378–2381. doi: 10.1073/pnas.75.5.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):459–477. doi: 10.1016/0022-2836(76)90240-0. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C., Cooper P. K., Ganesan A. K., Smith C. A. DNA repair in bacteria and mammalian cells. Annu Rev Biochem. 1979;48:783–836. doi: 10.1146/annurev.bi.48.070179.004031. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Huberman E., Fogel M. Activation of carcinogenic polycyclic hydrocarbons in polyoma-virus-transformed cells as a prerequisite for polyoma virus induction. Int J Cancer. 1975 Jan 15;15(1):91–98. doi: 10.1002/ijc.2910150111. [DOI] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer K. H., Coon H. G., Petinga R. A., Barrett S. F., Rahe A. E., Robbins J. H. National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20014, USA. Proc Natl Acad Sci U S A. 1975 Jan;72(1):59–63. doi: 10.1073/pnas.72.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Arlett C. F., Harcourt S. A., de Weerd-Kastelein E. A., Keijzer W., Hall-Smith P. Repair of ultraviolet light damage in a variety of human fibroblast cell strains. Cancer Res. 1977 Mar;37(3):904–910. [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Arlett C. F., Paterson M. C., Lohman P. H., de Weerd-Kastelein E. A., Bootsma D. Xeroderma pigmentosum cells with normal levels of excision repair have a defect in DNA synthesis after UV-irradiation. Proc Natl Acad Sci U S A. 1975 Jan;72(1):219–223. doi: 10.1073/pnas.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Harper J. E. Identification of the lexA gene product of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6147–6151. doi: 10.1073/pnas.76.12.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle C. D., Day R. S., 3rd, Hellman K. B., Bockstahler L. E. Infection of UV-irradiated xeroderma pigmentosum fibroblasts by herpes simplex virus: study of capacity and Weigle reactivation. Mutat Res. 1976 Sep;36(3):257–264. doi: 10.1016/0027-5107(76)90235-9. [DOI] [PubMed] [Google Scholar]
- Maher V. M., Dorney D. J., Mendrala A. L., Konze-Thomas B., McCormick J. J. DNA excision-repair processes in human cells can eliminate the cytotoxic and mutagenic consequences of ultraviolet irradiation. Mutat Res. 1979 Sep;62(2):311–323. doi: 10.1016/0027-5107(79)90087-3. [DOI] [PubMed] [Google Scholar]
- Miskin R., Easton T. G., Reich E. Plasminogen activator in chick embryo muscle cells: induction of enzyme by RSV, PMA and retinoic acid. Cell. 1978 Dec;15(4):1301–1312. doi: 10.1016/0092-8674(78)90055-7. [DOI] [PubMed] [Google Scholar]
- Miskin R., Reich E. Plasminogen activator: induction of synthesis by DNA damage. Cell. 1980 Jan;19(1):217–224. doi: 10.1016/0092-8674(80)90403-1. [DOI] [PubMed] [Google Scholar]
- Pawsey S. A., Magnus I. A., Ramsay C. A., Benson P. F., Giannelli F. Clinical, genetic and DNA repair studies on a consecutive series of patients with xeroderma pigmentosum. Q J Med. 1979 Apr;48(190):179–210. [PubMed] [Google Scholar]
- Radman M. Is there SOS induction in mammalian cells? Photochem Photobiol. 1980 Dec;32(6):823–830. doi: 10.1111/j.1751-1097.1980.tb04062.x. [DOI] [PubMed] [Google Scholar]
- Sarasin A. R., Hanawalt P. C. Carcinogens enhance survival of UV-irradiated simian virus 40 in treated monkey kidney cells: induction of a recovery pathway? Proc Natl Acad Sci U S A. 1978 Jan;75(1):346–350. doi: 10.1073/pnas.75.1.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasin A., Benoit A. Induction of an error-prone mode of DNA repair in UV-irradiated monkey kidney cells. Mutat Res. 1980 Mar;70(1):71–81. doi: 10.1016/0027-5107(80)90059-7. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. Repair deficient human disorders and cancer. Nature. 1978 Feb 23;271(5647):713–717. doi: 10.1038/271713a0. [DOI] [PubMed] [Google Scholar]
- Soreq H., Miskin R. Plasminogen activator in the rodent brain. Brain Res. 1981 Jul 20;216(2):361–374. doi: 10.1016/0006-8993(81)90138-4. [DOI] [PubMed] [Google Scholar]
- Swift M. Malignant disease in heterozygous carriers. Birth Defects Orig Artic Ser. 1976;12(1):133–144. [PubMed] [Google Scholar]
- Wigler M., Weinstein I. B. Tumour promotor induces plasminogen activator. Nature. 1976 Jan 22;259(5540):232–233. doi: 10.1038/259232a0. [DOI] [PubMed] [Google Scholar]
- Wilson E. L., Becker M. L., Hoal E. G., Dowdle E. B. Molecular species of plasminogen activators secreted by normal and neoplastic human cells. Cancer Res. 1980 Mar;40(3):933–938. [PubMed] [Google Scholar]
- Witkin E. M. Elevated mutability of polA derivatives of Escherichia coli B/r at sublethal doses of ultraviolet light: evidence for an inducible error-prone repair system ("SOS repair") and its anomalous expression in these strains. Genetics. 1975 Jun;79 (Suppl):199–213. [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]




