Summary
We report on five patients who were treated by stent-assisted coil embolization to preserve the patency of the parent artery. Three patients presented with subarachnoid haemorrhage and two with ischemic symptoms. Four patients were treated with stenting and then followed by coil embolization of the aneurysmal dilatation, and the remaining patient with stenting alone because the aneurysmal dilatation was too small to insert coils.
Complete obliteration of the aneurysm was achieved in three patients, but in one patient an aneurysmal rupture occurred during the insertion of the first coil and a parent artery occlusion was therefore performed. In the one patient treated with stenting alone, a small aneurysmal dilatation remained patent, but complete obliteration was confirmed by the follow-up angiography. Subsequent subarachnoid haemorrhage was not observed in any of the patients. Four of them achieved a good recovery, but one patient suffered severe disability due to the aneurysmal rupture during the procedure. Parent artery occlusion remains the treatment of choice. Stent-assisted coil embolization has a higher risk of rupture than does the parent artery occlusion during the procedure. Furthermore, recanalization or subsequent subarachnoid haemorrhage is more likely to occur in a stent-assisted coil embolization after the procedure. However, this procedure, which can maintain the patency of the parent artery, will become an alternative for patients who are at a high risk of developing ischemic symptoms in parent artery occlusions.
Key words: vertebral artery, dissecting aneurysm, stent, coil embolization
Introduction
Vertebral artery (VA) dissecting aneurysms are commonly treated with parent artery occlusion using the endovascular approach 1,2. However, this procedure cannot be applied to patients who are intolerant to the occlusion test. Moreover, ischemic complications, which cannot be predicted by the occlusion test, sometimes occur as a result of thrombosis of the perforating arteries 3. We previously reported on a patient who suffered from severe ischemic complication due to late thrombosis of the anterior spinal artery (ASA). We pointed out that catastrophic complications can occur if the ASA originates only in an affected VA in patients with vertebral aneurysms located distal to the origin of the posterior inferior cerebellar artery ("distal to PICA" type aneurysm) 4.
Recently, endovascular treatment using stents and coils for VA dissecting aneurysms has been reported 5-8. In this report, we therefore describe five patients who were treated by stent-assisted coil embolization in order to preserve the patency of the parent artery for various reasons.
Material and Methods
Five patients (4 men and 1 woman; age range 48-65 yrs; mean age 57.8 yrs) with VA dissecting aneurysms were treated by an endovascular approach using stents. The onset of subarachnoid haemorrhage (SAH) occurred in three patients, and ischemic symptoms in the other two. The angiography of all patients showed "distal to PICA" type aneurysms. Stent-assisted coil embolization was indicated for various reasons: hypoplasia of the contralateral VA, bilateral dissections (a string sign on the right and an aneurysmal dilatation on the left), and preservation of the ASA and posterior inferior cerebellar artery. Moreover, the aneurysms projected unilaterally were chosen for this treatment. The intervals between the onset and the treatment were 5 to 46 days (mean 20.2 days). Endovascular treatment was performed under general anesthesia. Usually, two guiding catheters were introduced into the VA; one was used for placing the stent and the other for inserting coils. Balloon expandable S670 coronary stents (Medtronic AVE) were used in all the patients.
The stent size was selected to equal the diameter of the parent artery and to sufficiently cover the orifice of the aneurysm. A stent was positioned across the lesion and was released by inflating the balloon with nominal pressure.
When necessary, the balloon was slightly pulled out in order to check if the stent was anchored securely to the parent artery. If the stent was displaced backward, it was repositioned with maximum inflation of the balloon. The balloon was kept in the stent lumen so as to provide backup against a possible aneurysmal rupture during the procedure. A microcatheter was then introduced into the aneurysm through the stent strut, and the aneurysm was embolized using Guglielmi detachable coils (Boston Scientific). In case 5, a microcatheter was introduced into the aneurysm before stenting (table 1).
Table 1.
Characteristics of the Five Patients with VA Dissecting Aneurysms
| No. | Age(yr) /Sex |
Onset (H&K grading) |
Location of the aneurysm |
Reasons for stenting |
Interval between onset and treatment |
|---|---|---|---|---|---|
| 1 | 52/M | SAH (grade 3) |
distal to PICA | preservation of ASA hypoplasia of the contralateral VA |
5 days |
| 2 | 48/M | brain stem infarction |
distal to PICA | preservationof of ASA and PICA |
46 days |
| 3 | 60/M | SAH (grade 3) |
distal to PICA | bilateral dissections | 25 days |
| 4 | 65/F | SAH (grade 4) |
distal to PICA | hypoplasia of the contralateral VA |
15 days |
| 5 | 64/M | TIA | distal to PICA | hypoplasia of the contralateral VA |
10 days |
|
SAH: Subarachnoid haemorrhage; TIA: transient ischemic attack; PICA: posterior inferior cerebellar artery; ASA: anterior spinal artery; VA: vertebral artery | |||||
Results
The S670 stents were positioned across the lesions in all the patients and were deployed by inflating the balloon with nominal pressure. In cases one and three, however, the released stents were displaced backward during removal of the balloon. In case one, the stent was compressed in the proximal VA with maximum inflation of the balloon, and then another S67O coronary stent of a larger diameter was deployed to sufficiently anchor the parent artery. In case three, the released stent was fortunately repositioned across the lesion by pushing the balloon distally, and then it was anchored to the parent artery with maximum inflation of the balloon. Finally, the stents were placed to sufficiently cover the lesions in all the patients without rupture of the aneurysms.
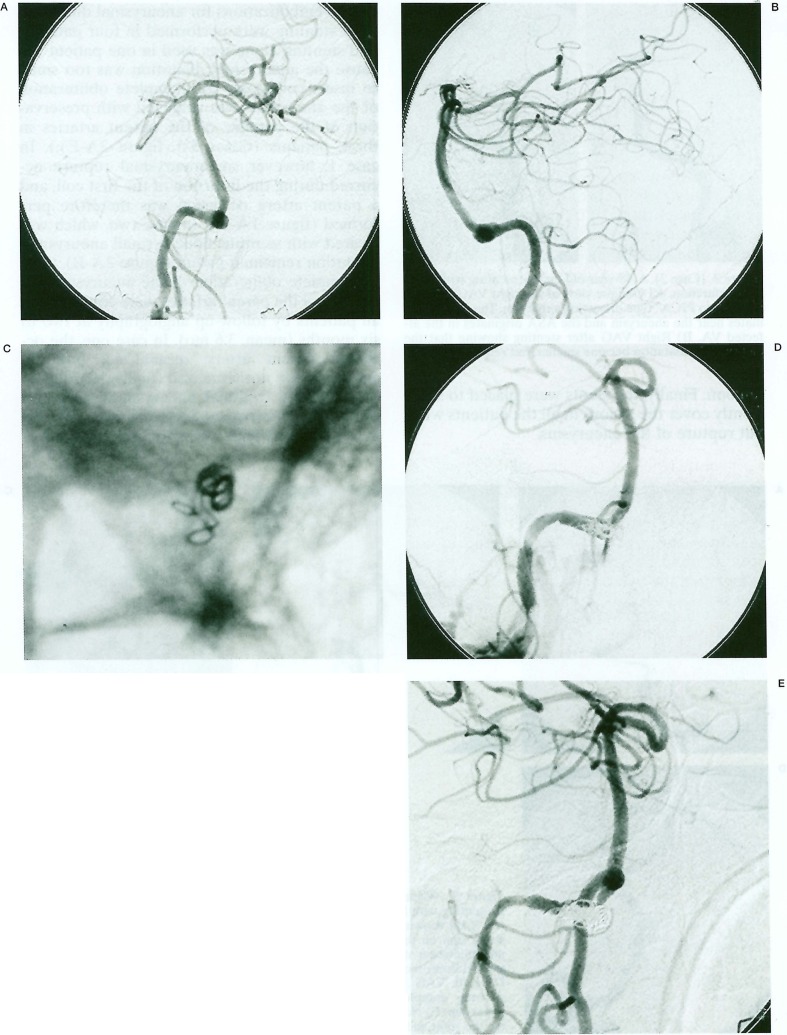
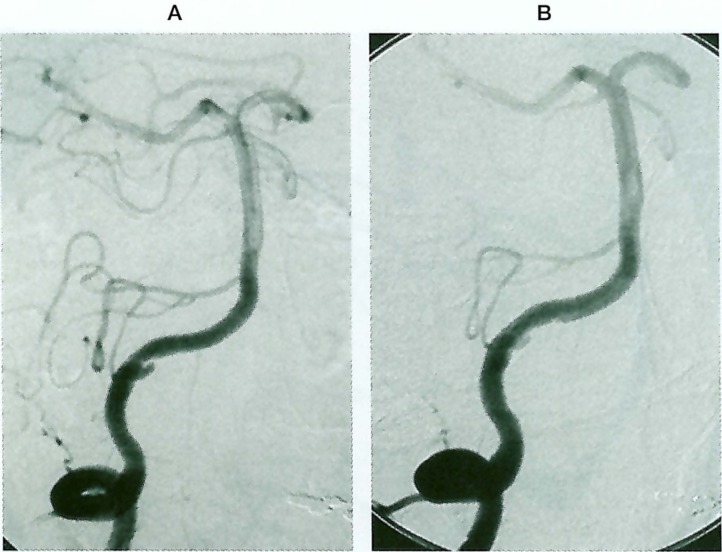
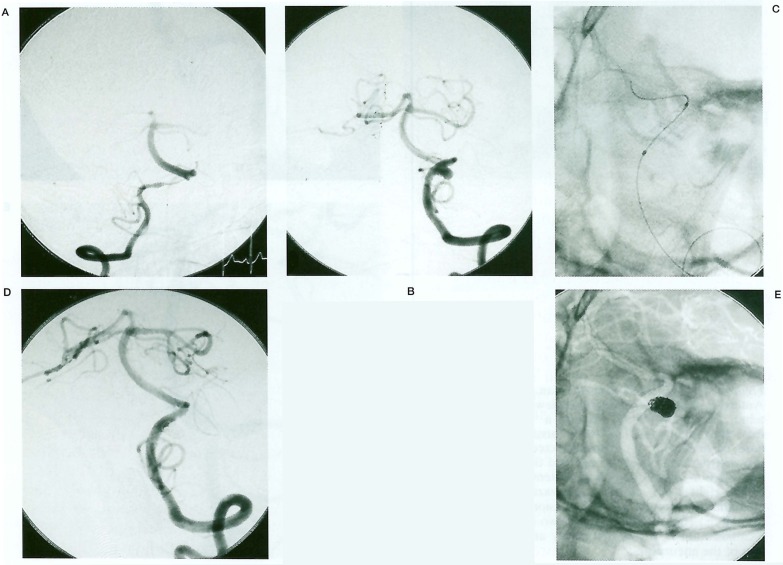
Coil embolizations for aneurysmal dilatation after stenting were performed in four patients, and stenting alone was used in one patient because the aneurysmal dilatation was too small to insert coils (case 2). Complete obliteration of the aneurysms was achieved with preservation of the patency of the parent arteries in three patients (Cases 3-5, figure 3A-E).). In case 1, however, an aneurysmal rupture occurred during the insertion of the first coil, and a parent artery occlusion was therefore performed (figure 1A-D). In case two, which was treated with stenting alone, a small aneurysmal dilatation remained patent (figure 2A-B).
Figure 1.
(Case 1). A 52-year-old man presenting with SAH. Antero-posterior (A) and lateral views (B) of the right vertebral angiogram (VAG) showing a "distal to PICA" type dissecting aneurysm. The ASA (arrow) originates in the base of aneurysm. C) Nonsubtracted fluoroscopic image showing perforation of the coil and the microcatheter during insertion of the first coil after stenting. D) Oblique view of the right VAG showing extravazation of the contrast medium. The parent artery occlusion was therefore performed. E) Right VAG obtained two months later showing recanalization of the parent artery and complete obliteration of the aneurysm.
Figure 2.
(Case 2). A 48-year-old man presenting with brain stem infarction. A) Oblique view of the right VAG showing a "distal to PICA" type dissecting aneurysm. The PICA originates near the aneurysm and the ASA originates in the affected VA. B) Right VAG after stenting showing that the aneurysmal dilatation became smaller, but remained patent.
Figure 3.
(Case 3). A 60-year-old man presenting with SAH. Right (A) and left (B) VAG showing bilateral dissections, a string sign on the right and an aneurysmal dilatation on the left. C) Nonsubtracted fluoroscopic image after stenting. D) Left VAG after coil embolization showing complete obliteration of the aneurysm. E) Nonsubtracted VAG showing the stent and coils.
Complete obliteration of the aneurysms and patency of the parent arteries were confirmed in all patients by follow-up angiography at two to six months (mean, 3.6 mo). In case one, the occluded parent artery recanalized, but the aneurysm was not visualized (figure 1E), and, in case two, the residual aneurysm disappeared completely. Subsequent SAH was not observed in any of the patients during follow-up period of three to 24 months (mean, 11.6 mo). Four of the patients achieved a good recovery, but one patient suffered severe disability due to the aneurysmal rupture during the procedure (table 2).
Table 2.
Results of the Treatment
| No. | Procedures | Immediate angiographic results |
Complications | Follow-up angiographic findings |
Subsequent rupture |
Outcome (GOS) |
|---|---|---|---|---|---|---|
| 1 | stenting & coils (S670 3.5 x 12 mm |
parent artery occlusion including the aneurysm |
stent displacement rupture |
Recanalization of the parent artery complete obliteration (2 mo) |
none (24 mo) |
SD |
| 2 | stenting (S67O 3.0 x 9 mm |
remnant of the aneurysm |
none | complete obliteration (6 mo) |
none (12 mo) |
GR |
| 3 | stenting & coils (S67O 3.5 x 12 mm |
complete obliteration |
stent displacement | complete obliteration (4 mo) |
none (12 mo) |
GR |
| 4 | stenting & coils (S67O 3.5 x 12 mm |
complete obliteration |
none | complete obliteration (3 mo) |
none (7 mo) |
GR |
| 5 | stenting & coils (S67O 3.5 x 12 mm |
complete obliteration |
none | complete obliteration (3 mo) |
none (3 mo) |
GR |
| GOS: Glasgow outcome scale; SD: severe disability; GR: goodrecovery | ||||||
Discussion
Indication of Stent-assisted Coil Embolization
Parent artery occlusion for VA dissecting aneurysms remains the treatment of choice. Stent-assisted coil embolization has a higher risk of rupture than does the parent artery occlusion during the procedure. Furthermore, recanalization or subsequent SAH is more apt to occur after a stent-assisted coil embolization procedure. Therefore, indication of this procedure should be limited to patients who cannot tolerate to the occlusion test due to hypoplasia or aplasia of the contralateral VA.
It has been reported that patients with bilateral dissections, who were treated with the parent artery occlusion on one side, suffered from rupture of the contralateral lesion 9-11. Increased haemodynamic stress is considered to be the cause of rupture. The patency of the VA should therefore be preserved in patients with bilateral dissections, if possible 6.
ASA is formed by the paired anterior ven tral spinal arteries originating in the VA and has anastomoses with the anterior radicular arteries 12. Therefore, the occlusion of the anterior ventral spinal artery on one side may rarely give rise to ischemic symptoms. However, catastrophic complication can occur when the ASA originates only in the affected VA and has poor anastomoses with the anterior radicular arteries in patients with "distal to PICA" type aneurysms 4. Stent-assisted coil embolizations can be indicated in such patients, though the evaluation of the latent anastomoses is difficult. As for the shape of the aneurysm, the indication for a stent-assisted coil embolization is a unilaterally projecting aneurysm, and a circumferentially dilated aneurysm should be excluded.
Technical Considerations
Careful selection of the appropriate stent size is very important. If an over-sized stent is selected, an aneurysmal rupture may occur. On the contrary, an under-sized stent may be displaced downward during removal of the balloon. Usually, the stent size is selected to equal the diameter of the parent artery and to sufficiently cover the orifice of the aneurysm, and the stent is deployed by inflating the balloon with nominal pressure. If the released stent is displaced, it is compressed in the parent artery with maximum inflation of the balloon. A self-expandable stent, which can be anchored securely to the parent artery with less stress, is better than a balloon expandable stent.
The stent restricts the movability of the coils and the microcatheter as the coils are being inserted, especially when the stent is placed after the introduction of the microcatheter into the aneurysm. Therefore, the fragile aneurysmal wall is exposed to higher pressure creating a greater possibility of an aneurysmal rupture. To prepare for an unexpected rupture, the balloon used for deploying the stent should be left inside the stent lumen. This balloon is also available for preventing the coil protrusion into the parent artery from the stent mesh.
Conclusions
Parent artery occlusion is a safe and effective treatment for VA dissecting aneurysms, and it remains the treatment of choice. Stent-assisted coil embolization may be a reasonable alternative in patients who are at a high risk of developing ischemic symptoms in parent artery occlusions.
References
- 1.Halbach VV, Higashida, et al. Endovascular treatment of vertebral artery dissections and pseudoaneurysms. J Neurosurg. 1993;79:183–191. doi: 10.3171/jns.1993.79.2.0183. [DOI] [PubMed] [Google Scholar]
- 2.Yamaura I, Tani E, et al. Endovascular treatment of ruptured dissecting aneurysms aimed at occlusion of the dissected site by using Guglielmi detachable coils. J Neurosurg. 1999;90:853–856. doi: 10.3171/jns.1999.90.5.0853. [DOI] [PubMed] [Google Scholar]
- 3.Naito I, Iwai T, Sasaki T. Management of intracranial vertebral artery dissections initially presenting without subarachnoid haemorrhage. Neurosurgery. 2002;51:930–937. doi: 10.1097/00006123-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Iwai T, Naito I, et al. Angiographic findings and clinical significance of the anterior and posterior spinal arteries in therapeutic parent artery occlusion for vertebral artery aneurysms. Interventional Neuroradiology. 2000;6:299–309. doi: 10.1177/159101990000600404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benndorf G, Herbon U, et al. Treatment of a ruptured dissecting vertebral artery aneurysm with double stent placement: case report. Am J Neuroradiol. 2001;22:1844–1848. [PMC free article] [PubMed] [Google Scholar]
- 6.Chiaradio JC, Guzman L, et al. Intravascular graft stent treatment of a ruptured fusiform dissecting aneurysm of the intracranial vertebral artery: technical case report. Neurosurgery. 2002;50:213–216. doi: 10.1097/00006123-200201000-00034. [DOI] [PubMed] [Google Scholar]
- 7.Lylyk P, Ceratto R, et al. Treatment of a vertebral dissecting aneurysm with stents and coils: technical case report. Neurosurgery. 1998;43:385–388. doi: 10.1097/00006123-199808000-00132. [DOI] [PubMed] [Google Scholar]
- 8.Lylyk P, Cohen JE, et al. Combined endovascular treatment of dissecting vertebral artery aneurysms by using stents and coils. J Neurosurg. 2001;94:427–432. doi: 10.3171/jns.2001.94.3.0427. [DOI] [PubMed] [Google Scholar]
- 9.Otawara Y, Ogasawara K, et al. Dissecting aneurysms of the bilateral vertebral arteries with subarachnoid haemorrhage: report of three cases. Neurosurgery. 2002;50:1372–1274. doi: 10.1097/00006123-200206000-00033. [DOI] [PubMed] [Google Scholar]
- 10.Redekop G, terBrugge K, Willinsky R. Subarachnoid haemorrhage from vertebrobasilar dissecting aneurysm treated with staged bilateral vertebral artery occlusion: the importance of early follow-up angiography: technical case report. Neurosurgery. 1999;45:1258–1262. doi: 10.1097/00006123-199911000-00056. [DOI] [PubMed] [Google Scholar]
- 11.Yasui T, Sakamoto H, et al. Bilateral dissecting aneurysms of the vertebral arteries resulting in subarachnoid haemorrhage: case report. Neurosurgery. 1998;42:162–165. doi: 10.1097/00006123-199801000-00035. [DOI] [PubMed] [Google Scholar]
- 12.de Oliveira E, Rhoton AL, Jr, et al. Microsurgical anatomy of the region of the foramen magnum. Surg Neurol. 1988;24:293–352. doi: 10.1016/0090-3019(85)90042-4. [DOI] [PubMed] [Google Scholar]