Abstract
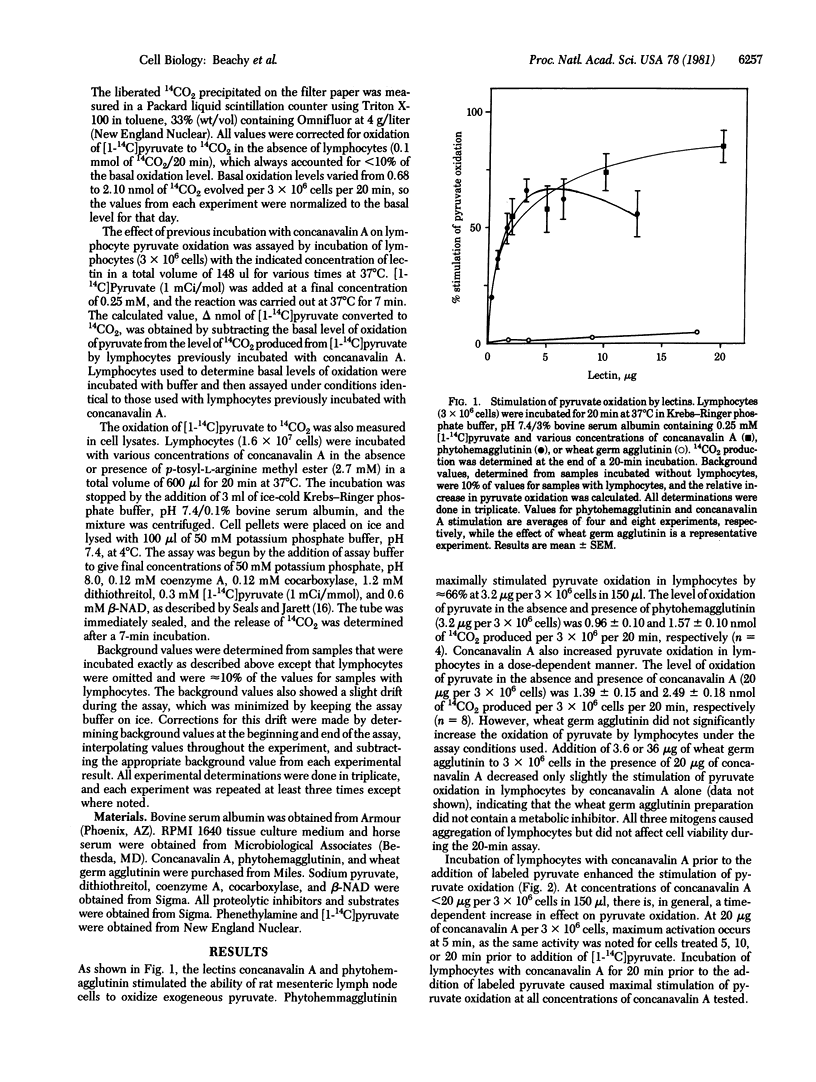
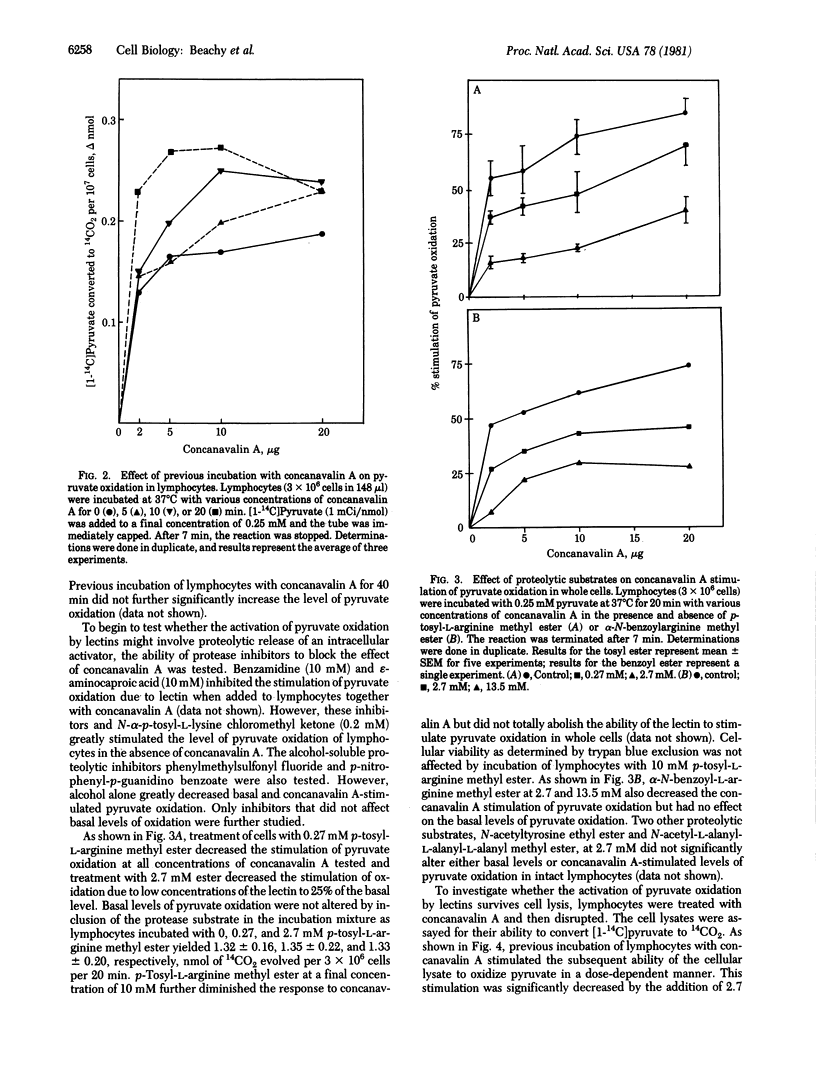
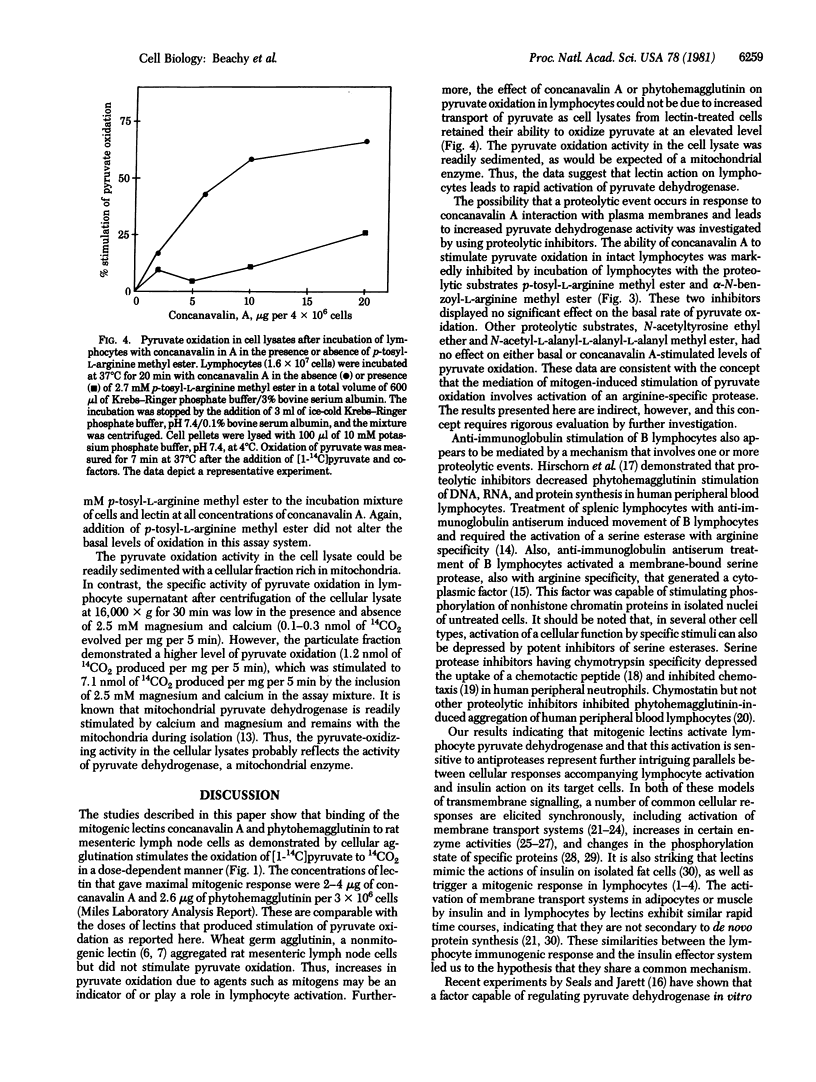
The mitogenic lectins concanavalin A and phytohemagglutinin were found to stimulate pyruvate oxidation in rat mesenteric lymphocytes. Marked cell agglutination accompanied this response. Wheat germ agglutinin, a nonmitogenic lectin, also aggregated lymphocytes but did not cause alteration of pyruvate oxidation. Cell lysates from lectin-treated cells retained their ability to oxidize pyruvate at an elevated rate, indicating that the observed stimulation of pyruvate oxidation was not due to increased transport of labeled pyruvate into the cells. Pyruvate oxidation activity in such lysates was readily sedimented in a mitochondria-enriched cellular fraction, indicating that it reflects mitochondrial pyruvate dehydrogenase. Stimulation of this activity by lectins in intact lymphocytes was inhibited when the cells were incubated under conditions expected to inhibit trypsin-like proteases. Thus, esters of arginine, but not of alanine or tyrosine, blocked stimulation of pyruvate dehydrogenase by the lectins. The data indicate that pyruvate dehydrogenase is activated in lymphocytes treated with mitogenic lectins by a mechanism involving one or more proteolytic reactions. The similarity between the results presented here and those recently reported for insulin action on its target cells [Seals, J. R. & Czech, M. P. (1980) J. Biol. Chem. 255, 6529-6531] suggests that these systems may have similar modes of transmembrane signalling.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker E. L., Unanue E. R. The requirement for esterase activation in the anti-immunoglobulin-triggered movement of B lymphocytes. J Immunol. 1976 Jul;117(1):27–32. [PubMed] [Google Scholar]
- Chaplin D. D., Wedner H. J., Parker C. W. Protein phosphorlyation in human peripheral blood lymphocytes. Phosphorylation of endogenous plasma membrane and cytoplasmic proteins. Biochem J. 1979 Aug 15;182(2):537–546. doi: 10.1042/bj1820537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech M. P. Insulin action and the regulation of hexose transport. Diabetes. 1980 May;29(5):399–409. doi: 10.2337/diab.29.5.399. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Lawrence J. C., Jr, Lynn W. S. Activation of hexose transport by concanavalin A in isolated brown fat cells. Effects of cell surface modification with neuraminidase and trypsin on lectin and insulin action. J Biol Chem. 1974 Dec 10;249(23):7499–7505. [PubMed] [Google Scholar]
- Freedman M. H. Early biochemical events in lymphocyte activation. I. Investigations on the nature and significance of early calcium fluxes observed in mitogen-induced T and B lymphocytes. Cell Immunol. 1979 May;44(2):290–313. doi: 10.1016/0008-8749(79)90007-8. [DOI] [PubMed] [Google Scholar]
- Freedman M. H., Raff M. C. Induction of increased calcium uptake in mouse T lymphocytes by concanavalin A and its modulation by cyclic nucleotides. Nature. 1975 May 29;255(5507):378–382. doi: 10.1038/255378a0. [DOI] [PubMed] [Google Scholar]
- Grady P. G., Davis A. T., Shapira E. The effect of some protease substrates and inhibitors on chemotaxis and protease activity of human polymorphonuclear leukocytes. J Infect Dis. 1979 Dec;140(6):999–1003. doi: 10.1093/infdis/140.6.999. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Grossman J., Troll W., Weissmann G. The effect of epsilon amino caproic acid and other inhibitors of proteolysis upon the response of human peripheral blood lymphocytes to phytohemagglutinin. J Clin Invest. 1971 Jun;50(6):1206–1217. doi: 10.1172/JCI106598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T., Kikutani H., Nishizawa Y., Sakaguchi N., Yamamura Y. Involvement of anti-Ig-activated serine protease in the generation of cytoplasmic factor(s) that are responsible for the transmission of Ig-receptor-mediated signals. J Immunol. 1979 Oct;123(4):1504–1510. [PubMed] [Google Scholar]
- Kono T., Barham F. W. Insulin-like effects of trypsin on fat cells. Localization of the metabolic steps and the cellular site affected by the enzyme. J Biol Chem. 1971 Oct 25;246(20):6204–6209. [PubMed] [Google Scholar]
- Lane J. T., Lo F. M., Prasad C. Chymostatin inhibits cellular aggregation of activated human peripheral blood lymphocytes. Life Sci. 1980 Aug 11;27(6):451–456. doi: 10.1016/0024-3205(80)90125-3. [DOI] [PubMed] [Google Scholar]
- Larsson E. L., Coutinho A. Mechanism of T cell activation. I. A screening of "step one" ligands. Eur J Immunol. 1980 Feb;10(2):93–99. doi: 10.1002/eji.1830100205. [DOI] [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. Electrophoretic resolution of three major insulin receptor structures with unique subunit stoichiometries. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7137–7141. doi: 10.1073/pnas.77.12.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely A. N., Sitzmann J. V., Kersey J. H. EGTA and proteinase reversal of cellular aggregation of activated lymphocytes. Nature. 1976 Dec 23;264(5588):770–771. doi: 10.1038/264770a0. [DOI] [PubMed] [Google Scholar]
- Niedel J., Frothingham R., Cuatrecasas P. Inhibition of 125I-chemotactic peptide uptake by protease inhibitors. Biochem Biophys Res Commun. 1980 May 30;94(2):667–673. doi: 10.1016/0006-291x(80)91284-x. [DOI] [PubMed] [Google Scholar]
- Peters J. H., Hausen P. Effect of phytohemagglutinin on lymphocyte membrane transport. 2. Stimulation of "facilitated diffusion" of 3-O-methyl-glucose. Eur J Biochem. 1971 Apr 30;19(4):509–513. doi: 10.1111/j.1432-1033.1971.tb01342.x. [DOI] [PubMed] [Google Scholar]
- Seals J. R., Czech M. P. Evidence that insulin activates an intrinsic plasma membrane protease in generating a secondary chemical mediator. J Biol Chem. 1980 Jul 25;255(14):6529–6531. [PubMed] [Google Scholar]
- Seals J. R., Jarett L. Activation of pyruvate dehydrogenase by direct addition of insulin to an isolated plasma membrane/mitochondria mixture: evidence for generated of insulin's second messenger in a subcellular system. Proc Natl Acad Sci U S A. 1980 Jan;77(1):77–81. doi: 10.1073/pnas.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals J. R., McDonald J. M., Jarett L. Insulin effect on protein phosphorylation of plasma membranes and mitochondria in a subcellular system from rat adipocytes. I. Identification of insulin-sensitive phosphoproteins. J Biol Chem. 1979 Aug 10;254(15):6991–6996. [PubMed] [Google Scholar]
- Stein-Streilein J., Hart D. A. Exogenous protease promotes specific antibody forming cell response in vitro. Cell Immunol. 1980 Sep 1;54(2):284–292. doi: 10.1016/0008-8749(80)90210-5. [DOI] [PubMed] [Google Scholar]
- Vischer T. L. Stimulation of mouse B lymphocytes by trypsin. J Immunol. 1974 Jul;113(1):58–62. [PubMed] [Google Scholar]
- Wang T., Foker J. E., Tsai M. Y. The shift of an increase in phosphofructokinase activity from protein synthesis-dependent to -independent mode during concanavalin A induced lymphocyte proliferation. Biochem Biophys Res Commun. 1980 Jul 16;95(1):13–19. doi: 10.1016/0006-291x(80)90697-x. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Kishimoto T., Miyake T., Nishizawa Y., Inoue H. Triggering mechanism of B lymphocytes. II. Induction of ornithine decarboxylase in B cells by anti-immunoglobulin and enhancing soluble factor. J Immunol. 1975 Nov;115(5):1185–1190. [PubMed] [Google Scholar]
- Witters L. A., Moriarity D., Martin D. B. Regulation of hepatic acetyl coenzyme A carboxylase by insulin and glucagon. J Biol Chem. 1979 Jul 25;254(14):6644–6649. [PubMed] [Google Scholar]



