Summary
Percutaneous transluminal angioplasty (PTA) has been a useful therapy to treat stenosis of cervical internal carotid artery (ICA) or vertebral artery (VA). Here we show that our clinical results regarding preventive effect of stenting on restenosis after PTA in the ICA and VA origin. We also show our experimental studies with gene transfer techniques aiming reduction of restenosis after balloon-injury in rat carotid artery. It has been reported that drug-eluting stent inhibits restenosis after coronary angioplasty. Further understanding of the mechanism of restenosis and application of these new modalities may lead to better clinical results in angioplasty of the craniocervical arteries.
Introduction
Recently, percutaneous transluminal angioplasty (PTA) has been regarded as a useful therapy for stenosis of supraaortic arteries including internal carotid artery (ICA) and vertebral artery (VA) 8,10,11. Although this therapy has lots of advantages because of its less-invasiveness, restenosis is observed three to six months after angioplasty in significant percentage of the patients 12. It was reported that stenting was useful for the stenosis of the ICA and VA 8,10,11. To reduce restenosis after PTA, we have also employed stenting for the stenosis of the ICA, VA, and intracranial arteries. On the other hand, we have been studying molecular mechanisms of restenosis after balloon injury in rat carotid artery to find a way to prevent restenosis after procedure 6,13.
Here we show that retrospective analysis of our clinical results of PTA/stenting, and experimental studies regarding restenosis prevention after balloon injury. We also refer to possible impacts of new treatments, including gene therapy and drug-eluting stent 3,5,9, on inhibition of neointimal hyperplasia after PTA.
Clinical Study
Material and Methods
Under local anesthesia, PTA or PTA/stenting was performed in a total of 137 patients with high grade stenosis of cervical ICA or VA origin in our clinic: ICA, 105 cases, 14 females and 91 males, age; 43-84 (mean 65.9) year-old; VA origin, 32 cases, five females and 27 males, age; 52-78 (mean 63.8) y.o. Indication of PTA/stenting was based on NASCET 7 or ACAS 2 criterions. Follow-up angiography was performed three to six months after procedures. Restenosis was diagnosed when it was more than 50% on the angiograms 7.
Results of Clinical Study
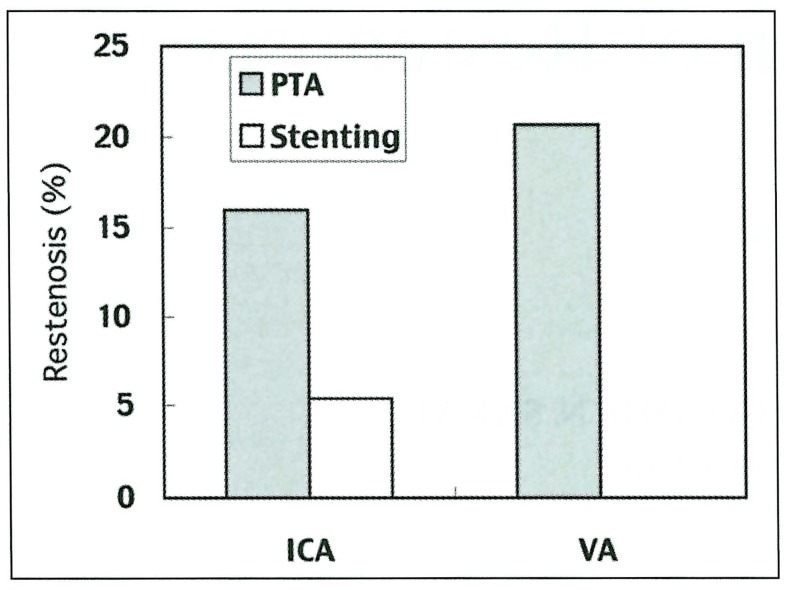
Restenosis was observed on the follow-up angiography as follows (figure 1):
Figure 1.
1- IC stenosis: PTA group, 8 cases (16.0%), Stenting group, three cases (5.4%).
2- VA origin stenosis: PTA group, five cases (20.7%), Stenting group, none (0%).
Representative Case
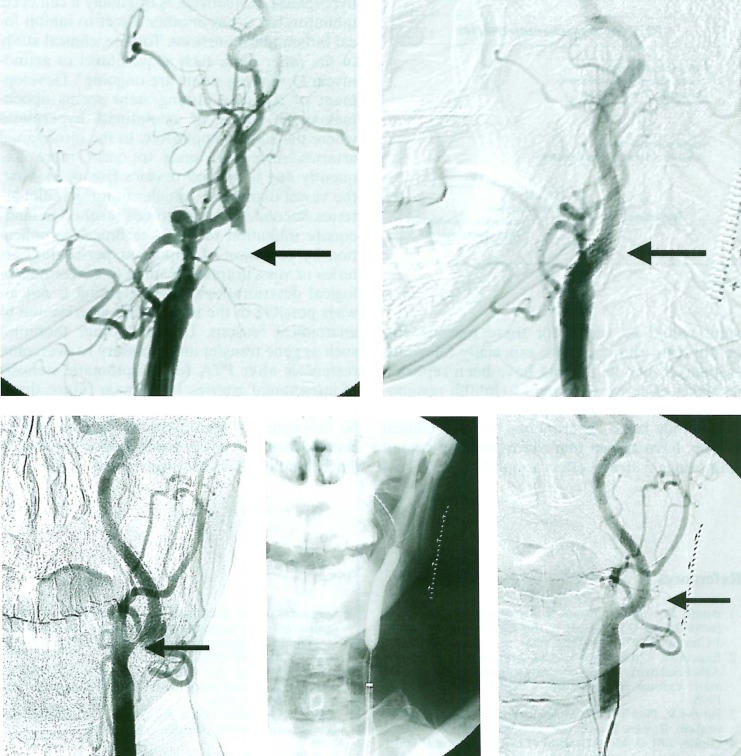
A 70-year-old man had right hemiparesis and motor aphasia due to previous cerebral infarction in the territory of the left middle cerebral artery. A high grade stenosis of the left ICA was confirmed by carotid angiography, and successfully treated with PTA with a self-expandable stent (SMART stent, J&J) (figure 2 A,B). However, restenosis was observed on the follow-up angiography performed 3 months after the procedure. The restenosis needed to be treated again with PTA and stenting (figure 2 C,D,E).
Figure 2.
Basic Study
Material and Methods
Male SD rats were anesthetized with ketamine and the left common carotid artery (CCA) was surgically exposed. Vascular injury of the CCA was produced by the 3 times passages of inflated balloon catheter (2 Fr. Fogarty catheter) through an arteriotomy in the external carotid artery (ECA). In case of gene transfer, a cannula was introduced into the CCA via the ECA, and the injured site was incubated with 200 microliter HVJ liposome complexes containing decoy oligonucleotide (ODN) or antisense 6. One or two weeks after injury, each carotid artery was processed for pathological and molecular experiments.
Results of Basic Experiments
In vivo transfection of NFkB decoy ODN, into balloon-injured carotid artery resulted in the inhibition of neointimal formation at 14 days after injury as compared with vessels transfected with control ODN (table 1). Gene expression of ICAM-l/VCAM-1 and the migration of macrophages/T-lymphocytes were markedly decreased by NFkB decoy transfer 13. These results suggested that clinical application of gene transfer would be possible to inhibit neointimal hyperplasia after angioplasty.
Table 1.
Effect of decoy oligonucleotide transfection on neointilman formation of rat carotid artery after ballon injury
| intimal/medial ratio | |
| untreated | 1.75 ± 0.2 |
| scrambled decoy | 1.81 ± 0.3 |
| nuclear factor-kB | 0.62 ± 0.4* |
| AP-1 | 1.86 ± 0.5 |
| * : p<0.01 versus untreated, scrambled decoy and AP-1 | |
Discussion
Our clinical study indicated that application of stenting following PTA significantly reduced restenosis on the follow-up angiography. By application of stenting, restenosis was reduced from 16.0% to 5.4% in the cervical ICA, and 20.7 to 0% in the VA origin. This effect was possibly caused by prevention of elastic recoil and vascular remodeling after PTA.
On the other hand, stenting is known to be ineffective to prevent intimal hyperplasia. Also, in the field of the intracranial arteries, stenting is not always possible because of existence of the important perforating artery and difficulty of the stent insertion to tortuous vessels. Therefore, to reduce restenosis after stenting, intimal hyperplasia should be inhibited by other treatments.
The molecular mechanisms of restenosis after angioplasty have been studied in rat carotid artery after balloon injury. Using this model, we have also tried to inhibit intimal hyperplasia with drug or gene transfer after angioplasty. NFkB is known to enhance restenosis or atheroscrelosis by activating adhesion molecules such as ICAM-1 or VCAM-1 1,4.
Then, we have tried to inhibit this molecule by in vivo transfection of decoy oligonucleotide by incubating the intraluminal space of the carotid artery just after balloon injury.
In our study, several molecules were targeted such as NFkB and AP-1. As shown in table 1, transfection of NFkB decoy ODN, but not AP-1 decoy, inhibited neointimal hyperplasia in injured carotid artery of rat. In the same experiment, NFkB transfection inhibited local inflammatory actions through the downregulation of adhesion molecules 13. These results suggested that gene transfer technique targeting these molecules would be applicable after repeated evaluation using the other types of animals. On the other hand, gene transfer technique should be modified for the cerebral arteries.
1- To avoid embolic complications, vectors should be changed for cerebral artery.
2- To avoid gene transfer to the brain, a special catheter, such as a double balloon infusion catheter, should be developed. A gene-eluting stent would be useful for these reasons. Recently drug-eluting stents, especially Silorimus (rapamycin)-coated stents, have been reported as highly effective treatment to inhibit restenosis after coronary angioplasty 5,9.
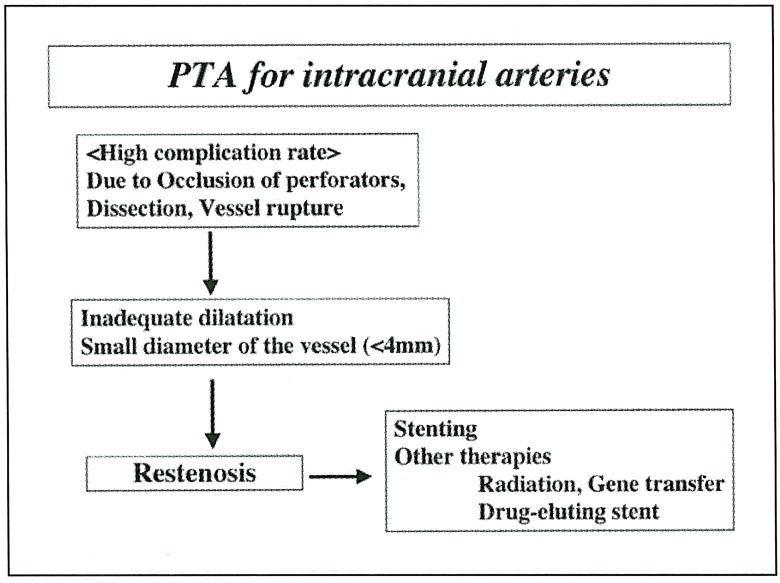
Not only basic experiments, but also clinical studies, have shown that the rapamycin-coated stents have a strong effect to inhibit neointimal hyperplasia. Rapamycin is originally a cell cycle inhibitor, but it has another effect to inhibit local inflammatory actions. To date, clinical study of the other drug, such as paclitaxel or actinomycin D, -eluting stents are ongoing 3. Development of the drug-eluting stent seems epochmaking in terms of neointimal hyperplasia where the stent is applicable. In the intracranial arteries, restenosis tends to occur more frequently due to several reasons (figure 3). First, the vessel diameter is smaller in intracranial arteries. Second, PTA sometimes resulted in inadequate dilatation to avoid technical complication by occlusion of important perforating arteries or vessel rupture leading to severe neurological deteriorations. Third, stenting is not always possible in the intracranial arteries due to anatomical reasons. Thus, the other therapies such as gene transfer are necessary to overcome restenosis after PTA for symptomatic stenosis of intracranial arteries. In the near future, drugeluting stent or gene transfer technique would be theoretically applicable for intracranial or cervical arteries when enough modification is performed in a site-specific manner.
Figure 3.
References
- 1.Brand K, et al. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 3.Fattori R, Piva T. Drug-eluting stents in vascular intervention. Review Lancet. 2003;361:247–249. doi: 10.1016/S0140-6736(03)12275-1. [DOI] [PubMed] [Google Scholar]
- 4.Landry DB, et al. Activation of the NF-kappa B and I kappa B system in smooth muscle cells after rat arterial injury. Induction of vascular cell adhesion molecule-1 and monocyte chemoattractant protein-1. Am J Pathol. 1997;151:1085–1095. [PMC free article] [PubMed] [Google Scholar]
- 5.Morice MC, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 6.Morishita R, et al. Novel intraluminal molecular delivery of antisense cdc2 kinase and PCNA oligonucleotide results in chronic inhibition of neointimal hyperplasia. Proc Natl Acad Sci USA. 1993;90:8474–8478. doi: 10.1073/pnas.90.18.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 8.Seoley GS, Marks MP, et al. Vertebral artery stenting following percutaneous transluminal angioplasty. J Neurosurg. 1996;84:883–887. doi: 10.3171/jns.1996.84.5.0883. [DOI] [PubMed] [Google Scholar]
- 9.Sousa JE, et al. Sustained suppression of neointimal proliferation by sirolimus-eluting stents: one-year angiographic and intravascular ultrasound follow-up. Circulation. 2001;104:2007–2011. doi: 10.1161/hc4201.098056. [DOI] [PubMed] [Google Scholar]
- 10.Wholey MH, et al. Global experience in cervical carotid artery stent placement. Catheter Cardiovasc Interv. 2000;50:160–167. doi: 10.1002/(sici)1522-726x(200006)50:2<160::aid-ccd2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Yadav VS, Roubin GS, et al. Elective stenting of the extracranial carotid arteries. Circulation. 1997;95:375–381. doi: 10.1161/01.cir.95.2.376. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura S, Kaku Y, et al. Results and problems in percutaneous transluminal angioplasty for internal carotid artery stenosis. Interventional Neuroradiology. 1998;4(suppl 1):37–40. doi: 10.1177/15910199980040S105. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura S, Morishita R, et al. Inhibition of intimal hyperplasia after balloon injury in rat carotid artery model using cis-element ’decoy’ of nuclear factor-kB binding site as a novel molecular strategy. Gene Ther. 2001;8:1635–1642. doi: 10.1038/sj.gt.3301566. [DOI] [PubMed] [Google Scholar]