Abstract
Treg are important in maintaining immune homeostasis and in regulating a variety of immune responses, making them attractive targets for modulating immune-related diseases. Success in using induction or transfer of Treg in mice to mediate transplant tolerance suggests Treg-based therapies as mechanisms of long-term drug free transplant tolerance in human patients. While more work is needed, critical analyses suggest that key factors in Treg induction, migration, and function are important areas to concentrate investigative efforts and therapeutic development. Elucidation of basic biology will aid in translating data gleaned from mice to humans so that Treg therapies become reality for patients.
Introduction
Regulatory T cells (Treg) are essential to maintain immune homeostasis, and are critical regulators for a variety of immune responses, including tolerance induction and maintenance for organ transplantation (1, 2). There are two main types of Treg: thymus-derived CD4+CD25+Foxp3+ natural Treg (nTreg), and adaptive/induced Treg (iTreg) that develop from naïve T cells in the periphery under tolerogenic conditions (1). It has long been established that Fork-head box P3 (Foxp3) is the major transcription factor (TF) that determines the fate, identity, and function of Treg, and Treg regulate immune functions by producing cytokines such as TGFβ, IL-10, and IL-35 (3, 4). However, there are subsets of Treg that do not express Foxp3. For example, TGFβ-producing Th3 and IL-10-secreting Tr1 regulatory T cells also can be potent suppressors in some experimental systems. This review will be restricted to CD4+CD25+Foxp3+ Treg.
Treg in transplantation: Treg Induction
The basic requirements for the induction of both natural (nTreg) and adaptive or inducible (iTreg) regulatory T cells are similar. Both nTreg and iTreg require TGFβ and IL-2 for induction of Foxp3 (5–7). Without Foxp3 expression, suppressive function of both subsets is lost (8), and both mice (9) and humans (10) succumb to autoimmune disease.
Although the basic requirements are similar, the generation of nTreg and iTreg varies in a plethora of ways. nTreg mature in the thymus during T cell receptor (TCR) chain selection (11) based on their high affinity for self-peptides (12), although alloreactive nTreg have been reported (13) and can be selected by intrathymic presentation of transplant-derived antigen (14). Both iTreg and effector T cells (Teff) enter the periphery as naïve T cells, but iTreg go on to acquire a suppressive phenotype (13, 15). Furthermore, TCR transgenic CD4 T cells of a single specificity can differentiate into either iTreg or Teff depending upon the timing and context in which alloantigen is presented (16). This suggests that bulk iTreg and Teff TCR repertoires may be broadly similar. As foreign antigen is required for the induction of iTreg, it follows that iTreg may be more likely to recognize alloantigen presented indirectly and act in a more transplant-specific fashion compared to nTreg.
In murine models of transplantation, iTreg are generated by recipient treatment with donor-specific splenocyte transfusion (DST) in combination with anti-CD4 non-depleting antibody (17) or costimulatory blockade with anti-CD40L mAb (16). iTreg induction is likely due to the absence of sufficient T cell costimulation. Furthermore, negative costimulatory engagement via PD-1–PD-L1 interactions supports the development of iTreg (18), while costimulatory signals via OX40 inhibit iTreg development (19).
Human Treg are more difficult to study than murine Treg as in humans Foxp3 can also be induced transiently and at low levels in recently activated CD4+ T cells (20). Hence, there is an ongoing search for alternative markers of Treg in humans, with the combination of the markers CD127(lo)CD25+CD4+ the current standard. Nevertheless, methods of expanding human Treg have been established. Rabbit anti-thymocyte globulin induces in vitro conversion and expansion of human Treg, likely by increasing NFAT1 expression (21) or inducing tolerogenic DC, which can then induce Treg conversion as discussed in the following paragraph (22). Conversely, others have suggested that rabbit anti-thymocyte globulin induces transient Foxp3 expression associated with the generation of Teff as opposed to Treg (23). Culture with stimulatory anti-CD3 plus anti-CD28 mAbs in combination with IL-2 and rapamycin (24) or donor-derived leukocytes (25) are also common methods of Treg induction.
The nature of maturation signals to which naïve T cells are exposed also affects their fate. Tolerogenic dendritic cells (Tol-DC) are so named as they induce donor-specific Treg (26). Tol-DC have an immature phenotype defined by low expression of MHC class II, CD40, CD80/86, and IL-12 (26). Tol-DC can be generated ex vivo, using either donor or recipient DC pulsed with donor alloantigen (27). Pharmacologic interventions can also prevent DC maturation, and these interventions include in vitro or in vivo exposure to IL-10, TGFβ, vitamin D3, histamine, or clinically relevant immunosuppressants including corticosteroids, cyclosporine, rapamycin, and mycophenolate (28). Lastly, a positive feedback loop exists in which Tol-DC induce Treg and in turn Treg induce Tol-DC (26). Hence, donor-reactive Treg have the ability to propagate their lineage by limiting the immunogenicity of DC that encounter donor antigen. This may be significant in the maintenance phase of graft tolerance as the graft remains a constant source of antigen in the periphery.
Plasmacytoid dendritic cells (pDC) are integral to the establishment of graft tolerance in mice as they induce iTreg in the LN of tolerant recipients (29). pDC have also been implicated in the generation of nTreg that specifically recognize donor antigen. pDC can acquire antigen in the periphery, maintain an immature phenotype, and home to the thymus in a CCR9 dependent fashion (14). Once in the thymus, immature pDC delete peripheral antigen-reactive T cells, and it is possible that these same pDC are able to mediate the selection of peripheral-antigen specific nTreg. Together, these findings emphasize that both the location and type of cell presenting alloantigen are integral to the generation of Treg.
Immunosuppressants and Transplant Tolerance
Common immunosuppressants used post-transplantation in humans have opposing effects on Treg induction. Both mycophenolate mofetil, an inosine 5’-monophosphate dehydrogenase inhibitor, and rapamycin, the eponymous inhibitor of the mammalian target of rapamycin (mTOR), expand iTreg in vitro and in vivo (30–32). In humans, rapamycin (sirolimus) given following treatment with a lympho-depleting anti-CD52 mAb favors Treg expansion (33).
Sphingosphine 1-phosphate receptor 1 (S1P1) engagement by sphingosphine 1-phosphate inhibits the generation and function of both iTreg and nTreg, and inhibiting S1P1 with FTY720 results in both iTreg and nTreg generation (34). S1P1 engagement results in deficient Smad3 activation following TGFβ signaling, disrupting signals integral for Treg development (35). Interestingly, both FTY720 and rapamycin target the mTOR pathway (34), further illustrating the importance of mTOR inhibition in Treg generation.
Conversely, calcineurin inhibitors may prevent Treg induction (36). Cyclosporine A inhibits Treg function and proliferation (37), likely by inhibiting IL-2 production (38). This inhibition of Treg function and proliferation allows for effector T cell proliferation (38) and prevention of peripheral repopulation of Treg following anti-CD52 mAb treatment (33). FK506 (tacrolimus) also inhibits the transmission of TCR signaling, thus inhibiting IL-2 production. Reports regarding the impact of FK506 on Treg induction vary; it has been reported to inhibit, not affect, or favor Treg induction, likely in a dose-dependent fashion (39–41). Thus, post-transplantation immunosuppressive regimens should be chosen with care in order to promote the generation and function of protective Treg and suppress anti-graft immune responses.
Adhesion, migration and trafficking of Treg
Much progress has been made in understanding conventional T cell (Tconv) recirculation and trafficking and the molecules that mediate these processes. T cell trafficking to LN and peripheral tissues occurs predominantly from blood, and is a highly regulated, multi-step process involving a number of different classes of adhesive and inflammation-sensing molecules. Briefly, the steps can be divided into rolling, mediated primarily by selectins and their ligands (42); arrest and firm adhesion, mediated by integrins and chemokine receptors (43); and diapedesis into the target tissue. Chemokine receptors are important both in activating firm adhesion via modulation of integrin conformation and affinity, and in chemotaxis in tissue parenchyma after diapedesis (44). Different selectins, integrins, and chemokine receptors are involved in homing to different tissues.
These general trafficking rules apply to Treg as well as Tconv. However, the question of where Treg must exert their functions, and therefore to what sites they must migrate in order to prevent allograft rejection, is as yet incompletely answered. Tregs have been found in kidney (45), cardiac (46), and skin grafts (47), and there is evidence that Treg must be able to home to grafts in order to protect against graft rejection (48–50). Concordantly, subsets of Tregs that appear to be antigen experienced can express multiple molecules implicated in trafficking into and retention within inflamed target tissues, including but not limited to E and P selectin ligands, LFA-1, CD103 and other integrins, and the chemokine receptors CCR2, CCR6, and CXCR3 (51). Other work has found that CCR4 is involved in Treg recruitment to cardiac allografts (46), and CCL5 has been implicated in attracting Treg to kidney allografts (52). Studies in humans demonstrate that Treg are present in heart and skin allografts and they specifically control immune responses to donor alloantigens (53, 54). Recent studies have suggested that Treg use helper T cell (Th) cell transcription factors (TFs) to suppress specific Th subsets (55–60). These TFs are important for expression of suppressive molecules as well as molecules that enable Treg to migrate to sites of inflammation for interaction with and suppression of Teff functions.
There is also evidence from mice that Treg must home to LN in order for tolerance to be established (50, 61, 62). In humans the role of LN in the control of allogeneic immune responses is unknown as these tissues can only rarely be examined in patients, and usually only under conditions of significant organ dysfunction. The selectin CD62L and chemokine receptor CCR7 are the primary molecules involved in recruitment of T cells to LN from the blood (63, 64). Interestingly, we found that CD62L blockade, and subsequent depletion of T cells from LN, prevented induction of tolerance and appearance of Treg in a vascularized cardiac allograft model (61). Treg were not increased in organs other than the LN of tolerant mice. Likewise, the decrease in Treg after L-selectin blockade was also confined to LN. This finding indicates that Treg develop in the LN from naïve T cells in the cardiac allograft model, and also that these are iTreg as opposed to nTreg. Thus, in situations where induction of iTreg is important in regulating immune responses, the trafficking of the naïve T cells is integral. However, while this study indicates that LN are the sites of iTreg generation it does not specify where iTreg need to be to exert their suppressive function; the site of suppressive function could be the LN, the graft, or both.
Results from our lab in an islet transplant model suggest an explanation for disparities in where Tregs can be found or must home in order to prevent graft rejection (50). In this study, we found that nTreg trafficked sequentially to the graft and from there to draining lymph nodes, and that this process was required for their optimal suppressive function. E and P selectin ligands were important in Treg homing to the graft, while CCR7, CCR2, and CCR5 were required for the subsequent migration to draining lymph nodes (50). Thus, nTreg and iTreg appear to exhibit different patterns of migration, at least in part explained by differences in where and when they are generated. While iTreg, like Teff, must be generated in lymph nodes before homing to the graft, nTreg, like memory T cells, are already present in the periphery and can home directly to inflamed grafts. The different patterns of migration and sites of activation of these cells may speak to a division of labor that has yet to be fully elucidated. Importantly, different types of transplants in different locations may require one, the other, or both types of Treg in order for graft acceptance to be achieved.
Treg function
Timing
There is evidence that Treg must function at different times relative to transplantation: both in induction and maintenance of tolerance. Tolerogenic drug regimens given before transplantation can induce Treg that have important roles in both induction and maintenance of alloantigenic tolerance in mice. Pretreatment with anti-CD4 mAb plus DST before transplantation generates donor-specific Treg that prevent skin and cardiac allograft rejection without furthur immunosuppression (17, 65). Blockade of the CD40-CD154 pathway at the time of transplantation generates donor alloantigen-specific Treg that suppress CD154-independent, CD8+ T cell mediated allograft rejection (66). These results indicate a role for Treg in tolerance induction. On the other hand, Treg have been detected in long-term tolerized islet, skin, and cardiac allograft recipients, suggesting that they also function to maintain tolerance (30, 46, 49, 54, 67). In one study tolerant mice were treated with stimulatory anti-OX40, which is known to impair Treg suppressive function (19), 30 days after allogeneic cardiac transplant (68). This resulted in chronic graft rejection, and implied that functional Treg were required to maintain tolerance.
These studies have not differentiated between induction and maintenance of tolerance by nTreg, iTreg or both subsets. Donor alloantigen presentation is required prior to transplantation for tolerance induction to occur, suggesting that preexisting nTreg are insufficient to mediate tolerance. Therfore, tolerance could require either expansion of nTreg (69), induction of iTreg, or both prior to transplantation. One or both Treg subsets is also required to maintain tolerance. Thus, Treg function throughout tolerance in distinct induction and maintenance phases.
Mechanism
Treg employ different mechanisms of suppression dependent upon temporal and microenvironmental pressures, and the dominance of a given mechanism may be biased due to particular experimental protocols and the assays performed. Treg suppress target T cells by secreting cytokines such as IL-10 and TGFβ, or via cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) (17, 70, 71). CTLA-4 can function either in a cell-intrinsic manner by impairing costimulation of Treg, thus preserving their suppressive capacity (17), or in a cell-extrinsic manner by mediating trans-endocytosis of CD80 and CD86 (72–74), resulting in depletion of these costimulatory molecules. In a murine model, blocking IL-10 and CTLA-4 abrogated the suppression of skin allograft rejection by Treg during the induction phase (17). Blockade of IL-10 and TGFβ abrogates Treg mediated unresponsiveness to alloantigen during the maintenance phase in skin and cardiac transplant models (67, 75). Spontaneous renal allograft acceptance involves TGFβ (76). In a rat model, TGFβ expression is increased in tolerated cardiac allografts, TGFβ neutralization results in graft rejection, and over-expression leads to prolonged graft survival (77). CD4+CD25+ cells constitutively express CTLA-4 in mice (71) and humans (78, 79), and Treg can be activated by CTLA-4 ligation by CD80 or CD86 on DC or monocytes (80–82). CTLA-4 blockade prevents suppression by alloantigen-specific Treg in islet and skin transplantation and enhances rejection of these tissues (17, 83).
Mouse Treg also function by rendering APC unable to activate Tconv (84) by inducing expression of the enzyme indoleamine 2,3-dioxygenase (IDO) in APC (80). CTLA-4 is a potent activator of IDO in mouse DC, and resting Treg are dependent on CTLA-4 expression for IDO induction. However, Treg activated by anti-CD3 mAb or LPS rely less on CTLA-4 and instead produce IFNγ, which also induces IDO, suggesting a mechanism for this shift (80). Additionally, IDO-expressing DC may regulate the cytokine balance to favor the expansion of nTreg and induce iTreg to prolong skin and cardiac transplant survival (85). IFNγ-induced IDO in DC may attenuate acute liver rejection in a rat model (86), possibly by a similar Treg-mediated mechanism.
Recently, several studies have demonstrated that Treg cooperate with mast cells in skin transplantation (87, 88). Treg and mast cells are increased in tolerated grafts, as are mast cell-associated genes including mast cell proteases 1 and 5 (Mcpt1 and Mcpt5), tryptophan hydroxylase (Tph1), and the high-affinity IgE receptor (Fcer1a). Mice with reduced numbers of mast cells fail to be tolerized, and tolerance was restored when mast cells were reconstituted. Treg-secreted IL-9 is a potent chemoattractant and activator of mast cells. Neutralizing IL-9 prevented graft survival, positioning IL-9 as the functional link between Treg and mast cells to mediate immune suppression. Although the exact mechanism of suppression is unknown, data suggest that TGFβ or TPH produced by Treg-activated mast cells could play a role. TPH, a tryptophan-metabolizing enzyme, could create a tryptophan-deficient environment that limits T cell activation (88, 89).
It is likely that Treg interactions with non-immune cells, such as endothelial cells, also promote tolerance. During inflammation, Treg suppress endothelial cell activation and leukocyte recruitment (90). Endothelial cells can enhance the regulatory activity of Treg by inducing their upregulation of PD-L1, IL-10, and TGFβ (91). Thus, Treg suppress anti-graft immune responses via a number of mechanisms, including direct suppression of Teff as well as modulation of APC, and endothelial cell status, however, more research is required to elucidate the relative contributions by and functional differences between between nTreg and iTreg in transplantation.
Therapeutic application of Treg in transplantation
Despite extensive research over the past two decades, definitive evidence linking Treg and tolerance in human organ transplant recipients is still lacking. There are reports demonstrating the contribution of Treg in clinically tolerant recipients in which Treg inhibit the activation of effector cells and control alloresponses at the graft (53, 92). Liver transplant recipients with operational tolerance exhibited significantly more circulating Treg than non-tolerant patients and healthy individuals (93). On the other hand, Foxp3 mRNA in urine or from renal transplant biopsies is higher in kidney transplant patients with acute rejection (94, 95), however, in humans FOXP3 can be expressed in recently activated conventional CD4+ T cells (20). Therefore, non-Treg may account for the increase in FOXP3 during acute rejection.
Regardless, Treg are currently being tested in clinical trials as a potential therapy in cell and solid organ transplantation (96–98). Recently, Hester et al. demonstrated that low-dose rapamycin and subtherapeutic Treg numbers suppressed T cell proliferation in arterial transplantation (99), supporting the concept of using rapamycin as an adjunctive therapy to improve the efficacy of Treg-based immunosuppressive protocols in clinical practice. Selective expansion of Treg by injection of IL-2/antibody complexes leads to permanent acceptance of islet transplants in the absence of immunosuppression in mice (100). These recent advances in Treg expansion in vivo and vitro may point the way toward future clinical use of Treg in transplant.
Effects on specific effector arms and mechanisms
Treg exert their regulatory function on various phases of alloresponses in transplantation, including alloantibody production, antigen presenting, CD8 T cell cytotoxicity, delayed type hypersensitivity (DTH) and other inflammatory responses (2, 15, 101–104). Treg inhibit alloantibody production indirectly by suppressing T helper responses in secondary lymphoid tissues, and directly by suppressing B cell proliferation, immunoglobulin (Ig) production, and class switch recombination within germinal centers (GC) of lymphoid tissues (104, 105). Treg suppress antigen presentation by APC in mice and humans, thus abrogating their abilities to activate Tconv cells (84, 106). Treg can also kill target cells through granzyme-or perforin-dependent mechanisms (107, 108), or by inducing apoptotic pathways (109, 110). Treg suppress DTH and other inflammatory responses by inhibiting not only the activation and differentiation of innate and adaptive immune cells, but also the proliferation and cytokine production of effector cells via direct cell-cell contact-dependent suppression (101). Additionally, Treg can impair cytotoxic T lymphocyte (CTL) lytic function without affecting their proliferation or IFNγ production, which does not require prolonged physical Treg-CTL contact (111). Moreover, Treg can inhibit a wide range of immune responses through antigen non-specific “bystander suppression” (112), and establish and maintain a state of dominant and stable immunosuppression through “infectious tolerance”, by which Treg create a regulatory environment that expands their suppressive abilities and promotes the outgrowth of a new Treg population with distinct antigen specificities (113, 114).
nTreg vs. iTreg
Both nTreg from mice that are naïve or stimulated by tolerogen, and iTreg induced by tolerizing protocols can confer donor specific tolerance in transplant models (115–117). In other models, there is evidence of functional differences between iTreg and nTreg: by adoptive transfer of nTreg or iTreg into newborn Foxp3-deficient mice, a recent study showed that only nTreg prevented allergic disease lethality, but did not suppress chronic inflammation and autoimmunity, demonstrating distinct roles for iTreg and nTreg (118). However, functional differences in transplant models are less clear. In one study the ratio of nTreg to effector T cells in tolerized allografts and draining LN was higher than the ratio of iTreg to effector T cells at two weeks after transplantation. However, the ratios of nTreg and iTreg in the spleen, blood and nondraining LN during tolerance maintance were highly variable, so the relative importance of each subset at different times was not clear (119). Another study showed that iTreg are more critical than nTreg to suppress autoimmune diabetes (120), which may be important in islet transplantation. As few studies of transplant tolerance have distinguished between nTreg and iTreg, and those that have have shown inconsistent, contradictory, or inconclusive results, more study is needed to dissect the relative contributions of these Treg subsets to establishment and maintenance of tolerance.
Conclusions
Organ transplantation presents both enticing opportunities and challenges for the use of Treg-based therapies. In transplantation, unlike infections, there is a significant physical surgical insult, a persistent source of foreign antigen, and HLA mismatch. These factors may contribute to the development of detrimental T cell responses. In order to protect the graft Treg likely need to suppress generation of inflammatory immune responses in the draining LN as well as suppress inflammation in the transplanted tissue itself. Treg, and their progeny or other successors, must continue to do this for a long time, and in the presence of various immunomodulatory compounds that may make this task either easier or more difficult, and hopefully all without compromising broader immunity.
Despite these substantial hurdles, experimentally we can induce Treg in vivo that mediate long-term allograft tolerance, or expand them ex vivo and via their transfer into transplanted hosts, also achieve tolerance. While progress has been made in understanding the basic biology of Treg in mice, there is much we do not fully comprehend: how or if Treg selectively traffic to specific organs; the full complement of their suppressive mechanisms; the relative importance of Treg in induction or maintenance of tolerance; the effects of immunosuppressants on the induction, migration, and function of Treg; and to what extent are there distinct roles for nTreg vs. iTreg. Furthermore, we still do not completely understand the differences between Treg in mice and humans, and differences in identification of these subsets have confounded translation of our ever-growing understanding of murine Treg in tolerance to clinical practice. Hence, definitive evidence linking Treg and tolerance in human organ transplant recipients is still lacking. However, manipulation of the generation, migration, and function of Treg remains an attractive and potentially potent strategy to induce donor-specific tolerance while avoiding the pitfalls of current immunosuppressive protocols.
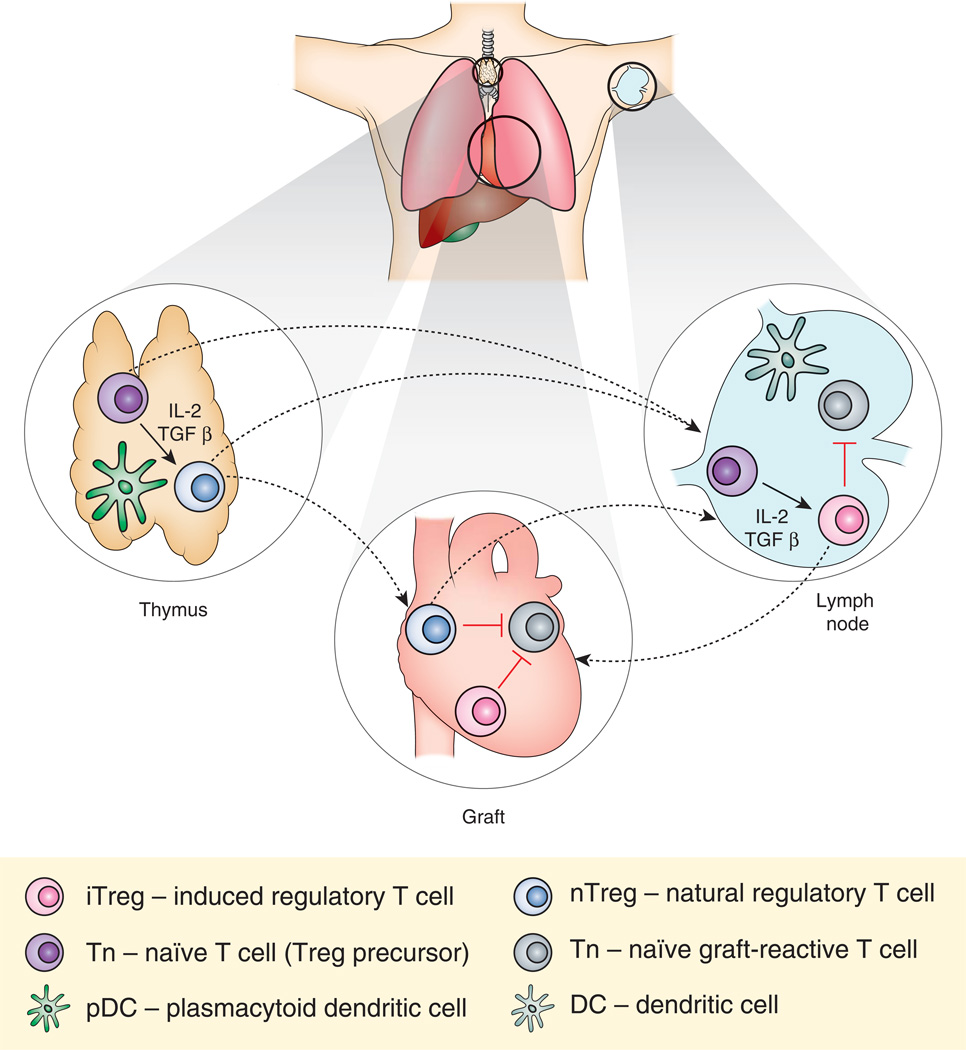
Figure.
In the thymus, naïve T cells (Tn) exposed to IL-2 and TGFβ mature into natural regulatory T cells (nTreg). Transplanted antigen may be presented by plasmacytoid dendritic cells (pDC) to generate alloantigen specific nTreg. nTreg can then migrate to the graft or to the lymph node to suppress potentially graft-reactive T cell activation and inflammation. In the lymph node, Tn exposed to IL-2, TGFβ, and donor antigen presented by tolerogenic dendritic cells mature into iTreg. These cells suppress the activation of potentially graft-reactive cells in the lymph node and graft. Dashed lines denote movement of T cells.
Acknowledgments
Support: NIH RO1 AI41428, NIH RO1 AI62765, and NIH R56 AI72039 (all to JSB).
Abbreviations
- APC
antigen presenting cells
- iTreg
induced regulatory T cells
- DST
donor specific transfusion
- DTH
delayed type hypersensitivity
- LAG3
lymphocyte activation gene 3
- LN
lymph node
- mTOR
mammalian target of rapamycin
- nTreg
natural regulatory T cells
- pDC
plasmacytoid dendritic cells
- Tconv
Conventional T cells
- Teff
Effector T cells
- Th
Helper T cells
- TF
Transcription factor
- TLR
Toll-like receptor
- Tol-DC
tolerogenic dendritic cells
- Treg
regulatory T cells
References
- 1.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorantla VS, Schneeberger S, Brandacher G, Sucher R, Zhang D, Lee WP, Zheng XX. T regulatory cells and transplantation tolerance. Transplant Rev (Orlando) 2010;24:147–159. doi: 10.1016/j.trre.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 4.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 7.Malek TR, Yu A, Zhu L, Matsutani T, Adeegbe D, Bayer AL. IL-2 family of cytokines in T regulatory cell development and homeostasis. J Clin Immunol. 2008;28:635–639. doi: 10.1007/s10875-008-9235-y. [DOI] [PubMed] [Google Scholar]
- 8.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 9.Clark LB, Appleby MW, Brunkow ME, Wilkinson JE, Ziegler SF, Ramsdell F. Cellular and molecular characterization of the scurfy mouse mutant. J Immunol. 1999;162:2546–2554. [PubMed] [Google Scholar]
- 10.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–545. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 13.Li XC, Turka LA. An update on regulatory T cells in transplant tolerance and rejection. Nat Rev Nephrol. 2010;6:577–583. doi: 10.1038/nrneph.2010.101. [DOI] [PubMed] [Google Scholar]
- 14.Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R, Nguyen L, Ghodsi A, Adler S, Butcher EC. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36:438–450. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang SM, Tang Q, Bluestone JA. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am J Transplant. 2007;7:1457–1463. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 16.Burrell BE, Bromberg JS. Fates of CD4+ T cells in a tolerant environment depend on timing and place of antigen exposure. Am J Transplant. 2012;12:576–589. doi: 10.1111/j.1600-6143.2011.03879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 18.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, Killeen N, Ishii N, Li XC. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wieckiewicz J, Goto R, Wood KJ. T regulatory cells and the control of alloimmunity: from characterisation to clinical application. Curr Opin Immunol. 2010;22:662–668. doi: 10.1016/j.coi.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng X, Kajigaya S, Solomou EE, Keyvanfar K, Xu X, Raghavachari N, Munson PJ, Herndon TM, Chen J, Young NS. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood. 2008;111:3675–3683. doi: 10.1182/blood-2008-01-130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boenisch O, Lopez M, Elyaman W, Magee CN, Ahmad U, Najafian N. Ex vivo expansion of human Tregs by rabbit ATG is dependent on intact STAT3-signaling in CD4(+) T cells and requires the presence of monocytes. Am J Transplant. 2012;12:856–866. doi: 10.1111/j.1600-6143.2011.03978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broady R, Yu J, Levings MK. ATG-induced expression of FOXP3 in human CD4(+) T cells in vitro is associated with T-cell activation and not the induction of FOXP3(+) T regulatory cells. Blood. 2009;114:5003–5006. doi: 10.1182/blood-2009-04-214437. [DOI] [PubMed] [Google Scholar]
- 24.Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, Mysliwska J, Hellmann A. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127− T regulatory cells. Clin Immunol. 2009;133:22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Peters JH, Hilbrands LB, Koenen HJ, Joosten I. Ex vivo generation of human alloantigen-specific regulatory T cells from CD4(pos)CD25(high) T cells for immunotherapy. PLoS One. 2008;3:e2233. doi: 10.1371/journal.pone.0002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Min WP, Zhou D, Ichim TE, Strejan GH, Xia X, Yang J, Huang X, Garcia B, White D, Dutartre P, Jevnikar AM, Zhong R. Inhibitory feedback loop between tolerogenic dendritic cells and regulatory T cells in transplant tolerance. J Immunol. 2003;170:1304–1312. doi: 10.4049/jimmunol.170.3.1304. [DOI] [PubMed] [Google Scholar]
- 27.Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Semin Immunol. 2011;23:252–263. doi: 10.1016/j.smim.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 29.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 30.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945–1953. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 31.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 32.Ruggenenti P, Perico N, Gotti E, Cravedi P, D'Agati V, Gagliardini E, Abbate M, Gaspari F, Cattaneo D, Noris M, Casiraghi F, Todeschini M, Cugini D, Conti S, Remuzzi G. Sirolimus versus cyclosporine therapy increases circulating regulatory T cells, but does not protect renal transplant patients given alemtuzumab induction from chronic allograft injury. Transplantation. 2007;84:956–964. doi: 10.1097/01.tp.0000284808.28353.2c. [DOI] [PubMed] [Google Scholar]
- 33.Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, Aiello S, Cassis L, Gotti E, Gaspari F, Cattaneo D, Perico N, Remuzzi G. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. J Am Soc Nephrol. 2007;18:1007–1018. doi: 10.1681/ASN.2006101143. [DOI] [PubMed] [Google Scholar]
- 34.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-beta. J Immunol. 2007;179 11 p following 1390. [PubMed] [Google Scholar]
- 36.De Serres SA, Sayegh MH, Najafian N. Immunosuppressive drugs and Tregs: a critical evaluation! Clin J Am Soc Nephrol. 2009;4:1661–1669. doi: 10.2215/CJN.03180509. [DOI] [PubMed] [Google Scholar]
- 37.Miroux C, Morales O, Carpentier A, Dharancy S, Conti F, Boleslowski E, Podevin P, Auriault C, Pancre V, Delhem N. Inhibitory effects of cyclosporine on human regulatory T cells in vitro. Transplant Proc. 2009;41:3371–3374. doi: 10.1016/j.transproceed.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 38.Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, Contag CH, Negrin RS. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108:390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Shi B, Jin H, Xiao L, Chen Y, Qian Y. Low-dose of tacrolimus favors the induction of functional CD4(+)CD25(+)FoxP3(+) regulatory T cells in solid-organ transplantation. Int Immunopharmacol. 2009;9:564–569. doi: 10.1016/j.intimp.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 40.Sewgobind VD, van der Laan LJ, Kho MM, Kraaijeveld R, Korevaar SS, Mol W, Weimar W, Baan CC. The calcineurin inhibitor tacrolimus allows the induction of functional CD4CD25 regulatory T cells by rabbit anti-thymocyte globulins. Clin Exp Immunol. 2010;161:364–377. doi: 10.1111/j.1365-2249.2010.04183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levitsky J, Gallon L, Miller J, Tambur AR, Leventhal J, Flaa C, Huang X, Sarraj B, Wang E, Mathew JM. Allospecific regulatory effects of sirolimus and tacrolimus in the human mixed lymphocyte reaction. Transplantation. 2011;91:199–206. doi: 10.1097/TP.0b013e318200e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 43.Denucci CC, Mitchell JS, Shimizu Y. Integrin function in T-cell homing to lymphoid and nonlymphoid sites: getting there and staying there. Crit Rev Immunol. 2009;29:87–109. doi: 10.1615/critrevimmunol.v29.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 45.Sawitzki B, Lehmann M, Ritter T, Graser E, Kupiec-Weglinski JW, Volk HD. Regulatory tolerance-mediating T cells in transplantation tolerance. Transplant Proc. 2001;33:2092–2093. doi: 10.1016/s0041-1345(01)01960-1. [DOI] [PubMed] [Google Scholar]
- 46.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. 2002;195:1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin CY, Graca L, Cobbold SP, Waldmann H. Dominant transplantation tolerance impairs CD8+ T cell function but not expansion. Nat Immunol. 2002;3:1208–1213. doi: 10.1038/ni853. [DOI] [PubMed] [Google Scholar]
- 49.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 50.Zhang N, Schroppel B, Lal G, Jakubzick C, Mao X, Chen D, Yin N, Jessberger R, Ochando JC, Ding Y, Bromberg JS. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, Niesner U, de la Rosa M, Schmidt CA, Brauer R, Buer J, Scheffold A, Hamann A. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dilek N, Poirier N, Usal C, Martinet B, Blancho G, Vanhove B. Control of Transplant Tolerance and Intragraft Regulatory T Cell Localization by Myeloid-Derived Suppressor Cells and CCL5. J Immunol. 2012;188:4209–4216. doi: 10.4049/jimmunol.1101512. [DOI] [PubMed] [Google Scholar]
- 53.Baan CC, Dijke IE, Weimar W. Regulatory T cells in alloreactivity after clinical heart transplantation. Curr Opin Organ Transplant. 2009;14:577–582. doi: 10.1097/MOT.0b013e32833037e8. [DOI] [PubMed] [Google Scholar]
- 54.Eljaafari A, Badet L, Kanitakis J, Ferrand C, Farre A, Petruzzo P, Morelon E, Dubosson M, Tiberghien P, Dubois V, Martin X, Miossec P, Dubernard JM. Isolation of regulatory T cells in the skin of a human hand-allograft, up to six years posttransplantation. Transplantation. 2006;82:1764–1768. doi: 10.1097/01.tp.0000250937.46187.ca. [DOI] [PubMed] [Google Scholar]
- 55.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, Fan HM, Liu ZM, Neelapu SS, Dong C. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pallandre JR, Brillard E, Crehange G, Radlovic A, Remy-Martin JP, Saas P, Rohrlich PS, Pivot X, Ling X, Tiberghien P, Borg C. Role of STAT3 in CD4+CD25+FOXP3+ regulatory lymphocyte generation: implications in graft-versus-host disease and antitumor immunity. J Immunol. 2007;179:7593–7604. doi: 10.4049/jimmunol.179.11.7593. [DOI] [PubMed] [Google Scholar]
- 60.Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M, Belz GT, Smyth GK, Busslinger M, Nutt SL, Kallies A. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 61.Ochando JC, Yopp AC, Yang Y, Garin A, Li Y, Boros P, Llodra J, Ding Y, Lira SA, Krieger NR, Bromberg JS. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J Immunol. 2005;174:6993–7005. doi: 10.4049/jimmunol.174.11.6993. [DOI] [PubMed] [Google Scholar]
- 62.Bai Y, Liu J, Wang Y, Honig S, Qin L, Boros P, Bromberg JS. L-selectin-dependent lymphoid occupancy is required to induce alloantigen-specific tolerance. J Immunol. 2002;168:1579–1589. doi: 10.4049/jimmunol.168.4.1579. [DOI] [PubMed] [Google Scholar]
- 63.Warnock RA, Askari S, Butcher EC, von Andrian UH. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J Exp Med. 1998;187:205–216. doi: 10.1084/jem.187.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 65.Bushell A, Niimi M, Morris PJ, Wood KJ. Evidence for immune regulation in the induction of transplantation tolerance: a conditional but limited role for IL-4. J Immunol. 1999;162:1359–1366. [PubMed] [Google Scholar]
- 66.van Maurik A, Herber M, Wood KJ, Jones ND. Cutting edge: CD4+CD25+ alloantigen-specific immunoregulatory cells that can prevent CD8+ T cell-mediated graft rejection: implications for anti-CD154 immunotherapy. J Immunol. 2002;169:5401–5404. doi: 10.4049/jimmunol.169.10.5401. [DOI] [PubMed] [Google Scholar]
- 67.Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, Morris PJ, Powrie F, Wood KJ. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 68.Burrell BE, Lu G, Li XC, Bishop DK. OX40 costimulation prevents allograft acceptance induced by CD40-CD40L blockade. J Immunol. 2009;182:379–390. doi: 10.4049/jimmunol.182.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagahama K, Fehervari Z, Oida T, Yamaguchi T, Ogawa O, Sakaguchi S. Differential control of allo-antigen-specific regulatory T cells and effector T cells by anti-CD4 and other agents in establishing transplantation tolerance. Int Immunol. 2009;21:379–391. doi: 10.1093/intimm/dxp005. [DOI] [PubMed] [Google Scholar]
- 70.Karim M, Bushell AR, Wood KJ. Regulatory T cells in transplantation. Curr Opin Immunol. 2002;14:584–591. doi: 10.1016/s0952-7915(02)00379-5. [DOI] [PubMed] [Google Scholar]
- 71.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang CJ, Kenefeck R, Wardzinski L, Attridge K, Manzotti C, Schmidt EM, Qureshi OS, Sansom DM, Walker LS. Cutting edge: cell-extrinsic immune regulation by ctla-4 expressed on conventional T cells. J Immunol. 2012;189:1118–1122. doi: 10.4049/jimmunol.1200972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corse E, Allison JP. Cutting edge: ctla-4 on effector T cells inhibits in trans. J Immunol. 2012;189:1123–1127. doi: 10.4049/jimmunol.1200695. [DOI] [PubMed] [Google Scholar]
- 75.Bickerstaff AA, VanBuskirk AM, Wakely E, Orosz CG. Transforming growth factor-beta and interleukin-10 subvert alloreactive delayed type hypersensitivity in cardiac allograft acceptor mice. Transplantation. 2000;69:1517–1520. doi: 10.1097/00007890-200004150-00055. [DOI] [PubMed] [Google Scholar]
- 76.Bickerstaff AA, Wang JJ, Pelletier RP, Orosz CG. Murine renal allografts: spontaneous acceptance is associated with regulated T cell-mediated immunity. J Immunol. 2001;167:4821–4827. doi: 10.4049/jimmunol.167.9.4821. [DOI] [PubMed] [Google Scholar]
- 77.Josien R, Douillard P, Guillot C, Muschen M, Anegon I, Chetritt J, Menoret S, Vignes C, Soulillou JP, Cuturi MC. A critical role for transforming growth factor-beta in donor transfusion-induced allograft tolerance. J Clin Invest. 1998;102:1920–1926. doi: 10.1172/JCI4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 81.Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, Koni PA, Iwashima M, Munn DH. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol. 2003;171:1652–1655. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 82.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 83.Zheng XX, Markees TG, Hancock WW, Li Y, Greiner DL, Li XC, Mordes JP, Sayegh MH, Rossini AA, Strom TB. CTLA4 signals are required to optimally induce allograft tolerance with combined donor-specific transfusion and anti-CD154 monoclonal antibody treatment. J Immunol. 1999;162:4983–4990. [PubMed] [Google Scholar]
- 84.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 85.Yu G, Fang M, Gong M, Liu L, Zhong J, Feng W, Xiong P, Wang CY, Gong F. Steady state dendritic cells with forced IDO expression induce skin allograft tolerance by upregulation of regulatory T cells. Transpl Immunol. 2008;18:208–219. doi: 10.1016/j.trim.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 86.Sun X, Gong ZJ, Wang ZW, Li T, Zhang JY, Sun HC, Liu S, Huang L, Huang C, Peng ZH. IDO-Competent-DCs Induced by IFN-gamma Attenuate Acute Rejection in rat Liver Transplantation. J Clin Immunol. 2012 doi: 10.1007/s10875-012-9681-4. [DOI] [PubMed] [Google Scholar]
- 87.de Vries VC, Wasiuk A, Bennett KA, Benson MJ, Elgueta R, Waldschmidt TJ, Noelle RJ. Mast cell degranulation breaks peripheral tolerance. Am J Transplant. 2009;9:2270–2280. doi: 10.1111/j.1600-6143.2009.02755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, Noelle RJ. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 89.Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107:452–460. doi: 10.1046/j.1365-2567.2002.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maganto-Garcia E, Bu DX, Tarrio ML, Alcaide P, Newton G, Griffin GK, Croce KJ, Luscinskas FW, Lichtman AH, Grabie N. Foxp3+-inducible regulatory T cells suppress endothelial activation and leukocyte recruitment. J Immunol. 2011;187:3521–3529. doi: 10.4049/jimmunol.1003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bedke T, Pretsch L, Karakhanova S, Enk AH, Mahnke K. Endothelial cells augment the suppressive function of CD4+ CD25+ Foxp3+ regulatory T cells: involvement of programmed death-1 and IL-10. J Immunol. 2010;184:5562–5570. doi: 10.4049/jimmunol.0902458. [DOI] [PubMed] [Google Scholar]
- 92.Koshiba T, Li Y, Takemura M, Wu Y, Sakaguchi S, Minato N, Wood KJ, Haga H, Ueda M, Uemoto S. Clinical, immunological, and pathological aspects of operational tolerance after pediatric living-donor liver transplantation. Transpl Immunol. 2007;17:94–97. doi: 10.1016/j.trim.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 93.Martinez-Llordella M, Puig-Pey I, Orlando G, Ramoni M, Tisone G, Rimola A, Lerut J, Latinne D, Margarit C, Bilbao I, Brouard S, Hernandez-Fuentes M, Soulillou JP, Sanchez-Fueyo A. Multiparameter immune profiling of operational tolerance in liver transplantation. Am J Transplant. 2007;7:309–319. doi: 10.1111/j.1600-6143.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 94.Bunnag S, Allanach K, Jhangri GS, Sis B, Einecke G, Mengel M, Mueller TF, Halloran PF. FOXP3 expression in human kidney transplant biopsies is associated with rejection and time post transplant but not with favorable outcomes. Am J Transplant. 2008;8:1423–1433. doi: 10.1111/j.1600-6143.2008.02268.x. [DOI] [PubMed] [Google Scholar]
- 95.Muthukumar T, Dadhania D, Ding R, Snopkowski C, Naqvi R, Lee JB, Hartono C, Li B, Sharma VK, Seshan SV, Kapur S, Hancock WW, Schwartz JE, Suthanthiran M. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353:2342–2351. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 96.Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol. 2012;4:11–21. doi: 10.1093/jmcb/mjr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL, Riley JL, June CH, Scheinberg P, Douek DC, Miller JS, Wagner JE, Blazar BR. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001809. 83ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 99.Hester J, Schiopu A, Nadig SN, Wood KJ. Low-dose rapamycin treatment increases the ability of human regulatory T cells to inhibit transplant arteriosclerosis in vivo. Am J Transplant. 2012;12:2008–2016. doi: 10.1111/j.1600-6143.2012.04065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boyman O, Krieg C, Letourneau S, Webster K, Surh CD, Sprent J. Selectively expanding subsets of T cells in mice by injection of interleukin-2/antibody complexes: implications for transplantation tolerance. Transplant Proc. 2012;44:1032–1034. doi: 10.1016/j.transproceed.2012.01.093. [DOI] [PubMed] [Google Scholar]
- 101.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 103.Warnecke G, Chapman SJ, Bushell A, Hernandez-Fuentes M, Wood KJ. Dependency of the trans vivo delayed type hypersensitivity response on the action of regulatory T cells: implications for monitoring transplant tolerance. Transplantation. 2007;84:392–399. doi: 10.1097/01.tp.0000269705.94545.3a. [DOI] [PubMed] [Google Scholar]
- 104.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 105.Lim HW, Hillsamer P, Kim CH. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J Clin Invest. 2004;114:1640–1649. doi: 10.1172/JCI22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 107.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 108.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 109.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 110.Nikolova M, Lelievre JD, Carriere M, Bensussan A, Levy Y. Regulatory T cells differentially modulate the maturation and apoptosis of human CD8+ T-cell subsets. Blood. 2009;113:4556–4565. doi: 10.1182/blood-2008-04-151407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 112.Karim M, Feng G, Wood KJ, Bushell AR. CD25+CD4+ regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: antigen-specific reactivation in vivo is critical for bystander regulation. Blood. 2005;105:4871–4877. doi: 10.1182/blood-2004-10-3888. [DOI] [PubMed] [Google Scholar]
- 113.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H. "Infectious" transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 114.Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O'Shea JJ, Shevach EM. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med. 2008;205:1975–1981. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen J, Huoam C, Plain K, He XY, Hodgkinson SJ, Hall BM. CD4(+), CD25(+) T cells as regulators of alloimmune responses. Transplant Proc. 2001;33:163–164. doi: 10.1016/s0041-1345(00)01956-4. [DOI] [PubMed] [Google Scholar]
- 116.Fan Z, Spencer JA, Lu Y, Pitsillides CM, Singh G, Kim P, Yun SH, Toxavidis V, Strom TB, Lin CP, Koulmanda M. In vivo tracking of 'color-coded' effector, natural and induced regulatory T cells in the allograft response. Nat Med. 2010;16:718–722. doi: 10.1038/nm.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Waldmann H, Graca L, Adams E, Fairchild P, Cobbold S. Regulatory T cells in transplantation tolerance. Curr Top Microbiol Immunol. 2005;293:249–264. doi: 10.1007/3-540-27702-1_11. [DOI] [PubMed] [Google Scholar]
- 118.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 119.Fan H, Cao P, Game DS, Dazzi F, Liu Z, Jiang S. Regulatory T cell therapy for the induction of clinical organ transplantation tolerance. Semin Immunol. 2011;23:453–461. doi: 10.1016/j.smim.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 120.You S, Leforban B, Garcia C, Bach JF, Bluestone JA, Chatenoud L. Adaptive TGF-beta-dependent regulatory T cells control autoimmune diabetes and are a privileged target of anti-CD3 antibody treatment. Proc Natl Acad Sci U S A. 2007;104:6335–6340. doi: 10.1073/pnas.0701171104. [DOI] [PMC free article] [PubMed] [Google Scholar]