Abstract
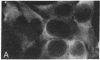
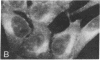
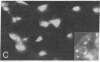
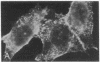
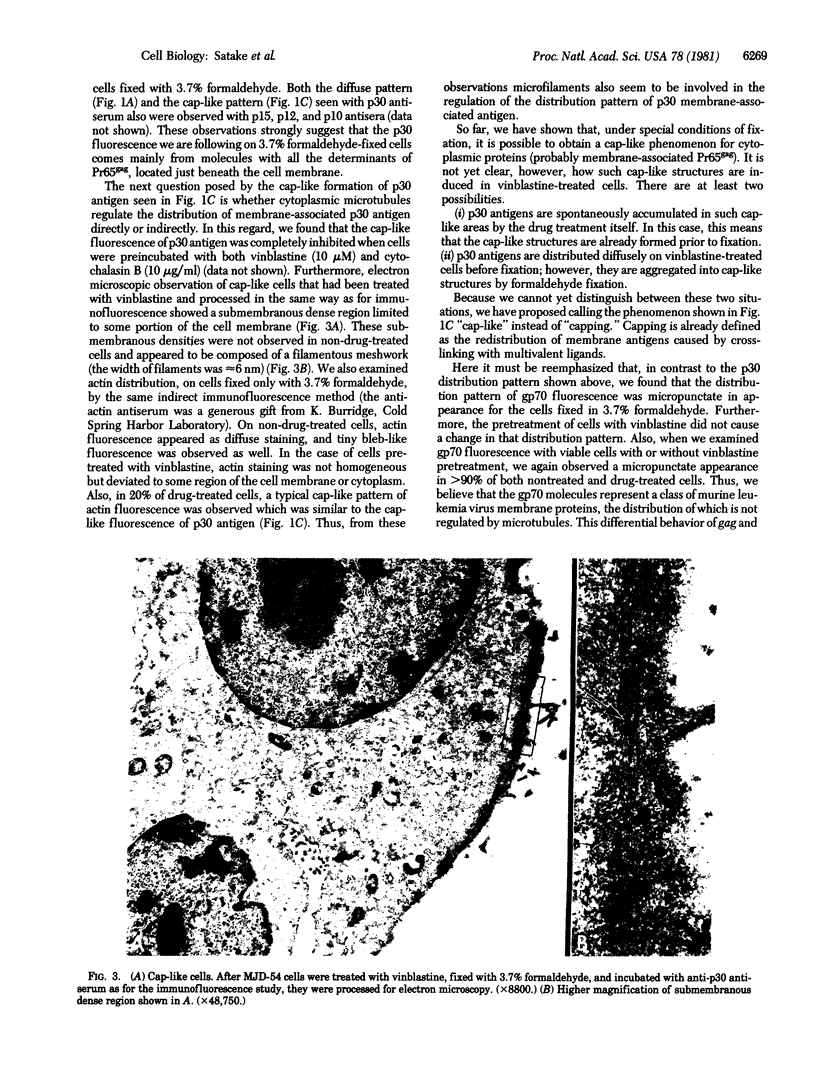
The effect of vinblastine on the distribution of murine leukemia virus-derived membrane-associated antigens was examined by using the indirect immunofluorescence of 3.7% formaldehyde-fixed MJD-54 (Moloney murine leukemia virus-infected) cells. On fixed, non-drug-treated cells, p30 antigen was distributed homogeneously and diffusely over the cell membrane. When cells were incubated with 10 microM vinblastine for 1 hr before fixation, the distribution of p30 antigen was greatly changed, fluorescence now being collected into poles (cap-like formation). In contrast to this distribution pattern for p30 antigen, gp70 antigen was distributed in a micropunctate pattern on the cell surface, with or without vinblastine pretreatment. These observations indicate that the distribution patterns of p30 and gp70 membrane antigens are completely different and that they are differently controlled by cytoplasmic microtubules. In addition, because the p30 membrane antigen visualized in these studies most likely represents viral Pr65gag precursor molecules which are localized directly under and associated with the plasma membrane, these results suggest that, under special conditions of fixation, it is possible to obtain a cap-like phenomenon for cytoplasmic (internal) membrane-oriented proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash J. F., Louvard D., Singer S. J. Antibody-induced linkages of plasma membrane proteins to intracellular actomyosin-containing filaments in cultured fibroblasts. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5584–5588. doi: 10.1073/pnas.74.12.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Aaronson S. A. Membrane properties of the gag gene-coded p15 protein of mouse type-C RNA tumor viruses. J Biol Chem. 1978 Mar 10;253(5):1408–1414. [PubMed] [Google Scholar]
- Berlin R. D. Microtubules and the fluidity of the cell surface. Ann N Y Acad Sci. 1975 Jun 30;253:445–454. doi: 10.1111/j.1749-6632.1975.tb19220.x. [DOI] [PubMed] [Google Scholar]
- Buetti E., Diggelmann H. Murine leukemia virus proteins expressed on the surface of infected cells in culture. J Virol. 1980 Mar;33(3):936–944. doi: 10.1128/jvi.33.3.936-944.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Hanke K., Henning R. Simian virus 40 T-antigen-related cell surface antigen: serological demonstration on simian virus 40-transformed monolayer cells in situ. J Virol. 1980 Aug;35(2):505–518. doi: 10.1128/jvi.35.2.505-518.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. A., Fan H. gag-Related polyproteins of Moloney murine leukemia virus: evidence for independent synthesis of glycosylated and unglycosylated forms. J Virol. 1979 May;30(2):551–563. doi: 10.1128/jvi.30.2.551-563.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Nowinski R. C., Eisenman R. N. Biosynthesis and metabolism of viral proteins expressed on the surface of murine leukemia virus-infected cells. Virology. 1978 Nov;91(1):116–129. doi: 10.1016/0042-6822(78)90360-4. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., McMillan P. N., Bolognesi D. P. An ultrastructural study of C-type virion assembly in mouse cells. Cancer Res. 1974 Dec;34(12):3303–3310. [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Rosen O. M., Rubin C. S. Organelle relationships in cultured 3T3-L1 preadipocytes. J Cell Biol. 1980 Oct;87(1):180–196. doi: 10.1083/jcb.87.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M., Gelfand E. W., Pearson C. B., Pfeiffer J. R., Dosch H. M. Microtubule assembly and conanavalin A capping in lymphocytes: reappraisal using normal and abnormal human peripheral blood cells. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3499–3503. doi: 10.1073/pnas.77.6.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M., Ukena T. E., Berlin R. D. Effects of phagocytosis and colchicine on the distribution of lectin-binding sites on cell surfaces. Proc Natl Acad Sci U S A. 1974 Feb;71(2):394–398. doi: 10.1073/pnas.71.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Nowinski R. C. Lysis of retroviruses with monoclonal antibodies against viral envelope proteins. Virology. 1980 Feb;101(1):296–299. doi: 10.1016/0042-6822(80)90507-3. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Rabin E. H., Oroszlan S. Post-translational modification of Rauscher leukemia virus precursor polyproteins encoded by the gag gene. J Virol. 1979 Apr;30(1):255–266. doi: 10.1128/jvi.30.1.255-266.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz H., Hunsmann G., Moenning V., Schäfer W. Properties of mouse leukemia viruses. XI. Immunoelectron microscopic studies on viral structural antigens on the cell surface. Virology. 1976 Jan;69(1):169–178. doi: 10.1016/0042-6822(76)90204-x. [DOI] [PubMed] [Google Scholar]
- Solomon F., Magendantz M., Salzman A. Identification with cellular microtubules of one of the co-assemlbing microtubule-associated proteins. Cell. 1979 Oct;18(2):431–438. doi: 10.1016/0092-8674(79)90062-x. [DOI] [PubMed] [Google Scholar]
- Stern P. L., Bretscher M. S. Capping of exogenous Forssman glycolipid on cells. J Cell Biol. 1979 Sep;82(3):829–833. doi: 10.1083/jcb.82.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. W., Sanford K. K., Frankel R. Tubulin and actin in paired nonneoplastic and spontaneously transformed neoplastic cell lines in vitro: fluorescent antibody studies. Cell. 1978 Apr;13(4):629–642. doi: 10.1016/0092-8674(78)90213-1. [DOI] [PubMed] [Google Scholar]
- Tung J. S., Yoshiki T., Fleissner E. A core polyprotein of murine leukemia virus on the surface of mouse leukemia cells. Cell. 1976 Dec;9(4 Pt 1):573–578. doi: 10.1016/0092-8674(76)90039-8. [DOI] [PubMed] [Google Scholar]
- Yahara I., Kakimoto-Sameshima F. Analysis of ligand-independent cap formation induced in hypertonic medium. Exp Cell Res. 1979 Mar 15;119(2):237–252. doi: 10.1016/0014-4827(79)90352-5. [DOI] [PubMed] [Google Scholar]
- Yeger H., Kalnins V. I. Immunocytochemical localization of gp70 over virus-related submembranous densities in ts mutant Rauscher murine leukemia virus-infected cells at the nonpermissive temperature. Virology. 1978 Dec;91(2):489–492. doi: 10.1016/0042-6822(78)90397-5. [DOI] [PubMed] [Google Scholar]
- Yeger H., Kalnins V. I., Stephenson J. R. Type-C retrovirus maturation and assembly: post-translational cleavage of the gag-gene coded precursor polypeptide occurs at the cell membrane. Virology. 1978 Aug;89(1):34–44. doi: 10.1016/0042-6822(78)90037-5. [DOI] [PubMed] [Google Scholar]
- Yoshiki T., Mellors R. C., Hardy W. D., Jr, Fleissner E. Common cell surface antigen associated with mammalian C-type RNA viruses. Cell membrane-bound gs antigen. J Exp Med. 1974 Apr 1;139(4):925–942. doi: 10.1084/jem.139.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]