Abstract
The leaves and especially the roots of chicory (Cichorium intybus L.) contain high concentrations of bitter sesquiterpene lactones such as the guianolides lactupicrin, lactucin, and 8-deoxylactucin. Eudesmanolides and germacranolides are present in smaller amounts. Their postulated biosynthesis through the mevalonate-farnesyl diphosphate-germacradiene pathway has now been confirmed by the isolation of a (+)-germacrene A synthase from chicory roots. This sesquiterpene cyclase was purified 200-fold using a combination of anion-exchange and dye-ligand chromatography. It has a Km value of 6.6 μm, an estimated molecular mass of 54 kD, and a (broad) pH optimum around 6.7. Germacrene A, the enzymatic product, proved to be much more stable than reported in literature. Its heat-induced Cope rearrangement into (−)-β-elemene was utilized to determine its absolute configuration on an enantioselective gas chromatography column. To our knowledge, until now in sesquiterpene biosynthesis, germacrene A has only been reported as an (postulated) enzyme-bound intermediate, which, instead of being released, is subjected to additional cyclization(s) by the same enzyme that generated it from farnesyl diphosphate. However, in chicory germacrene A is released from the sesquiterpene cyclase. Apparently, subsequent oxidations and/or glucosylation of the germacrane skeleton, together with a germacrene cyclase, determine whether guaiane- or eudesmane-type sesquiterpene lactones are produced.
The sprouts of chicory (Cichorium intybus L.), a vegetable (French endive) grown in the dark, are known for their slightly bitter taste originating from sesquiterpene lactones. The tap roots of chicory in particular are extremely bitter due to these components with antifeedant properties (Rodriguez et al., 1976; Rees and Harborne, 1985; van Beek et al., 1990). In the past, these roots were roasted and used as a coffee substitute; now they are regarded as a waste product of chicory cultivation. About 100,000 tons of chicory roots are produced annually in The Netherlands, but because of their bitter taste, it is not possible to use them as cattle feed. In searching for a way to enhance the value for this waste product, we have been investigating the bitter sesquiterpene lactones (van Beek et al., 1990; Leclercq, 1992), and we are interested in the biosynthesis of these compounds because it involves stereoselective oxidizing enzymes that might be useful as catalysts in organic syntheses.
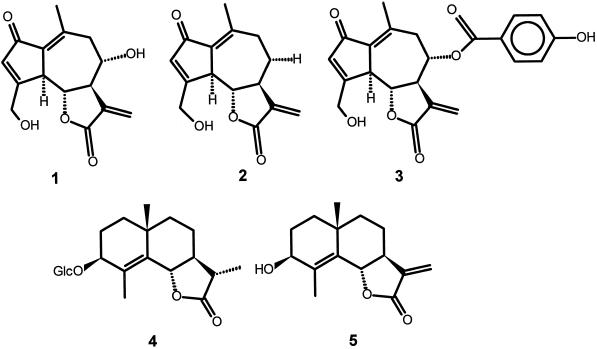
The average sesquiterpene lactone content is measured at 0.42% dry weight in the roots and 0.26% in the leaves (Rees and Harborne, 1985). The three major sesquiterpene lactones in chicory are the guaianolides lactucin (see Fig. 1, 1), 8-deoxylactucin (Fig. 1, 2), and lactupicrin (Fig. 1, 3), which are present in both leaves and roots of chicory (Pyrek, 1985; van Beek et al., 1990). The two eudesmanolides sonchuside C (Fig. 1, 4) and cichoriolide A (Fig. 1, 5) are also present, together with the germacranolides sonchuside A and cichorioside C (Seto et al., 1988).
Figure 1.
The three major guaianolides of chicory, lactucin (1), 8-deoxylactucin (2), and lactupicrin (3), and its two eudesmanolides, sonchuside C (4) and cichoriolide A (5).
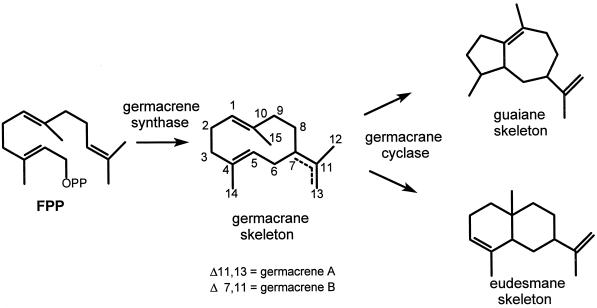
It is assumed that both the guaiane- and eudesmane-type lactones originate from a common germacrane precursor that is formed via the acetate-mevalonate-FPP pathway by a germacrene synthase, an enzyme belonging to the group of sesquiterpene cyclases (Herz, 1977; Bohlman and Zdero, 1978; Fischer, 1990; Song et al., 1995). Whether this common germacrane precursor is transformed into a guaiane skeleton or a eudesmane skeleton would depend on the position of enzyme-mediated epoxidations. A germacrene C4-C5-epoxide would lead to a guaiane, whereas a germacrene C1-C10-epoxide would lead to a eudesmane (Brown et al., 1975; Teisseire, 1994; Piet et al., 1995b). For this reason we postulated that, apart from the oxidizing enzymes, two different cyclizing enzymes are involved in the biosynthesis of the guaianolides and eudesmanolides: an enzyme that cyclizes FPP to a germacrane skeleton, and a separate enzyme that cyclizes the germacrane skeleton to a guaiane or eudesmane skeleton (Fig. 2) (Piet et al., 1995b, 1996).
Figure 2.
A simplified scheme (without oxidative steps) for the formation of eudesmane- and guaiane-type compounds involves two cyclizing enzymes, a germacrene synthase and a germacrane cyclase. Literature suggests that either germacrene A or germacrene B is the germacrane intermediate.
Biosynthetic studies with a hairy-root culture of blue-flowered lettuce supplied with 13C-labeled precursors of secondary plant metabolism (acetate and mevalolactone) seem to confirm this acetate-mevalonate-FPP-germacradiene pathway. From the patterns of 13C-enrichment in the produced guaianolides, Song et al. (1995) deduced that the C12 and the C13 atoms of the germacrane intermediate are chemically not identical; this indicates the formation of either germacrene A (7) or germacrene B. Formation of germacrene B would be supported by the existence of C8-oxygenated sesquiterpene lactones such as lactucin and lactupicrin, because in germacrene B the C8 position is activated for allylic oxidations (Fischer, 1990).
Sesquiterpene cyclases catalyze the conversion of FPP to over 200 different cyclic skeletons, and a growing number of these enzymes have been isolated and characterized in recent years. cDNA sequences (Cane, 1990; McCaskill, 1997) are available, and protein crystal structures have been published recently (Lesburg et al., 1997; Starks et al., 1997). Although germacrenes, especially germacrene D, are important constituents of many essential oils, to our knowledge, no germacrene synthase has so far been described. The biosynthesis of germacrene C by a homogenate of immature seeds of Kadsura japonica has been reported (Morikawa et al., 1971), as well as the partial purification of a synthase for β-selinene (Belingheri et al., 1992), a germacrene A-related compound, from the outer peels of Citrofortunella mitis.
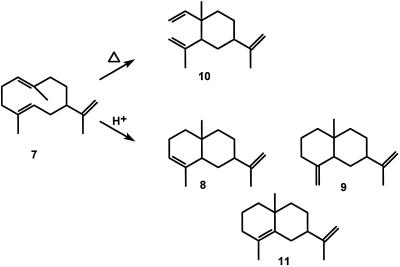
A problem in studying germacrene synthases may be the reported instability of all four known germacrenes. Germacrene A (Fig. 3, 7) is reported to be particularly susceptible to proton-induced cyclizations toward α-selinene (Fig. 3, 8) and β-selinene (Fig. 3, 9) on silica gel, or to Cope rearrangement toward β-elemene (Fig. 3, 10), even during freezer storage (Weinheimer, 1970; Bowers et al., 1977; Teisseire, 1994). Germacrene A itself has often been postulated as an intermediate (bound to the sesquiterpene cyclase) in the biosynthesis of patchoulol and phytoalexins, such as aristolochene, 5-epi-aristolochene, capsidiol, debneyol, and vetispiradiene (Threlfall et al., 1988; Hohn et al., 1989; Whitehead et al., 1989; Beale, 1990; Cane, 1990; Munck and Croteau, 1990; Cane et al., 1993; Back and Chappell, 1995).
Figure 3.
The reported high sensitivity of germacrene A (7) to heat and slightly acidic conditions gives rearrangement toward β-elemene (10), respectively, cyclization toward α-selinene (8) and β-selinene (9). Selina-4,11-diene (11) would be another acid-induced cyclization product.
The aim of our research was to identify the germacrane intermediate involved in the sesquiterpene lactone biosynthesis of chicory and to isolate and characterize the sesquiterpene cyclase responsible for its formation.
MATERIALS AND METHODS
Fresh roots of cultivated chicory (Cichorium intybus L. cv Focus) were harvested during late summer and obtained from a grower in Veenendaal, The Netherlands. Roots of wild chicory were collected in October in the forelands of the Rhine near Wageningen. The chicory roots were cut into small pieces, frozen in liquid nitrogen, and stored at −80°C. Unlabeled FPP was obtained from Sigma in a solution of 70% (v/v) methanol in 10 mm aqueous ammonium hydroxide. The solvent was evaporated in vacuo using a Gyrovap GT (Howe, Oxon, UK), and a 10 mm FPP stock solution was prepared with 50% (v/v) ethanol in 200 mm aqueous ammonium bicarbonate. [1-3H]FPP dissolved in a solution of 50% (v/v) ethanol with 100 mm aqueous ammonium bicarbonate (16.0 Ci mmol−1, 200 μCi mL−1) was purchased from Amersham.
Enzyme Isolation and Assay I
Fifty grams of frozen root material from either cultivated or wild chicory was homogenized in a blender with 5 g of insoluble polyvinylpolypyrrolidone and 80 mL of buffer containing 50 mm Mopso (3-[N-morpholino]2-hydroxy-propanesulfonic acid) (pH 7.0), 50 mm sodium meta-bisulfite, 50 mm ascorbic acid, 10 mm MgCl2, 5 mm DTT, and 20% glycerol (buffer A). The homogenate was transferred to a beaker using another 40 mL of buffer A, mixed with 5 g of polystyrene resin (Amberlite XAD-4, Serva) and allowed to stand on ice for several minutes. The slurry was filtered through premoistened cheesecloth and centrifuged for 20 min at 20,000g at 4°C. The supernatant was centrifuged once more for 90 min at 100,000g at 4°C; after that it was desalted with an Econo-Pac 10DG column (Bio-Rad) to 1 mm ascorbic acid in buffer B. This less concentrated buffer B contained 15 mm Mopso (pH 7.0), 10 mm MgCl2, 2 mm DTT, and 10% glycerol. A 1.0-mL aliquot of the desalted supernatant was incubated for 1 h at 30°C with either 50 μm unlabeled FPP or 20 μm [3H]FPP (50 Ci mol−1), using an overlay of 1 mL of pentane (assay I). As a control, both types of incubations were also performed with supernatant that had been boiled for 5 min. Incubations were stopped by vigorous shaking. For analysis, the pentane phase was filtered through a dimethyl chlorosilane-treated glass wool (Chrompack, Bergen op Zoom, The Netherlands) plugged Pasteur pipette that contained 0.90 g of aluminum oxide (grade III) and a little anhydrous magnesium sulfate. The extraction was repeated with 1 mL of 20% (v/v) ether in pentane, and the aluminum oxide column was washed with this extract and an additional amount of 1.5 mL of 20% (v/v) ether in pentane. The combined pentane eluate contained sesquiterpenoid hydrocarbons that do not bind to aluminum oxide under these conditions. The same assay was then reextracted with an equal portion of ether, and the ether extracts were passed through the same column. At the end of this extraction-filtration procedure, the column was rinsed with 1.5 mL of ether to ensure a complete elution of (oxygenated) products. The separately collected pentane/ether and ether phases were carefully concentrated to approximately 50 μL under a stream of nitrogen. Both the pentane and ether (diethyl ether) were redistilled before use in the filtration-extraction procedure described above.
Identification of Sesquiterpenoid Products
Before the concentrated extracts of the [3H]FPP incubated assays were analyzed by radio-GC, 1 μL of a sesquiterpene standard was added containing (each 1 mg mL−1 in pentane) germacrene B (prepared according to the method of Piet et al., 1995b), γ-elemene (prepared from germacrene B by heating at 160°C under argon for 16 h), nerolidol, and farnesol. In later experiments this sesquiterpene standard was replaced by either 5 μL of an alkane set (n = 7–22, 1 mg mL−1 each in pentane) to calculate Kovats' indices, or 5 μL of a liverwort (Frullania tamarisci) extract containing germacrene A as one of its major constituents (Hardt et al., 1995; W.A. König, unpublished results). Radio-GC analysis was performed on a Carlo-Erba 4160 (Milano, Italy) series gas chromatograph coupled to a RAGA 93 radioactivity detector (5-mL counting tube, Raytest, Straubenhart, Germany). The GC was equipped with a CP-WAX 52 column (25 m × 0.32 mm, film thickness of 0.25 μm) and programmed at 5°C min−1 from 70°C to 210°C using a helium flow rate of 2.7 mL min−1. Samples of 1 μL were injected in the cold on-column mode. The compounds eluting from the column were split in a ratio of 3:1 between the radioactivity detector and a flame-ionization detector at 210°C. Before entering the radioactivity detector, eluted compounds were quantitatively reduced by addition of hydrogen at 3 mL min−1 and passage through a conversion reactor filled with platinum chips at 800°C. After reduction, methane was added as a quenching gas to give a total flow of 36 mL min−1 through the counting tube of the radioactivity detector. The incubations with unlabeled FPP were analyzed by GC-MS using a 5890 series II gas chromatograph (Hewlett-Packard) equipped with a mass selective detector (model 5927A, Hewlett-Packard) and a capillary HP-5MS column (30 m × 0.25 mm, film thickness of 0.25 μm) at a helium flow rate of 0.969 mL min−1. The splitless injection of the 1-μL sample was initially done at an injection port temperature of 210°C, and at 150°C in later experiments because of the sensitivity of germacrenes to high temperatures. After an initial temperature of 55°C for 4 min, the column was programmed at 5°C min−1 to 210°C. The mass spectra were recorded at 70 eV scanning from 30 to 250 atomic mass units. MS data were compared with those recorded from compounds present in the natural oil of Mentha mirennea (β-elemene [Fig. 3, 10]; Maat et al., 1992) and the extract of F. tamarisci (germacrene A [Fig. 3, 7], α-selinene [Fig. 3, 8], β-selinene [Fig. 3, 9], and selina-4,11-diene [Fig. 3, 11]; W.A. König, unpublished results).
Determination of the Absolute Configuration of Germacrene A
The absolute configuration of germacrene A (Fig. 3, 7) was determined by means of its Cope rearrangement to β-elemene (Fig. 3, 10), a reaction that occurs with retention of stereochemical configuration at C7 (Weinheimer et al., 1970; Takeda, 1974; March, 1992). The GC-MS was essentially used as described above, but with an injection port temperature of 250°C to induce the rearrangement of enzymatically produced germacrene A. The oven temperature was programmed to 45°C for 4 min followed by a ramp of 2°C min−1 to 170°C, and spectra were recorded in the selected ion-monitoring mode (m/z 121, 147, and 189). The apparatus itself was equipped with a 25-m (0.25-mm i.d.) heptakis(6-O-TBDMS-2,3-di-O-methyl)-β-cyclodextrin (50% in OV17) column that is able to separate the enantiomers of racemic β-elemene (König et al., 1994). A racemic β-elemene standard was isolated from a hydrodistillate of the liverwort Frullania macrocephalum (gift of Dr. L. Kraut, University of Saarbrücken, Germany), and the elution order of its enantiomers was determined with a (−)-β-elemene standard (König et al., 1994). To substantiate correct identification of the β-elemene enantiomer derived from chicory germacrene A, racemic β-elemene was co-injected with enzymatically produced germacrene A at injection port temperatures of 150°C and 250°C.
Sesquiterpene Cyclase Assay II and Protein Determination
For routine determination of germacrene A synthase activity, 10 μL of sample was added to an Eppendorf vial with 90 μL of buffer C (0.1% [v/v] Tween 20 in buffer B) and incubated at 30°C with 20 μm [3H]FPP (50 Ci mol−1). The reaction mixture was overlaid with 1 mL of hexane to trap formed, labeled olefins (assay II). After 30 min the vial was vigorously mixed and cooled to stop the reaction, then it was briefly centrifuged to separate phases. In the hexane phase, 750 μL was transferred to a new Eppendorf vial containing 40 mg of silica (0.06–0.2 mm) to bind farnesol produced from FPP by phosphohydrolases. After mixing and centrifugation, 450 μL of the hexane layer was removed for scintillation counting in 4.5 mL of Ultima Gold cocktail (Packard, Meriden, CT). Protein concentrations were determined using the microassay protocol of the Coomassie Plus Protein Assay (Pierce) and BSA as a protein standard. Mono-Q fractions containing Tween 20 were desalted to 50 mm ammonium bicarbonate using a HiTrap desalting column and assayed by the Micro BCA Protein Assay (Pierce).
Sesquiterpene Cyclase Purification
Cellular extracts and enzyme preparations were kept on ice throughout the purification. The purification was started by making a 100,000g supernatant as described above, but with a buffer containing 25 mm Mopso (pH 7.0), 25 mm sodium meta-bisulfite, 25 mm ascorbic acid, 10 mm MgCl2, and 2 mm DTT (buffer D). A column (Ø 2.5 cm) of 25 g of DEAE (preswollen DE52, Whatman) suspended in 150 mL of buffer containing 150 mm Mopso (pH 7.0), 100 mm MgCl2, and 20 mm sodium meta-bisulfite was prepared and washed at 1.6 mL min−1 with 150 mL of 2 mm sodium meta-bisulfite in buffer B (buffer E). Seventy-five milliliters of the 100,000g supernatant was loaded onto this column and washed with another 100 mL of buffer E to remove unbound proteins. A 100-mL gradient of 0 to 0.5 m KCl in buffer E was used to elute sesquiterpene cyclase activity. Fractions containing sesquiterpene cyclase activity were pooled and desalted to buffer B, after which glycerol was added to a final concentration of 30% (v/v). The enzyme preparation was frozen in liquid nitrogen and stored at −80°C in 1-mL aliquots. One aliquot was tested for the nature of its enzymatic sesquiterpenoid product, whereas the others served as a stock for further purification steps.
Prior to the second purification step, various dye resins from a dye resin test kit (no. RDL-9, Sigma) and Red A (Amicon, Beverly, MA) were screened for their affinity for chicory germacrene A synthase. The dye-resin columns were tested according to manufacturer's instructions by applying 180 μL of DEAE purified cyclase to each column. Best results were obtained for Reactive Green 5 (R2257, Sigma), and a column was prepared (Ø 1.0 cm) of a 5-mL suspension containing 1.5 mg mL−1 Reactive Green 5 that had been rinsed twice with an equal volume of buffer B. The column was equilibrated with 15 mL of buffer B, and an aliquot of DEAE-purified cyclase (thawed and warmed up to room temperature) was applied to this column at 0.5 mL min−1. Unbound proteins were washed off the column with buffer B while monitoring the A280; the sesquiterpene cyclase was eluted using a one-step gradient of 1.5 m KCl in buffer B. Sesquiterpene cyclase activity containing fractions were combined, desalted to buffer C with an Econo-Pac 10DG column, and applied to a Mono-Q fast-protein liquid chromatography column (HR5/5, Pharmacia Biotech) previously equilibrated with buffer C. The column was washed with 4 mL of buffer C at 0.75 mL min−1, after which bound proteins were eluted with a 26-mL gradient of 0 to 0.66 m KCl in buffer C. Fractions were assayed, and those containing enzyme activity were tested for the nature of their sesquiterpenoid product(s). The fraction (0.75 mL) containing the highest amount of germacrene A synthase activity was used to determine the Mr and Km (see below). After each purification step the purification was visualized by SDS-PAGE using preprepared 10% (w/v) polyacrylamide gels (Bio-Rad) according to the manufacturer's instructions. Gels were stained using a silver-staining kit (Pharmacia Biotech).
Km, pH Optimum, and Mr Determination
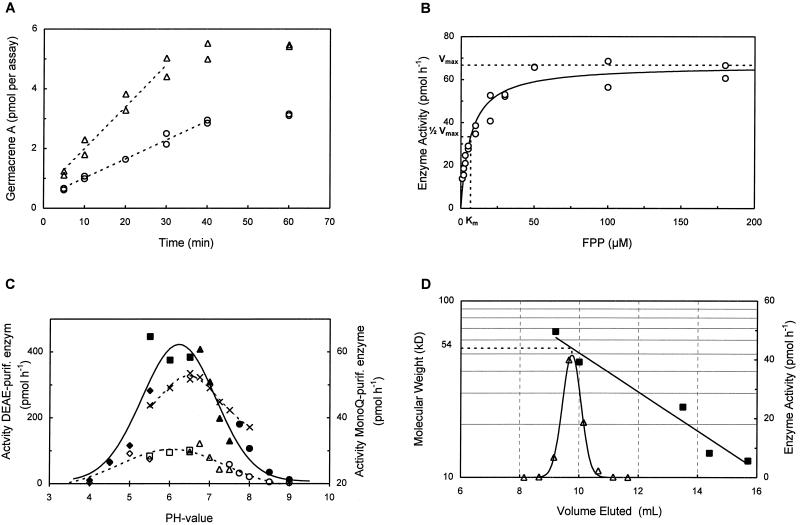
Before determining the Km of the chicory germacrene A synthase, assay II (with buffer C) was checked for its linearity using Mono-Q-purified enzyme. When the enzyme was diluted with an equal amount of buffer C, the assay was linear during the first 40 min (r2= 0.987) at a concentration of 2 μm FPP. When undiluted Mono-Q-purified enzyme was used, enzyme activity was twice as high and linear during 30 min (r2= 0.963) (Fig. 8A). For the kinetics study, enzyme activity was determined in the range of 0.5 to 80 μm FPP (enzyme diluted twice in buffer C). The pH optimum was determined with DEAE-purified germacrene A synthase in the pH range of 4.0 to 9.0 using the protocol of assay II and 5 μL of enzyme. For pH values of 4.0 to 5.5, 5.5 to 6.5, and 7.5 to 9.0, NaAc, Mes, and Tris-HCl, respectively, were used instead of Mopso. pH experiments were carried out in duplicate and in both the presence and the absence of 0.1% Tween 20. The Mr of the germacrene A synthase from chicory was estimated by exclusion chromatography on a Superdex 75 column (HR10/30, Pharmacia Biotech) in buffer C. The column was calibrated at 0.5 mL min−1 with cyt c (12.4 kD), RNase A (13.7 kD), α-chymotrypsinogen (25.0 kD), ovalbumin (45.0 kD), and BSA (67.0 kD), all purchased from Sigma. The column was loaded with 200 μL of Mono-Q-purified germacrene A synthase, and fractions of 0.5 mL were assayed for their sesquiterpene cyclase activity.
Figure 8.
Characterization of the (+)-germacrene A synthase. A, Linearity of the enzyme assay at 2 μm for undiluted Mono-Q eluent (▵) and 2-fold-diluted Mono-Q eluent (○). B, Michaelis-Menten curve featuring a Km of 6.6 μm and a Vmax of 66.8 pmol h−1. C, pH curve for DEAE-purified enzyme in the absence (- - -) and presence (——) of 0.1% Tween 20 using NaAc (◊, ♦), Mes (□, ▪), Mopso (▵, ▴), and Tris-HCl (○, •). Also shown is the pH curve for Mono-Q-purified enzyme in the presence of Tween 20 (×). D, Determination of the Mr by calibrated gel filtration. The column is calibrated by measuring the elution volume (▪) of Cyt c (12.4 kD), RNase A (13.7 kD), α-chymotrypsinogen (25.0 kD), ovalbumin (45.0 kD), and BSA (67.0 kD). The elution volume of germacrene A synthase activity (▵) corresponds to an estimated molecular mass of 54 kD.
RESULTS
Detection of the (+)-Germacrene A Synthase Activity in Chicory Roots
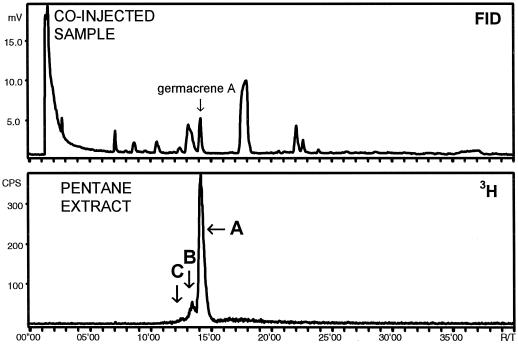
A 100,000g supernatant was prepared from both cultivated and wild chicory and incubated with [3H]FPP. The incubations were extracted subsequently with pentane and ether; the extracts were analyzed by radio-GC after being passed over a short aluminum oxide column. The pentane extracts of both types of plant material revealed one radioactive product, which in the ether extracts was accompanied by farnesol, a result of aspecific phosphohydrolase activity. The product peak did not coincide with that of germacrene B nor that of γ-elemene. The unknown product and farnesol were not present when the supernatant was boiled before incubation, and the amount of product was raised after the DEAE purification step in which almost all phosphohydrolase activity was discarded (Fig. 4).
Figure 4.
Identification by radio-GC of the unknown product formed by DEAE-purified chicory sesquiterpene cyclase. The upper trace represents the flame-ionization detector (FID) response to the hydrocarbons of the co-injected liverwort extract. The lower trace indicates the labeled compounds, extracted from the assay, detected by the radiodetector. The unknown product represented by peak A coincides with that of germacrene A present in the liverwort extract. Peak B and small peak C were, respectively, identified as selinene (8+9) and selina-4,11-diene (11).
The peak of the unknown product contained a small shoulder peak that became the major peak when silica instead of aluminum oxide was used during the extraction-filtration procedure. GC-MS analyses demonstrated that this shoulder peak consisted of α-selinene (Fig. 3, 8), β-selinene (Fig. 3, 9), and selina-4,11-diene (Fig. 3, 11), typical acid (silica)-induced cyclization products of germacrene A. The Kovats' index of our enzymatic product was 1737, which matches the value reported for germacrene A (1734; M.H. Boelens [1995] database Essential Oil, version 4.1, The Netherlands). The final proof for the identity of the unknown product was obtained by co-injection of an extract from the liverwort F. tamarisci, which contains germacrene A (Fig. 4).
Initially, the GC-MS identification of germacrene A (Fig. 3, 7) in both the liverwort extract and the extracts of the enzyme assay was troublesome because germacrene A rearranged into β-elemene (Fig. 3, 10) almost completely during the measurement. This problem was overcome by lowering the GC injection port temperature from 210°C to 150°C. At this lowered temperature, almost no Cope rearrangement of germacrene A occurred, although the germacrene A peak broadened significantly and was preceded by a “hump” in the baseline.
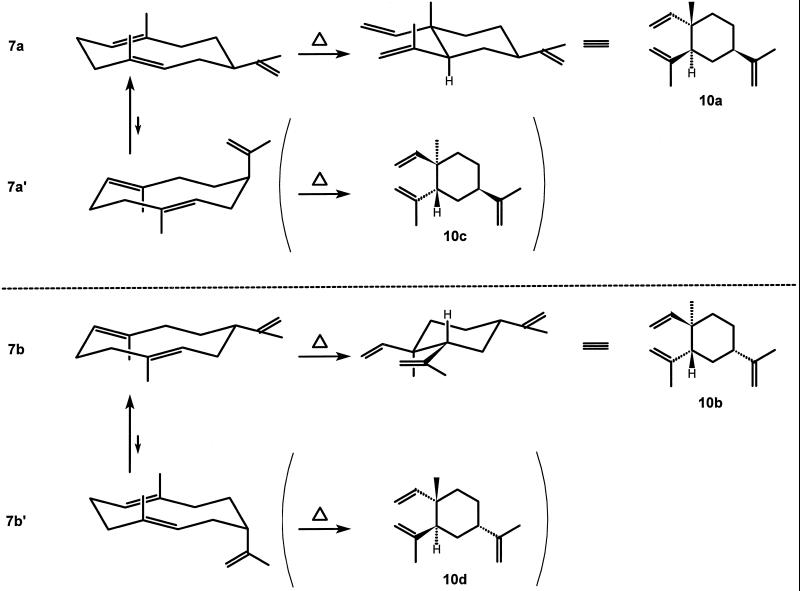
The Cope rearrangement is a stereospecific reaction that proceeds via a chair-like transition state (March, 1992). Since germacranes also prefer the chair-chair conformation, Cope rearrangement proceeds easily (Takeda, 1974). E,E-germacrenes are relatively flexible molecules, but in the case of a large substituent at C7, the conformation having the substituent at an equatorial position predominates (Takeda, 1974; Piet et al., 1995a). Hence, the two enantiomers of germacrene A (Fig. 5, 7a and 7b) will yield two enantiomers of β-elemene upon Cope rearrangement (Fig. 5, 10a and 10b, respectively); the diastereomers 10c and 10d will not be formed because the germacrene conformations 7a′ and 7b′ are energetically unfavorable. This also explains why the β-elemene diastereomers 10c and 10d have not been found in nature (Connolly and Hill, 1991).
Figure 5.
Conformations of the enantiomers of germacrene A ([+]-enantiomer 7a; [−]-enantiomer 7b), and their relation to the configuration of the β-elemenes formed by Cope rearrangement.
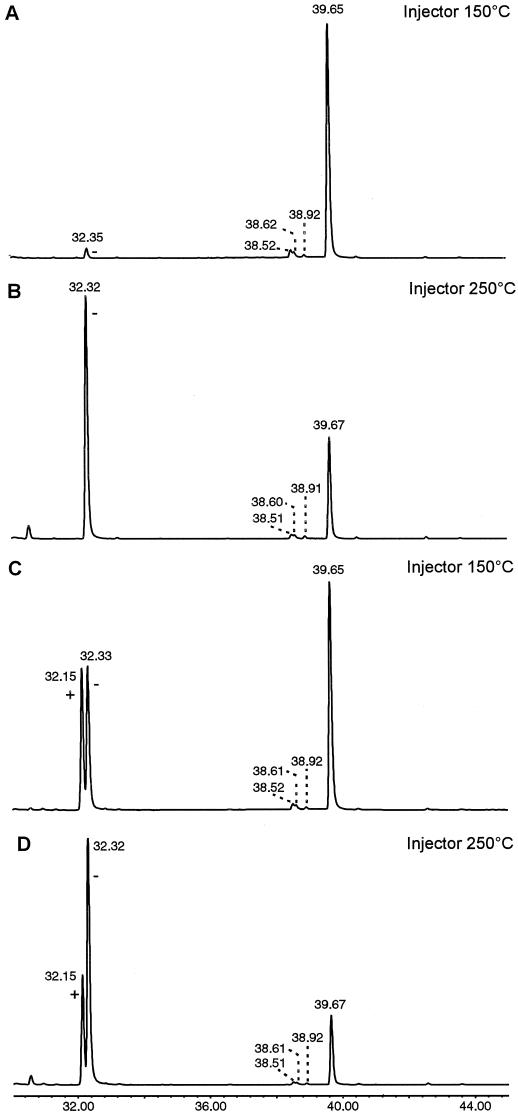
Using this knowledge, the Cope rearrangement can be used for the determination of the absolute configuration of germacrene A. Enzymatically produced germacrene A was injected on an enantioselective column at an injection port temperature of either 150°C or 250°C. Whereas a huge peak of germacrene A was visible at 150°C (besides smaller amounts of α-selinene [Fig. 3, 8], β-selinene [Fig. 3, 9], selina-4,11-diene [Fig. 3, 11], and (−)-β-elemene [Fig. 5, 10a]), the germacrene A was almost completely rearranged into (−)-β-elemene at an injection port temperature of 250°C (Fig. 6, A and B). This rearrangement of chicory germacrene A into (−)-β-elemene (and not into [+]-β-elemene) was substantiated by co-injection of the germacrene A with a racemic mixture of β-elemene at an injection port temperature of both 150°C and 250°C (Fig. 6, C and D). The (+)-enantiomer of β-elemene (10b) (König et al., 1994; Teisseire, 1994) has the same absolute configuration as (−)-germacrene A (7b), was determined by Weinheimer et al. (1970). Considering our experiment where no trace of (+)-β-elemene was observed and only its counterpart (−)-β-elemene (10a) was detected, we conclude that the germacrene A synthase of chicory produces exclusively (+)-germacrene A (7a) (Fig. 7).
Figure 6.
Determination of the absolute configuration of germacrene A using GC-MS equipped with a 25-m (0.25-mm i.d.) heptakis(6-O-TBDMS-2, 3-di-O-methyl)-β-cyclodextrin (50% in OV17) chiral column. Enzymatically (DEAE-purified enzyme) produced germacrene A (39.66 min) that is stable at an injection port temperature of 150°C (A) is rearranged into (−)-β-elemene (32.33 min) at an injection port temperature of 250°C (B). Co-injection of the germacrene A with a racemic β-elemene standard at 150°C (C) and 250°C (D) confirms the identity of its rearrangement product that co-elutes with (−)-β-elemene and is separated from the (+)-enantiomer of β-elemene (32.15 min). Small amounts of α-selinene (8) (38.52 min), β-selinene (9) (38.61 min), and selina-4,11-diene (11) (38.92 min) were detected during all measurements.
Figure 7.
Upon heating, the (+)-germacrene A (7a) produced in the enzyme assay rearranges toward (−)-β-elemene (10a) preserving its chiral center. Since chicory does not produce (−)-germacrene A, no (+)-β-elemene (10b) was observed. The stereochemical configuration of (+)-germacrene A (7a) is in accordance with the stereochemistry of the sesquiterpene lactones in chicory.
Purification of the (+)-Germacrene A Synthase from Chicory Roots
A summary of the protocol used in the purification of the chicory (+)-germacrene A synthase and its results are given in Table I. Purification was started by preparing a chicory root 100,000g supernatant and applying it to a DEAE column. The enzyme activity eluted from the column around 0.2 m KCl. Although the recovery of this first purification step was only 30%, it was very successful in discarding aspecific phosphohydrolase activity. Aliquots of 1 mL from this partially purified germacrene A synthase remained stable for several months in 30% (v/v) glycerol at −80°C, and they served as a stock for all further experiments.
Table I.
Purification of the chicory germacrene A synthase from 75 mL of a 100,000g supernatant (≈34 g of root material)
| Purification Step | Protein | Cyclase Activity | Specific Activity | Recovery | Purification Factor |
|---|---|---|---|---|---|
| mg | nmol h−1 | nmol h−1 mg−1 | % | ||
| Crude extract (pH 7.0) | 34.2 | 1073 | 31.3 | 100 | 1.0 |
| DEAE | 3.95 | 321 | 81.3 | 30 | 2.6 |
| Green 5 | 0.45 | 338 | 751 | 31.5 | 24 |
| Mono-Q | 0.033 | 206 | 6242 | 19.2 | 201 |
Several dye ligands were screened for their ability to bind and release the DEAE-purified germacrene A synthase; good results were obtained with Red A, Reactive Blue 72, Reactive Red 120, and Reactive Green 5. This seems to be in line with the results of Lanzaster and Croteau (1991) for the dye ligands Red A, Blue A, and Green A, and those of Moesta and West (1985) with Red A and Reactive Blue 2. Since Reactive Green 5 gave the best results and also since it had been used successfully in the purification of a trans-β-farnesene synthase from pine needles (Salin et al., 1995), we chose to carry out our experiments with it. As shown in Table I, we obtained a recovery slightly above 100% and a 9-fold purification. To ensure a good interaction of the germacrene A synthase with the matrix, it was important to warm the sample (at −80°C stored DEAE-stock; 1 mL) to room temperature before applying it to the Reactive Green 5 column. For enzyme stability the fractions containing enzyme activity required quick desalting into buffer C.
For the next purification step we used a Mono-Q column, which is often used in the purification of terpene cyclases (e.g. Savage et al., 1994). An additional advantage of this method is that it concentrated the enzyme activity, which was diluted over 8 mL after the dye-ligand purification step. Enzyme activity eluted from the Mono-Q column in two fractions of 0.75 mL at 0.15 m KCl with a recovery of 61%. A total purification fold of 201 was obtained. If this last purification step had been carried out in the absence of Tween 20, almost no sesquiterpene cyclase activity would have been detected.
Radio-GC analysis of the incubated Mono-Q-purified enzyme fractions showed only germacrene A (and its nonenzymatically produced derivatives). No trace of farnesol or any other sesquiterpene was detected.
SDS-PAGE showed that the purification was not complete, since at least three bands were detected after the last purification step (56, 59, and 62 kD). They were also visible when an excess of iodoacetamide was added to the sample immediately before applying it to gel; so these bands did not originate from keratin skin proteins, a common artifact in the range of 50 to 70 kD when using silver staining (Ochs, 1983; Görg et al., 1987).
Characterization of the (+)-Germacrene A Synthase
A fitted curve (r2 = 0.951) of the germacrene A synthase activities versus FPP concentration (between 0.5 and 180 μm FPP) gave rise to a typical hyperbolic saturation curve and yielded a Km value of 6.6 μm (Fig. 8B). The Vmax was estimated at 8.103 nmol h−1 mg−1 protein.
The DEAE-purified sesquiterpene cyclase showed a rather broad peak of activity at approximately pH 6.3 with half-maximal activities at pH 5.1 and 7.3 (Fig. 8C). After the last (Mono-Q) purification step, the optimum pH was slightly higher (6.7). In the absence of 0.1% Tween 20, enzyme activity was reduced 5-fold. This preserving/renaturing effect of Tween 20 on sesquiterpene cyclase activity has been described by Lewinsohn et al. (1992) and Davis et al. (1996).
The molecular mass of the (+)-germacrene A synthase was estimated at 54 kD (Fig. 8D) with calibrated gel filtration. This is in line with the results obtained from the protein gel. Recovery of enzyme activity from the gel-filtration column was 74%.
DISCUSSION
The sole enzymatically produced sesquiterpenoid product in incubations of a 100,000g chicory root supernatant with FPP was (+)-germacrene A (7). Additional enzymatic cyclization of this product did not occur. Therefore, we conclude that this (+)-germacrene A synthase activity, present in both wild and cultivated chicory, represents the first dedicated step in the biosynthesis of bitter compounds in chicory and, more generally, proves the previously proposed mevalonate-FPP-germacradiene pathway in sesquiterpene lactone biosynthesis (Herz, 1977; Bohlman and Zdero, 1978; Fischer, 1990; Song et al., 1995). Germacrene B, which would be a likely intermediate from a chemical point of view, appears not to be involved in the biosynthesis of C8-oxygenated guaianolides in chicory.
Incubations of [3H]germacrene A or [3H]FPP in the presence of NADPH and O2 carried out as described by Coolbear and Threlfall (1985) and Threlfall and Whitehead (1988) support this conclusion. Germacrene A is enzymatically oxidized to a compound having a mass spectrum that is identical to that of elema-1,3,11(13)-trien-12-ol (Maurer and Grieder, 1977; J.-W. de Kraker, unpublished results). Most probably, it is the Cope-rearrangement product of 1(10),4,11(13)-germacratrien-12-ol, indicating that, in chicory, germacrene A is further metabolized into a 12-hydroxylated germacrene.
In line with other enzymes belonging to the group of sesquiterpene cyclases, the (+)-germacrene A synthase from chicory operated optimally at approximately pH 6.7 with a rather broad peak of activity. A molecular mass of 54 kD, estimated by calibrated gel filtration, and a Km value of 6.6 μm were also in the same range as those observed for a number of higher plant sesquiterpene cyclases (Croteau and Cane, 1985; Cane, 1990) (Fig. 8).
Germacrene A itself is reported to be a highly unstable compound susceptible to both proton-induced cyclizations and heat-induced Cope rearrangement, and it would be unstable even at −20°C (Fig. 3) (Weinheimer et al., 1970; Bowers et al., 1977; Teisseire 1994). Our experiments showed otherwise. When silica was used during the assay extraction-filtration procedure, one-half of the germacrene A was cyclized toward α-selinene (8), β-selinene (9), and selina-4,11-diene (11). To our knowledge the latter compound has not been reported before in this context, but originates from the same intermediate carbocation as the other two selinenes. Using neutral aluminum oxide instead of the slightly acidic silica effectively minimized the nonenzymatic cyclization. Cope rearrangement of germacrene A did not occur, not even during incubations at 30°C overnight.
Cope rearrangement to β-elemene (10) can be a problem in GC measurements due to the high temperatures involved. However, reducing the injection port temperature to 150°C greatly diminished Cope rearrangement. If cold on-column injection is applied, no Cope rearrangement will be observed at all. Rearrangement of germacrene A and germacrene B during GC-MS measurement was detected by Wichtman and Stahl-Biskup (1987), whereas the influence of GC injection port temperature on Cope rearrangement was studied for germacrone by Reichardt et al. (1988). Nevertheless, high injection port temperatures in combination with enantioselective GC proved to be very useful in determining the absolute configuration of the germacrene A formed by the isolated enzyme. The heat-induced Cope rearrangement is stereospecific and the chiral center at C7 is not involved in this reaction (Weinheimer et al., 1970; Takeda, 1974; March, 1992) (Fig. 5). Since only (−)-β-elemene (10a) was obtained, we can designate our enzymatic product as the (+)-enantiomer of germacrene A (7a) (Figs. 6 and 7).
In chicory, just as in the majority of higher plants, sesquiterpene lactones possess an α-methylene-γ-lactone ring in which the proton at the C7-position of the sesquiterpenoid framework is, without exception, α-oriented (Bachelor and Ito, 1973; Seto et al., 1988; Fischer 1990; van Beek et al., 1990). Therefore, the absolute configuration of (+)-germacrene A corresponds with its biochemical fate, and the configuration is already determined in the first step of sesquiterpene lactone biosynthesis (Fig. 7).
Recently, Chappell and coworkers elucidated the crystal structure of 5-epi-aristolochene synthase and unraveled its enzymatic mechanism, which must be similar to that of vetispiradiene synthase, as several constructed epi-aristolochene-vetispiradiene chimeras demonstrated (Back and Chappell, 1996). In a first step FPP is bound to the enzyme and dephosphorylated, generating germacrene A. The germacrene A intermediate is then once more cyclized toward a eudesmane carbocation whose final destination, either epi-aristolochene or vetispiradiene, depends upon the particular active site conformation of the sesquiterpene cyclase involved (Starks et al., 1997).
The existence of the germacrene A intermediate has also been revealed in incubations of epi-aristolochene synthase using the anomalous substrate (7R)-6,7-dihydrofarnesyl diphosphate instead of FPP. In these experiments the intermediate dihydro-germacrene A is released because it cannot be further cyclized to the eudesmane (Cane and Tsantrizos, 1996).
It is generally assumed that the germacrene A intermediate is involved in the biosynthesis of numerous eudesmane- and eremophilane-type sesquiterpenes (Beale, 1990); however, so far it has never been detected because it remains bound to the sesquiterpene cyclase (Cane et al., 1990; Cane and Tsantrizos, 1996). Nevertheless, various species such as the liverwort F. tamarisci (W.A. König, unpublished results), caraway (Wichtman and Stahl-Biskup, 1987), the gorgonian Eunice mammosa (Weinheimer et al., 1970), and the spotted alfalfa aphid (Bowers et al., 1976) contain germacrene A and should therefore also contain the corresponding germacrene A synthase.
Despite thorough analyses, no trace of germacrene A (nor any other sesquiterpene hydrocarbon) has ever been detected in chicory (Leclercq, 1992). Consequently, it is rather peculiar that chicory contains an enzyme that releases germacrene A. Why is FPP not immediately cyclized, within one enzymatic step, toward a eudesmane or a guaiane by a (hypothetical) eudesmane synthase or guaiane synthase? In other words, why is germacrene A released by the chicory sesquiterpene cyclase instead of being subjected to a second cyclization step? It seems that in sesquiterpene lactone biosynthesis a different type of reaction is needed before such a second cyclization can take place.
Based upon the study of a large number of stereospecific biomimetic transformations of germacradienolides and their derivatives into methyleudesmanolides and guaianolides (Fischer, 1990), as well as the study of acid-induced cyclizations of germacrene B epoxides (Brown et al., 1975; Piet et al., 1995b), it has been postulated that this second cyclization is directed by epoxidations. A germacradienolide C4-C5-epoxide would be cyclized to a guaianolide, whereas a germacradienolide C1-C10-epoxide would be cyclized to a eudesmanolide (Fischer, 1990; Teisseire, 1994; Song et al., 1995). Such a cyclization of germacradienolide epoxides toward either a guaianolide or a eudesmanolide, depending on the position of the epoxide, is presumed to be catalyzed by one and the same germacrane cyclase that possesses a broad substrate specificity (Piet et al., 1996).
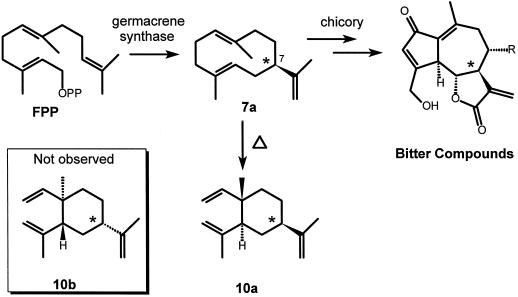
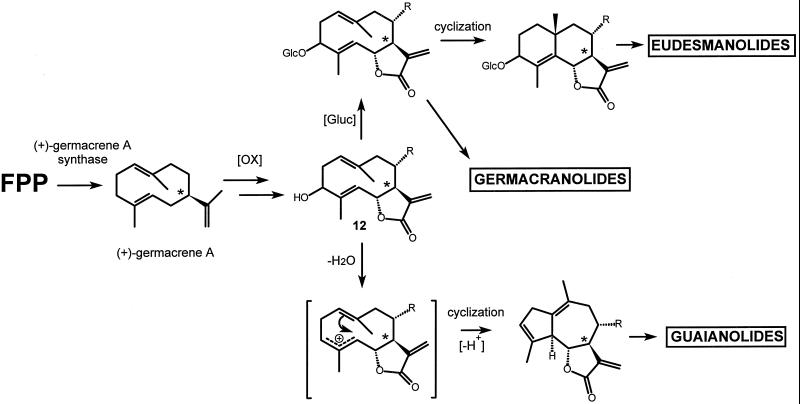
Piet et al. (1996) noticed that all germacranolides and almost all eudesmanolides of chicory posses a glucosilated hydroxyl function at C3, whereas the guaianolides lack this function and have an olefinic C3 atom. An alternative biopathway (Fig. 9) is proposed in which this C3-hydroxyl function plays a crucial role. As we have demonstrated for chicory, sesquiterpene lactone biosynthesis starts with the cyclization of FPP to (+)-germacrene A. Through several oxidative steps (+)-germacrene A might be transformed into a germacranolide (12), which would be the branching point in the biosynthesis of guaianolides, eudesmanolides, and germacranolides. So far, this compound has not been detected in chicory and is probably quickly subjected to the next biochemical step, just like germacrene A, that has not been detected in vivo either. Cyclization of 12 by a germacrane cyclase would start with the protonation of the C3-hydroxyl group, which is then released as a water molecule. The carbocation thus formed leads to guaianolides via 1,5-cyclization. Glucosylation of the C3-hydroxyl function would prevent this type of cyclization; it leaves the germacranolides as such or leads to the germacrane cyclase mediated cyclization toward eudesmanolides.
Figure 9.
Proposed pathway for the biosynthesis of sesquiterpene lactones in chicory using the (+)-germacrene A synthase as a starting point. It is not known at which stages the oxidative steps take place.
Several questions about the biosynthetic sequence remain unanswered, such as at which stages the hydroxylations take place and/or the lactone is formed. The moment at which glucosylation occurs is also unknown. Future research on the oxidative steps involved will shed more light on the biosynthesis of chicory sesquiterpene lactones.
ACKNOWLEDGMENTS
The authors would like to thank J. de Mik for the gift of chicory roots, Dr. D.P. Piet for synthesis of germacrene B and γ-elemene, Dr. M.A. Posthumus and J.A.R. Davies for their help with the GC-MS analyses, and M.C.J.M. Konings for technical assistance. We also thank the Koninklijke Landbouwkundige Vereniging for their support during the first stages of the work presented.
Abbreviation:
- FPP
farnesyl diphosphate
LITERATURE CITED
- Bachelor FW, Ito S. A revision of the stereochemistry of lactucin. Can J Chem. 1973;51:3626–3630. [Google Scholar]
- Back K, Chappell J. Cloning and bacterial expression of a sesquiterpene cyclase from Hyoscyamos muticus and its molecular comparison to related terpene cyclases. J Biol Chem. 1995;270:7375–7381. doi: 10.1074/jbc.270.13.7375. [DOI] [PubMed] [Google Scholar]
- Back K, Chappell J. Identifying functional domains within terpene cyclases using a domain swapping strategy. Proc Natl Acad Sci USA. 1996;93:6841–6845. doi: 10.1073/pnas.93.13.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale MH. The biosynthesis of C5-C20 terpenoid compounds. Nat Prod Rep. 1990;7:387–407. doi: 10.1039/np9900700025. [DOI] [PubMed] [Google Scholar]
- Belingheri L, Cartayrade A, Pauly G, Gleizes M. Partial purification and properties of the sesquiterpene β-selinene cyclase from Citronella mitis fruits. Plant Sci. 1992;84:129–136. [Google Scholar]
- Bohlman F, Zdero C. New sesquiterpenes and acetylenes from Athanasia and Pentzia species. Phytochemistry. 1978;17:1595–1599. [Google Scholar]
- Bowers WS, Nishino C, Montgomery ME, Nault LR, Nielson MW. Sesquiterpene progenitor, germacrene A: an alarm pheromone in aphids. Science. 1977;196:680–681. doi: 10.1126/science.558651. [DOI] [PubMed] [Google Scholar]
- Brown ED, Sutherland JK, Sam TW (1975) Medium-ring 1:5-dienes. Part III. Cyclization of germacra-1(10),4,7-(11)-triene oxides. J Chem Soc Perkin Trans I 2332–2336
- Cane DE. Enzymatic formation of sesquiterpenes. Chem Rev. 1990;90:1089–1103. [Google Scholar]
- Cane DE, Tsantrizos YS. Aristolochene synthase: elucidation of the cryptic germacrene A synthase activity using the anomalous substrate dihydrofarnesyl diphosphate. J Am Chem Soc. 1996;118:10037–10040. [Google Scholar]
- Cane DE, Wu Z, Proctor RH, Hohn TM. Arch Biochem Biophys. 1993;30:415–419. doi: 10.1006/abbi.1993.1369. [DOI] [PubMed] [Google Scholar]
- Connolly JA, Hill RA. Dictionary of Terpenoids, Vol 1. London: Chapman and Hall; 1991. [Google Scholar]
- Coolbear T, Threlfall DR. The biosynthesis of lubimin from [1-14C]isopentenyl pyrophosphate by cell-free extracts of potato tuber tissue inoculated with an elicitor preparation from phytophthora infestans. Phytochemistry. 1985;24:1963–1971. [Google Scholar]
- Croteau R, Cane DE. Monoterpene and sesquiterpene cyclases. Methods Enzymol. 1985;110:383–405. [Google Scholar]
- Davis EM, Tsuji J, Davis GD, Pierce ML, Essenberg M. Purification of (+)-δ-cadinene synthase, a sesquiterpene cyclase from bacteria inoculated cotton foliar tissue. Phytochemistry. 1996;41:1047–1055. doi: 10.1016/0031-9422(95)00771-7. [DOI] [PubMed] [Google Scholar]
- Fischer NH (1990) Sesquiterpene lactones: biogenesis and biomimetic transformations. In G Towers, H Towers, eds, Biochemistry of the Mevalonic Acid Pathway to Terpenoids. Plenum Press, New York, pp 161–201
- Görg A, Postel W, Weser J, Günter S, Strahler JR, Hanash JM, Somerlot L. Elimination of point streaking on silver stained two-dimensional gels by addition of iodoacetamide to the equilibrium buffer. Electrophoresis. 1987;8:122–124. [Google Scholar]
- Hardt IH, Rieck A, König WA, Muhle H. Isolepidizone, a diastereomer of bicyclogermacrene in some liverworts. Phytochemistry. 1995;40:605–606. [Google Scholar]
- Herz W. Sesquiterpene lactones in the compositae. In: Heywood VH, Harborne JB, Turner BL, editors. The Biology and Chemistry of the Compositae. London: Academic Press; 1977. pp. 337–357. [Google Scholar]
- Hohn TM, Plattner RD. Purification and characterization of the sesquiterpene cyclase aristolochene synthase from Penicillium roqueforti. Arch Biochem Biophys. 1989;272:137–143. doi: 10.1016/0003-9861(89)90204-x. [DOI] [PubMed] [Google Scholar]
- König WA, Rieck A, Hardt I, Gehrcke B, Kubeczka K-H, Muhle H. Enantiomeric composition of the chiral constituents of essential oils. Part 2. Sesquiterpene hydrocarbons. J High Resolut Chromatogr. 1994;17:315–320. [Google Scholar]
- Lanznaster N, Croteau R. Dye-ligand and immobilized metal ion interaction chromatography for the purification of enzymes of prenyl pyrophosphate metabolism. Protein Expr Purif. 1991;2:69–74. doi: 10.1016/1046-5928(91)90013-9. [DOI] [PubMed] [Google Scholar]
- Leclercq E (1992) Sesquiterpene lactones and inulin from chicory roots: extraction, identification, enzymatic release and sensory analysis. PhD thesis. Agricultural University, Wageningen, The Netherlands
- Lesburg CA, Zhai G, Cane DE, Cristianson DW. Crystal structure of pentalene synthase: mechanistic insight on terpenoid cyclization reactions in biology. Science. 1997;277:1820–1824. doi: 10.1126/science.277.5333.1820. [DOI] [PubMed] [Google Scholar]
- Lewinsohn E, Gijzen M, Croteau R. Wound-inducible pinene cyclase from grand fir: purification, characterization and renaturation after SDS-page. Arch Biochem Biophys. 1992;293:167–173. doi: 10.1016/0003-9861(92)90380-f. [DOI] [PubMed] [Google Scholar]
- Maat L, Straver EJM, van Beek TA, Posthumus MA, Piozzi F. Analysis of the essential oil of the so-called Menta mirennea Bruno by GC and GC-MS. J Essential Oil Res. 1992;4:615–618. [Google Scholar]
- March J (1992) Advanced Organic Chemistry, Ed 4. Wiley-Interscience, New York, pp 1132
- Maurer B, Grieder A. Sesquiterpenoids from costus root oil (Saussurea lappa Clarke) Helv Chim Acta. 1977;60:2177–2190. doi: 10.1002/hlca.19770600710. [DOI] [PubMed] [Google Scholar]
- McCaskill D, Croteau R (1997) Prospects for the bioengineering of isoprenoid biosynthesis. In R Berger, ed, Biotechnology of Aroma Compounds (Advances in Biochemical Engineering Biotechnology). Springer-Verlag, Berlin, pp 107−146 [DOI] [PubMed]
- Moesta P, West CA. Casbene synthetase regulation of phytoalexin biosynthesis in Ricinus communis L. seedlings. Arch Biochem Biophys. 1985;238:325–333. doi: 10.1016/0003-9861(85)90171-7. [DOI] [PubMed] [Google Scholar]
- Morikawa K, Hirose Y. Biosynthesis of germacrene C. Tetrahedron Lett. 1971;16:1131–1132. [Google Scholar]
- Munck SL, Croteau R. Purification and characterization of the sesquiterpene cyclase patchoulol synthase from Pogostemon cablin. Arch Biochem Biophys. 1990;282:58–64. doi: 10.1016/0003-9861(90)90086-e. [DOI] [PubMed] [Google Scholar]
- Ochs D. Protein contaminants of sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1983;135:470. doi: 10.1016/0003-2697(83)90714-5. [DOI] [PubMed] [Google Scholar]
- Piet DP, Franssen MCR, de Groot Ae Biotransformation of allylically activated (E,E)-cyclodeca-1,6-dienols by Cichorium intybus. Tetrahedron. 1996;52:11273–11280. [Google Scholar]
- Piet DP, Minnaard AJ, van der Heyden KA, Franssen MCR, Wijnberg JBPA, de Groot Ae Biotransformation of (±)-4,8-dimethylcyclodeca-3(E),7(E)-dien-1β-ol and (+)-hedycaryol by Cichorium intybus. Tetrahedron. 1995a;51:243–254. [Google Scholar]
- Piet DP, Schrijvers R, Franssen MCR, de Groot Ae Biotransformation of germacrene epoxides by Cichorium intybus L. Tetrahedron. 1995b;51:6303–6314. [Google Scholar]
- Pyrek JST. Sesquiterpene lactones of Cichorium intybus and Leontodon autumnalis. Phytochemistry. 1985;24:186–188. [Google Scholar]
- Rees BS, Harborne JB. The role of sesquiterpene lactones and phenolics in the chemical defense of the chicory plant. Phytochemistry. 1985;24:2225–2231. [Google Scholar]
- Reichardt PB, Anderson BJ, Clausen TP, Hoskins LC. Thermal instability of germacrone; implications for gas chromatographic analysis of thermally unstable analytes. Can J Chem. 1988;67:1174–1177. [Google Scholar]
- Rodriguez E, Towers GHN, Towers JC. Biological activities of sesquiterpene lactones. Phytochemistry. 1976;15:1573–1580. [Google Scholar]
- Salin F, Pauly G, Charon J, Gleizes M. Purification and characterization of trans-β-farnesene synthase from maritime pine (Pinus pinaster Ait.) needles. J Plant Physiol. 1995;146:203–209. [Google Scholar]
- Savage TJ, Hatch MW, Croteau R. Monoterpene synthases of Pinus cortorta and related conifers: a new class of terpenoid cyclase. J Biol Chem. 1994;269:4012–4020. [PubMed] [Google Scholar]
- Seto M, Miyase T, Umehara K, Ueno A, Hirano Y, Otani N. Sesquiterpene lactones from Cichorium endivia L. and C. intybus L. and cytotoxic activity. Chem Pharm Bull. 1988;36:2423–2429. doi: 10.1248/cpb.36.2423. [DOI] [PubMed] [Google Scholar]
- Song Q, Gomez-Barrios ML, Hopper EL, Hjortso MA, Fischer NH. Biosynthetic studies of lactucin derivatives in hairy root cultures of Lactuca floridana. Phytochemistry. 1995;40:1659–1665. [Google Scholar]
- Starks CM, Back K, Chappell J, Noel JP. Structural basis for cyclic terpene biosynthesis by tobacco 5-epi-aristolochene synthase. Science. 1997;277:1815–1819. doi: 10.1126/science.277.5333.1815. [DOI] [PubMed] [Google Scholar]
- Takeda K. Stereospecific Cope rearrangement of the germacrene-type sesquiterpenes. Tetrahedron. 1974;30:1525–1534. [Google Scholar]
- Teisseire PJ (1994) Chemistry of fragrant substances. VCH Publishers Inc., New York, pp 193–289
- Threlfall DR, Whitehead IM. Co-ordinated inhibition of squalene synthetase and induction of enzymes of sesquiterpenoid phytoalexin biosynthesis in cultures of Nicotiana tabacum. Phytochemistry. 1988;27:2567–2580. [Google Scholar]
- van Beek TA, Maas P, King BM, Leclercq E, Voragen AGJ, de Groot Ae Bitter sesquiterpene lactones from chicory roots. J Agric Food Chem. 1990;38:1035–1038. [Google Scholar]
- Weinheimer AJ, Youngblood WW, Washecheck PH, Karns TKB, Ciereszko LS. Isolation of the elusive (−)-germacrene-A from the gorgonian, Eunicea mammosa: chemistry of coelenterates XVIII. Tetrahedron Lett. 1970;7:497–500. [Google Scholar]
- Whitehead IM, Threlfall DR, Ewing DE. 5-Epi-aristolochene is a common precursor of the sesquiterpenoid phytoalexins capsidiol and debneyol. Phytochemistry. 1989;28:775–779. [Google Scholar]
- Wichtman E-M, Stahl-Biskup E. Composition of the essential oils from caraway herb and root. Flavour Fragrance J. 1987;2:83–89. [Google Scholar]