Abstract
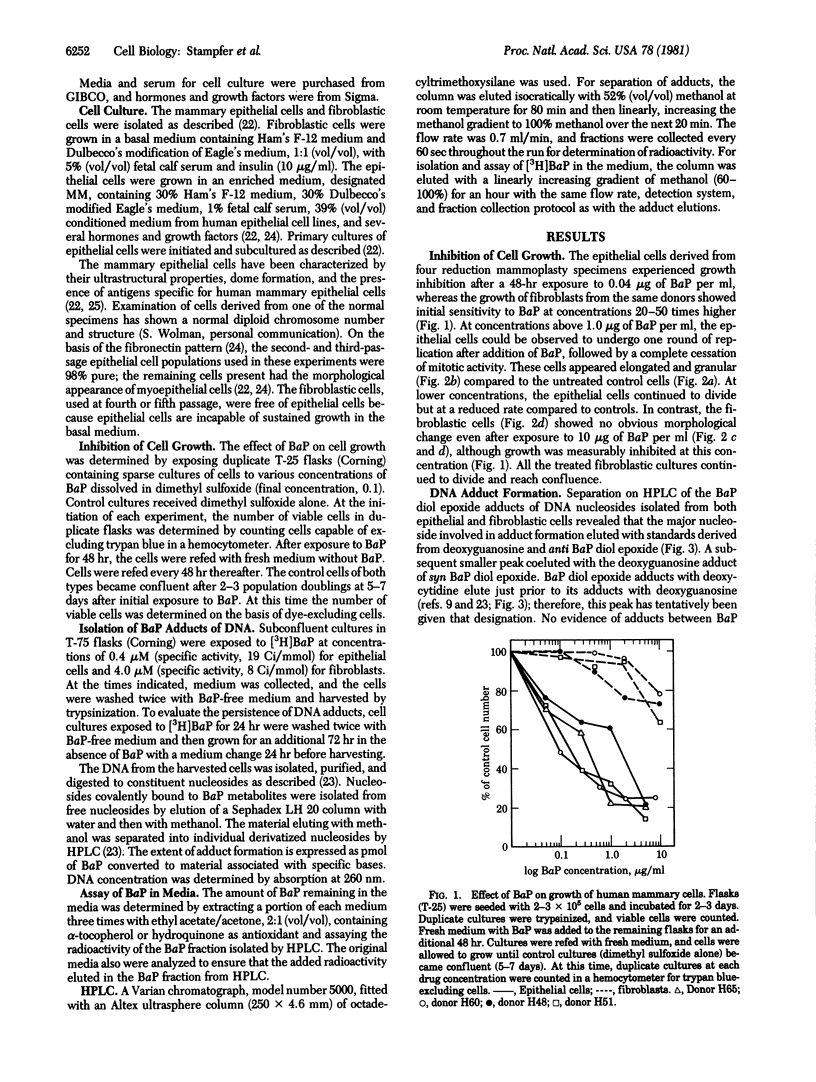
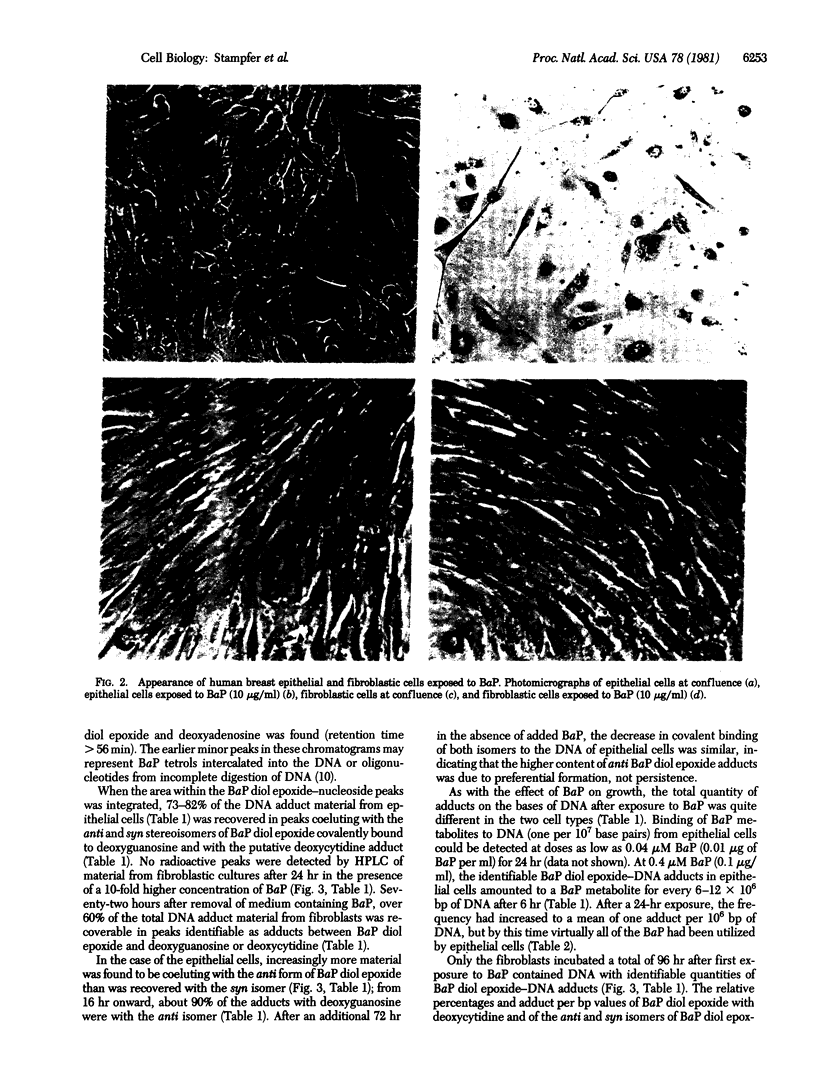
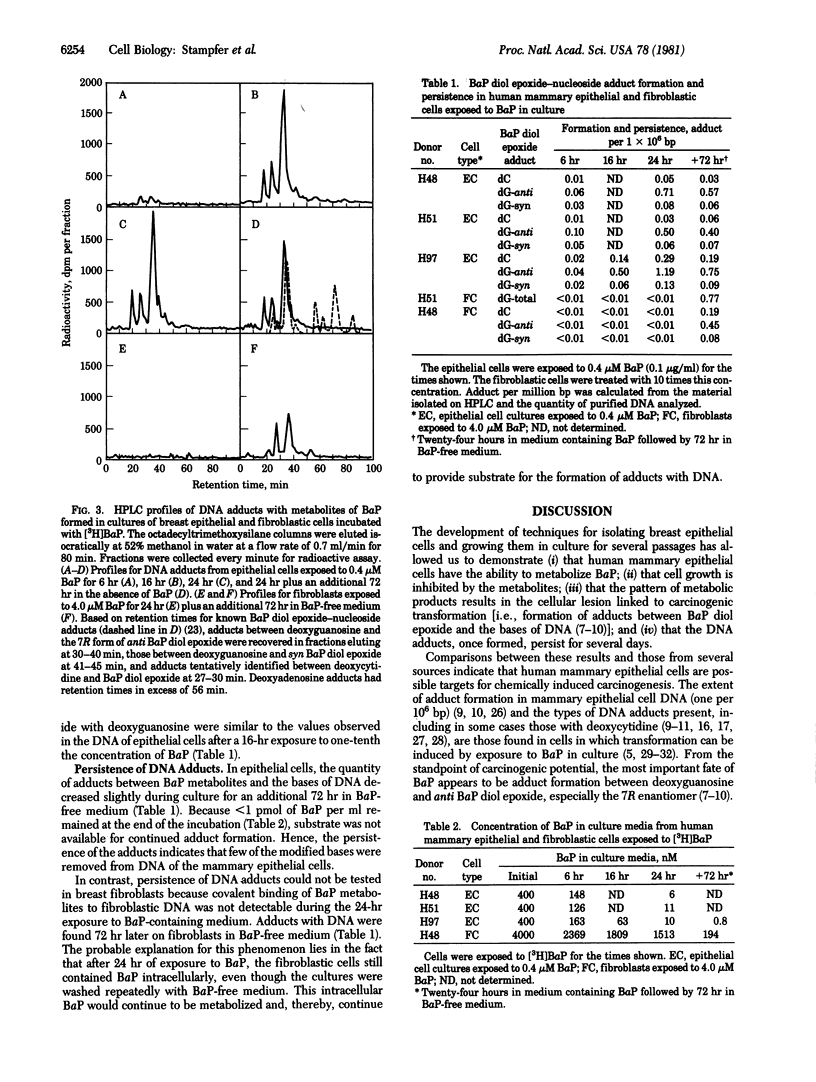
Pure cultures of human breast epithelial cells and of fibroblastic cells in early passage provided the opportunity to ask whether either cell type had the capability for metabolizing chemical carcinogens and, if so, was the fate of the metabolic products compatible with chemical carcinogens being a factor in the initiation of breast cancer in women. For this purpose, cells were exposed to benzo[a]pyrene (BaP), and (i) the influence on growth potential and (ii) the extent, type, and persistence of adducts between the metabolites of BaP and DNA were measured. Compared with fibroblasts, inhibition of growth by epithelial cells was 50-100 times more sensitive to BaP. Because of this differential sensitivity, epithelial cells were exposed to 0.4 microM BaP and fibroblasts were exposed to 4.0 microM BaP in the studies of DNA adduct formation. Separation by high-pressure liquid chromatography of adducts between (+/-)-7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene (BaP diol epoxide) and nucleosides from purified DNA revealed that epithelial cells contained modified DNA within 6 hr after adding BaP. Adducts between the 7R anti stereoisomer of BaP diol epoxide and deoxyguanosine predominated at all times. syn BaP diol epoxide adducts with deoxyguanosine and what appeared to be BaP diol epoxide adducts with deoxycytidine were consistently present but at much lower frequency. All three types of BaP diol epoxide--DNA adducts persisted in epithelial cells for 72 hr in BaP-free medium. No adducts were detected in fibroblastic cultures until 96 hr after first exposure to BaP. At this time, the type and extent of BaP diol epoxide--DNA adduct formation was similar to that in epithelial cells exposed to one-tenth the dose of BaP. The type, extent, rate of formation, and persistence of the adducts in human breast epithelial cells was similar to that in cells transformable by exposure to BaP, an indication that they may be targets for chemically induced carcinogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autrup H., Harris C. C., Trump B. F., Jeffrey A. M. Metabolism of benzo(a)pyrene and identification of the major benzo(a)pyrene-DNA adducts in cultured human colon. Cancer Res. 1978 Nov;38(11 Pt 1):3689–3696. [PubMed] [Google Scholar]
- Autrup H., Wefald F. C., Jeffrey A. M., Tate H., Schwartz R. D., Trump B. F., Harris C. C. Metabolism of benzo[a]pyrene by cultured tracheobronchial tissues from mice, rats, hamsters, bovines and humans. Int J Cancer. 1980 Feb 15;25(2):293–300. doi: 10.1002/ijc.2910250219. [DOI] [PubMed] [Google Scholar]
- Baird W. M., Chemerys R., Grinspan J. B., Mueller S. N., Levine E. M. Benzo(a)pyrene metabolism in bovine aortic endothelial and bovine lung fibroblast-like cell cultures. Cancer Res. 1980 Jun;40(6):1781–1786. [PubMed] [Google Scholar]
- Baird W. M., Diamond L. The nature of benzo(a)pyrene-DNA adducts formed in hamster embryo cells depends on the length of time of exposure to benzo(a)pyrene. Biochem Biophys Res Commun. 1977 Jul 11;77(1):162–167. doi: 10.1016/s0006-291x(77)80178-2. [DOI] [PubMed] [Google Scholar]
- Berwald Y., Sachs L. In vitro transformation of normal cells to tumor cells by carcinogenic hydrocarbons. J Natl Cancer Inst. 1965 Oct;35(4):641–661. [PubMed] [Google Scholar]
- Brown H. S., Jeffrey A. M., Weinstein I. B. Formation of DNA adducts in 10T1/2 mouse embryo fibroblasts incubated with benzo(a)pyrene or dihydrodiol oxide derivatives. Cancer Res. 1979 May;39(5):1673–1677. [PubMed] [Google Scholar]
- Bürki K., Stoming T. A., Bresnick E. Effects of an epoxide hydrase inhibitor on in vivo binding of polycyclic hydrocarbons to DNA and on skin carcinogenesis. J Natl Cancer Inst. 1974 Mar;52(3):785–788. doi: 10.1093/jnci/52.3.785. [DOI] [PubMed] [Google Scholar]
- Carroll K. K., Khor H. T. Effects of dietary fat and dose level of 7,12-dimethylbenz(alpha)-anthracene on mammary tumor incidence in rats. Cancer Res. 1970 Aug;30(8):2260–2264. [PubMed] [Google Scholar]
- Cohen G. M., Bracken W. M., Iyer R. P., Berry D. L., Selkirk J. K., Slaga T. J. Anticarcinogenic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on benzo(a)pyrene and 7,12-dimethylbenz(a)anthracene tumor initiation and its relationship to DNA binding. Cancer Res. 1979 Oct;39(10):4027–4033. [PubMed] [Google Scholar]
- Cohen G. M., MacLeod M. C., Moore C. J., Selkirk J. K. Metabolism and macromolecular binding of carcinogenic and noncarcinogenic metabolites of benzo(a)pyrene by hamster embryo cells. Cancer Res. 1980 Feb;40(2):207–211. [PubMed] [Google Scholar]
- Cohen G. M., Marchok A. C., Nettesheim P., Steele V. E., Nelson F., Huang S., Selkirk J. K. Comparative metabolism of benzo(a)pyrene in organ and cell cultures derived from rat tracheas. Cancer Res. 1979 Jun;39(6 Pt 1):1980–1984. [PubMed] [Google Scholar]
- DiPaolo J. A., Donovan P., Nelson R. Quantitative studies of in vitro transformation by chemical carcinogens. J Natl Cancer Inst. 1969 May;42(5):867–874. [PubMed] [Google Scholar]
- Feldman G., Remsen J., Shinohara K., Cerutti P. Excisability and persistence of benzo(a)pyrene DNA adducts in epithelioid human lung cells. Nature. 1978 Aug 24;274(5673):796–798. doi: 10.1038/274796a0. [DOI] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Persistence of O6-ethylguanine in rat-brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci U S A. 1974 Mar;71(3):639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover P. L., Hewer A., Pal K., Sims P. The involvement of a diol-epoxide in the metabolic activation of benzo(a)pyrene in human bronchial mucosa and in mouse skin. Int J Cancer. 1976 Jul 15;18(1):1–6. doi: 10.1002/ijc.2910180102. [DOI] [PubMed] [Google Scholar]
- Grover P. L., MacNicoll A. D., Sims P., Easty G. C., Neville A. M. Polycyclic hydrocarbon activation and metabolism in epithelial cell aggregates prepared from human mammary tissue. Int J Cancer. 1980 Oct 15;26(4):467–475. doi: 10.1002/ijc.2910260412. [DOI] [PubMed] [Google Scholar]
- HUGGINS C., BRIZIARELLI G., SUTTON H., Jr Rapid induction of mammary carcinoma in the rat and the influence of hormones on the tumors. J Exp Med. 1959 Jan 1;109(1):25–42. doi: 10.1084/jem.109.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. C., Autrup H., Stoner G. D., Trump B. F., Hillman E., Schafer P. W., Jeffrey A. M. Metabolism of benzo(a)pyrene, N-nitrosodimethylamine, and N-nitrosopyrrolidine and identification of the major carcinogen-DNA adducts formed in cultured human esophagus. Cancer Res. 1979 Nov;39(11):4401–4406. [PubMed] [Google Scholar]
- Heidelberger C. Chemical carcinogenesis. Annu Rev Biochem. 1975;44:79–121. doi: 10.1146/annurev.bi.44.070175.000455. [DOI] [PubMed] [Google Scholar]
- Huberman E., Sachs L., Yang S. K., Gelboin V. Identification of mutagenic metabolites of benzo(a)pyrene in mammalian cells. Proc Natl Acad Sci U S A. 1976 Feb;73(2):607–611. doi: 10.1073/pnas.73.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey A. M., Weinstein I. B., Jennette K. W., Grzeskowiak K., Nakanishi K., Harvey R. G., Autrup H., Harris C. Structures of benzo(a)pyrene--nucleic acid adducts formed in human and bovine bronchial explants. Nature. 1977 Sep 22;269(5626):348–350. doi: 10.1038/269348a0. [DOI] [PubMed] [Google Scholar]
- Kakunaga T. A quantitative system for assay of malignant transformation by chemical carcinogens using a clone derived from BALB-3T3. Int J Cancer. 1973 Sep 15;12(2):463–473. doi: 10.1002/ijc.2910120217. [DOI] [PubMed] [Google Scholar]
- Kapitulnik J., Wislocki P. G., Levin W., Yagi H., Thakker D. R., Akagi H., Koreeda M., Jerina D. M., Conney A. H. Marked differences in the carcinogenic activity of optically pure (+)- and (-)-trans-7,8-dihydroxy-7,8-dihydrobenzo(a)pyrene in newborn mice. Cancer Res. 1978 Sep;38(9):2661–2665. [PubMed] [Google Scholar]
- King M. M., Bailey D. M., Gibson D. D., Pitha J. V., McCay P. B. Incidence and growth of mammary tumors induced by 7,12-dimethylbenz[a]anthracene as related to the dietary content of fat and antioxidant. J Natl Cancer Inst. 1979 Sep;63(3):657–663. doi: 10.1093/jnci/63.3.657. [DOI] [PubMed] [Google Scholar]
- Levin W., Wood A. W., Chang R. L., Slaga T. J., Yagi H., Jerina D. M., Conney A. H. Marked differences in the tumor-initiating activity of optically pure (+)- and (-)-trans-7,8-dihydroxy-7,8-dihydrobenzo(a)pyrene on mouse skin. Cancer Res. 1977 Aug;37(8 Pt 1):2721–2725. [PubMed] [Google Scholar]
- Margison G. P., Margison J. M., Montesano R. Accumulation of O6-methylguanine in non-target-tissue deoxyribonucleic acid during chronic administration of dimethylnitrosamine. Biochem J. 1977 Sep 1;165(3):463–468. doi: 10.1042/bj1650463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S., Brankow D. W., Heidelberger C. Two-stage chemical oncogenesis in cultures of C3H/10T1/2 cells. Cancer Res. 1976 Jul;36(7 Pt 1):2254–2260. [PubMed] [Google Scholar]
- Parkinson E. K., Newbold R. F. Benzo(a)pyrene metabolism and DNA adduct formation in serially cultivated strains of human epidermal keratinocytes. Int J Cancer. 1980 Sep 15;26(3):289–299. doi: 10.1002/ijc.2910260307. [DOI] [PubMed] [Google Scholar]
- Peterson J. A., Bartholomew J. C., Stampfer M., Ceriani R. L. Analysis of expression of human mammary epithelial antigens in normal and malignant breast cells at the single cell level by flow cytofluorimetry. Exp Cell Biol. 1981;49(1):1–14. doi: 10.1159/000163773. [DOI] [PubMed] [Google Scholar]
- Selkirk J. K., Wiebel F. J. Comparative metabolism of benzo(a)pyrene in rodent liver and embryonic cells in tissue culture. Prog Exp Tumor Res. 1979;24:61–72. doi: 10.1159/000402084. [DOI] [PubMed] [Google Scholar]
- Shinohara K., Cerutti P. A. Excision repair of benzo[a]pyrene-deoxyguanosine adducts in baby hamster kidney 21/C13 cells and in secondary mouse embryo fibroblasts C57BL/6J. Proc Natl Acad Sci U S A. 1977 Mar;74(3):979–983. doi: 10.1073/pnas.74.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P., Grover P. L., Swaisland A., Pal K., Hewer A. Metabolic activation of benzo(a)pyrene proceeds by a diol-epoxide. Nature. 1974 Nov 22;252(5481):326–328. doi: 10.1038/252326a0. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Hallowes R. C., Hackett A. J. Growth of normal human mammary cells in culture. In Vitro. 1980 May;16(5):415–425. doi: 10.1007/BF02618365. [DOI] [PubMed] [Google Scholar]
- Steele V. E., Marchok A. C., Cohen G. M. Transformation of rat tracheal epithelial cells by benzo[a]pyrene and its metabolites. Cancer Lett. 1980 Feb;8(4):291–298. doi: 10.1016/0304-3835(80)90144-5. [DOI] [PubMed] [Google Scholar]
- Straub K. M., Meehan T., Burlingame A. L., Calvin M. Identification of the major adducts formed by reaction of benzo(a)pyrene diol epoxide with DNA in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5285–5289. doi: 10.1073/pnas.74.12.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch C. W., Nagasawa H. Prolactin and murine mammary tumorigenesis: a review. Cancer Res. 1977 Apr;37(4):951–963. [PubMed] [Google Scholar]
- Westra J. G., Kriek E., Hittenhausen H. Identification of the persistently bound form of the carcinogen N-acetyl-2-aminofluorene to rat liver DNA in vivo. Chem Biol Interact. 1976 Oct 2;15(2):149–164. doi: 10.1016/0009-2797(76)90160-5. [DOI] [PubMed] [Google Scholar]




