SUMMARY
Complement contributes to inflammation during pathogen infections, however less is known regarding its role during malaria and, the severest form of the disease, cerebral malaria. Recent studies have shown that deletion of the complement anaphylatoxins receptors, C3aR and C5aR, does alter disease susceptibility in experimental cerebral malaria (ECM). This does not however, preclude C3a- and C5a-mediated contributions to inflammation in ECM and raises the possibility that carboxypeptidase regulation of anaphylatoxin activity rapidly over rides their functions. To address this question we performed ECM using carboxypeptidase N-deficient (CPN−/−) mice. Unexpectedly, we found that CPN−/− mice survived longer than wild type mice but were fully susceptible to ECM. CD4+ and CD8+ T cell infiltration was not reduced at the peak of disease in CPN−/− mice and there was no corresponding reduction in pro-inflammatory cytokine production. Our results indicate that carboxypeptidases contribute to the pathogenesis of ECM and that, studies examining the contribution of other carboxypeptidase families and family members may provide greater insight into the role these enzymes play in malaria.
Keywords: cerebral malaria, complement anaphylatoxins, carboxypeptidases
Recent studies have demonstrated an important role for C5 in the pathogenesis of experimental cerebral malaria (ECM) utilizing in vivo and in vitro approaches (1, 2). These studies raised the question of the importance of the complement anaphylatoxins C5a in ECM. Subsequently studies using C5aR−/− mice showed that there was no difference between C5aR−/− and wild type mice, indicating that C5a did not contribute significantly to disease pathogenesis. One possible explanation for these results is that C3aR may substitute for C5aR in the development of ECM, in keeping with the known significant functional overlap between these two anaphylatoxin receptors (3). However C3aR−/− mice and C3aR−/−/C5aR−/− mice were both fully susceptible to ECM (4), suggesting that neither complement anaphylatoxin alone contributes significantly to ECM pathogenesis. One mechanism that may account for these observations is rapid turnover of the complement anaphylatoxins to their des-Arg forms by serum carboxypeptidases. The contribution of carboxypeptidases to human or murine cerebral malaria remains unexplored.
Carboxypeptidase N (CPN; EC 3.4.17.3) is a zinc-dependent metalloprotease and the prototypic member of the CPN subgroup of mammalian carboxypeptidase. CPN has a molecular weight of approximately 280 kD and is a tetramer composed of two large, heavily glycosylated subunits and two catalytic subunits that contain a zinc-binding site (5, 6). CPN is produced exclusively in the liver and is not an acute phase protein (7, 8). The catalytic subunit of CPN cleaves carboxy-terminal arginine and lysine residues found on as many as 250 proteins or peptides (9). The complement anaphylatoxins C5a and C3a are rapidly inactivated by CPN by removal of their C-terminal arginine residues (10). Inactivation of these biologically potent peptides markedly reduces the inflammatory and chemoattractant potential of complement activation and thus regulates the potential for autologous complement-mediated damage. Although C3aR- and C5aR-deficient mice are equally susceptible to ECM compared to wild type mice, we reasoned that deletion of CPN might reveal contributions of the complement anaphylatoxins not seen in the receptor deficient mice.
To address this possibility, we compared susceptibility and clinical severity of CPN−/− (11) and wild type mice in P. berghei ANKA-induced ECM as previously described (4). For these studies, Plasmodium berghei ANKA was maintained by passage in BALB/c mice (12). ECM was induced by injecting mice i.p. with 5× 105 PbA-infected RBCs. Peripheral parasitemia was monitored on day 6 post-infection by Giemsa-stained, thin-blood smears. Mice were monitored twice daily for clinical signs of neurologic disease, using the following scoring scale: 0, asymptomatic; 1, symptomatic (ruffled fur); 2, mild disease (slow righting); 3, moderate disease (difficulty righting); 4, severe disease (ataxia, seizures, coma); 5, dead. Mice observed having seizures were given a score of 4 regardless of other clinical signs of disease and, moribund animals were scored 4.5 and humanely sacrificed. Mice were classified as having ECM if they displayed these symptoms between days 5–9 post-infection and had a corresponding drop in external body temperature or succumbed to infection. Whole blood was collected via retro-orbital bleed on day six post ECM induction and samples were assayed for TNF-α, IFN-γ and platelet factor 4 by ELISA (Invitrogen) performed according to the manufacturers instructions. Mice were transcardially perfused with PBS for 2 min and brains were processed for flow cytometry as previously described (13).
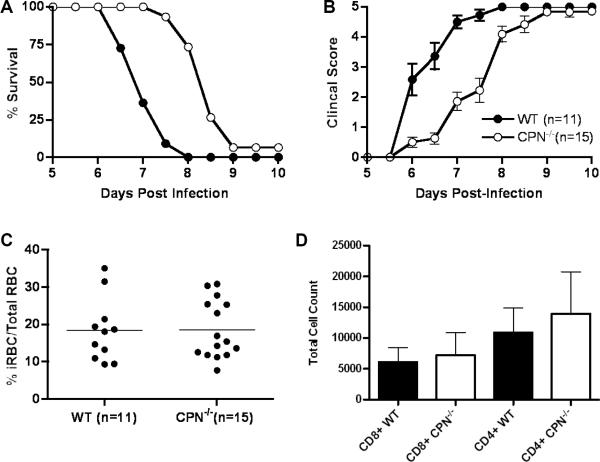
We found that CPN−/− mice survived longer than wild type mice (p<0.0001, Log rank test; Figure 1a): 50% of CPN−/− mice survived past day 8, a time point at which all wild type mice had succumbed to ECM. Disease severity in CPN−/− mice was also significantly reduced and corresponded well to survival (p<0.01, days 6–8.5, Wilcoxon rank-sum test; Figure 1b). Despite the level of protection we observed in CPN−/− mice, there was no difference in parasitemia at the onset of clinical signs of disease (p>0.05; Figure 1c). We also examined for differences in CD4+ and CD8+ T cell infiltration into the central nervous system in CPN−/− and wild type mice during ECM (Figure 1d). We observed no difference in T cell subset infiltration in CPN−/− mice compared to wild type mice. In fact the numbers of both subsets of T cells was slightly, but not significantly, elevated compared to wildtype mice. There was no difference in the levels of CXCL9 or CXCL10 in CPN−/− mice compared to wild type mice (data not shown). The serum levels of both IFN-γ and TNF-α were not reduced in CPN−/− mice compared to wild type mice at day 6 (data not shown). This latter observation likely contributes to a pro-inflammatory environment that ultimately leads to the development of ECM in CPN−/− mice.
Figure 1.
Survival is prolonged and clinical signs of disease are reduced in cerebral malaria in carboxypeptidase N-deficient mice (CPN−/−) compared to wild type mice. Wild type and CPN−/− mice were injected i.p. with 5 × 105 PbA-iRBC and clinical scores and survival were monitored twice daily for ten days as previously described (C5 paper). CPN−/− mice (n=15) were significantly resistant to disease-induced mortality (p=0.0001, Log rank test) compared to wild type mice (n=19) (a) and had reduced disease severity on days 6 through 8.5 (p<0.05, Wilcoxon rank-sum test) (b). The data shown in (a) and (b) are pooled from three independent experiments. The clinical score data shown is the mean and standard error of daily scores within each group of mice. These data indicate that experimental variation was minimal even during disease onset. Parasitemia, assessed at day 6 after infection, was not significantly different between wild type and CPN−/− mice (18.4 vs. 18.6 %iRBCs/total RBC, wild type vs. CPN−/− mice respectively). The data shown are pooled from three independent experiments and the horizontal line indicates the mean value for each group of mice. (c). Brain tissue was isolated from wild type and CPN−/− mice at day 6 (n=4/group) and subjected to flow cytometric analysis as previously described (12). The total number of CD4+ and CD8+ T cells was comparable the brains of CPN−/− and wild type mice (d). The data shown in (d) are the mean and standard error, pooled from three independent experiments. CD4+ T cells: wild type – 10888+/− 4035 vs CPN−/− - 13961+/−6794; CD8+ T cells: wild type – 6087 +/− 2363 vs. CPN−/− - 7197 +/− 3631.
Our original hypothesis was that deletion of CPN might reveal complement anaphylatoxin-mediated contributions to the development and progression of ECM, not seen with the individual anaphylatoxin receptor-deficient mice. Based on this hypothesis, we predicted a more severe course of disease due the lack of cleavage of C3a and C5a to their biologically less active des-Arg forms. In contrast, we found that CPN−/− mice survived on average one to two days longer than wild type mice. In parallel with the short-lived survival advantage, clinical scores were also slower to manifest in CPN−/− mice. Once CPN−/− mice began to develop ECM, they did so at a rate essentially identical to that of wild type mice. Thus deletion of CPN provided transient protection from ECM despite its role in modulating complement anaphylatoxin bioactivity (5, 6). These results support our previous studies indicating that C3a and C5a are not a major driving force behind the inflammatory processes (4), however, we cannot rule out compensatory contributions of other carboxypeptidases.
The complement anaphylatoxins represent a small percentage of peptides and proteins cleaved by CPN (9). Bradykinin is another biologically relevant protein cleaved by CPN (5) and the des-Arg form of bradykinin (des-Arg9-bradykinin) is found at higher levels in normal serum than bradykinin itself (14). The inability of CPN−/− mice to cleave plasma bradykinin to des-Arg9-bradykinin may account, in part, for the delay in development of ECM. des-Arg9-bradykinin binds to the B1 receptor for bradykinin and is thought to act as an agonist for the acute phase response (15–18). Thus in the absence of des-Arg9-bradykinin, the inflammatory response associated with the development of ECM may be transiently delayed until other inflammatory mechanisms compensate. Furthermore, given that the blood brain-barrier can be compromised in ECM (19), we cannot rule out a glial cell component in modulating ECM in CPN−/− mice. In the brain parenchyma, bradykinin interactions with the B1 and B2 receptors on glial cells are thought to promote anti-inflammatory and neuroprotective effects (20, 21). It is currently unclear if or how glial cell-derived mechanisms contribute to the delayed onset of ECM we observed in CPN−/− mice.
In addition to CPN, there are other carboxypeptidases that may contribute to the outcome of ECM. CPR and CPB, in particular may be of interest because it is are found in blood, has substrate specificity similar to CPN and has been shown to cleave the terminal arginine residue from both C3a and C5a (6, 22–25). Perhaps this carboxypeptidase compensates for the absence of CPN. Our data suggest that exploring the relative contribution of additional carboxypeptidases in ECM may provide useful insight into the role this class of enzymes plays in malaria. Recent progress in small molecular weight inhibitors for metallocarboxypeptidases (26) may offer a therapeutic approach to cerebral malaria should animal studies indicate a prominent role for one or more carboxypeptidases in the disease process.
ACKNOWLEDGEMENTS
This work was supported by NIH grants T32 AI07051 and NS077811 (to TNR), AI08382 (to SRB) and AI025011(to R.A.W.). The authors gratefully acknowledge the continuing support of Drs. Julian Rayner and Oliver Billker.
Footnotes
Disclosures: None
REFERENCES
- 1.Patel SN, Berghout J, Lovegrove FE, Ayi K, Conroy A, Serghides L, et al. C5 deficiency and C5a or C5aR blockade protects against cerebral malaria. J Exp Med. 2008 May 12;205(5):1133–43. doi: 10.1084/jem.20072248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conroy A, Serghides L, Finney C, Owino SO, Kumar S, Gowda DC, et al. C5a enhances dysregulated inflammatory and angiogenic responses to malaria in vitro: potential implications for placental malaria. PLoS ONE. 2009;4(3):e4953. doi: 10.1371/journal.pone.0004953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009 May 26; doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramos TN, Darley M, Bilker O, Rayner JC, Ahras M, Wohler JE, et al. The membrane attack complex of complement is required for the development of experimental cerebral malaria. J Immun. 2011 doi: 10.4049/jimmunol.1100603. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skidgel RA, Erdos EG. Structure and function of human plasma carboxypeptidase N, the anaphylatoxin inactivator. Int Immunopharmacol. 2007 Dec 20;7(14):1888–99. doi: 10.1016/j.intimp.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthews KW, Mueller-Ortiz SL, Wetsel RA. Carboxypeptidase N: a pleiotropic regulator of inflammation. Mol Immunol. 2004 Jan;40(11):785–93. doi: 10.1016/j.molimm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Oshima G, Kato J, Erdos EG. Plasma carboxypeptidase N, subunits and characteristics. Arch Biochem Biophys. 1975 Sep;170(1):132–8. doi: 10.1016/0003-9861(75)90104-6. [DOI] [PubMed] [Google Scholar]
- 8.Sato T, Miwa T, Akatsu H, Matsukawa N, Obata K, Okada N, et al. Procarboxypeptidase R is an acute phase protein in the mouse, whereas carboxypeptidase N is not. J Immunol. 2000 Jul 15;165(2):1053–8. doi: 10.4049/jimmunol.165.2.1053. [DOI] [PubMed] [Google Scholar]
- 9.Koomen JM, Li D, Xiao LC, Liu TC, Coombes KR, Abbruzzese J, et al. Direct tandem mass spectrometry reveals limitations in protein profiling experiments for plasma biomarker discovery. J Proteome Res. 2005 May-Jun;4(3):972–81. doi: 10.1021/pr050046x. [DOI] [PubMed] [Google Scholar]
- 10.Bokisch VA, Muller-Eberhard HJ, Cochrane CG. Isolation of a fragment (C3a) of the third component of human complement containing anaphylatoxin and chemotactic activity and description of an anaphylatoxin inactivator of human serum. J Exp Med. 1969 May 1;129(5):1109–30. doi: 10.1084/jem.129.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller-Ortiz SL, Wang D, Morales JE, Li L, Chang JY, Wetsel RA. Targeted disruption of the gene encoding the murine small subunit of carboxypeptidase N (CPN1) causes susceptibility to C5a anaphylatoxin-mediated shock. J Immunol. 2009 May 15;182(10):6533–39. doi: 10.4049/jimmunol.0804207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinden RE, Butcher GA, Beetsma AL. Maintenance of the Plasmodium berghei life cycle. Methods Mol Med. 2002;72:25–40. doi: 10.1385/1-59259-271-6:25. [DOI] [PubMed] [Google Scholar]
- 13.Wohler JE, Smith SS, Zinn KR, Bullard D, Barnum SR. gd T cells in experimental autoimmune encephalomyelitis: early trafficking events and cytokine requirements. Eur J Immunol. 2009;39:1516–26. doi: 10.1002/eji.200839176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odya CE, Wilgis FP, Walker JF, Oparil S. Immunoreactive bradykinin and [des-Arg9]-bradykinin in low-renin essential hypertension--before and after treatment with enalapril (MK 421) J Lab Clin Med. 1983 Nov;102(5):714–21. [PubMed] [Google Scholar]
- 15.Marceau F, Barabe J, St-Pierre S, Regoli D. Kinin receptors in experimental inflammation. Can J Physiol Pharmacol. 1980 May;58(5):536–42. doi: 10.1139/y80-088. [DOI] [PubMed] [Google Scholar]
- 16.Regoli D, Barabe J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- 17.Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992 Mar;44(1):1–80. [PubMed] [Google Scholar]
- 18.Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005 Mar;57(1):27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- 19.Amante FH, Haque A, Stanley AC, Rivera Fde L, Randall LM, Wilson YA, et al. Immune-mediated mechanisms of parasite tissue sequestration during experimental cerebral malaria. J Immunol. Sep 15;185(6):3632–42. doi: 10.4049/jimmunol.1000944. [DOI] [PubMed] [Google Scholar]
- 20.Noda M, Sasaki K, Ifuku M, Wada K. Multifunctional effects of bradykinin on glial cells in relation to potential anti-inflammatory effects. Neurochem Int. 2007 Jul-Sep;51(2–4):185–91. doi: 10.1016/j.neuint.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Noda M, Kariura Y, Pannasch U, Nishikawa K, Wang L, Seike T, et al. Neuroprotective role of bradykinin because of the attenuation of pro-inflammatory cytokine release from activated microglia. J Neurochem. 2007 Apr;101(2):397–410. doi: 10.1111/j.1471-4159.2006.04339.x. [DOI] [PubMed] [Google Scholar]
- 22.Skidgel RA. Structure and function of mammalian zinc carboxypeptidases. In: Hooper NM, editor. Zinc Metalloproteases in Health and Disease. Taylor and Francis Ltd.; London: 1996. pp. 241–83. [Google Scholar]
- 23.Myles T, Nishimura T, Yun TH, Nagashima M, Morser J, Patterson AJ, et al. Thrombin activatable fibrinolysis inhibitor, a potential regulator of vascular inflammation. J Biol Chem. 2003 Dec 19;278(51):51059–67. doi: 10.1074/jbc.M306977200. [DOI] [PubMed] [Google Scholar]
- 24.Campbell W, Okada N, Okada H. Carboxypeptidase R is an inactivator of complement-derived inflammatory peptides and an inhibitor of fibrinolysis. Immunol Rev. 2001 Apr;180:162–7. doi: 10.1034/j.1600-065x.2001.1800114.x. [DOI] [PubMed] [Google Scholar]
- 25.Campbell WD, Lazoura E, Okada N, Okada H. Inactivation of C3a and C5a octapeptides by carboxypeptidase R and carboxypeptidase N. Microbiol Immunol. 2002;46(2):131–4. doi: 10.1111/j.1348-0421.2002.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez D, Pallares I, Vendrell J, Aviles FX. Progress in metallocarboxypeptidases and their small molecular weight inhibitors. Biochimie. 2010 Nov;92(11):1484–500. doi: 10.1016/j.biochi.2010.05.002. [DOI] [PubMed] [Google Scholar]