Abstract
Background
Silymarin is the most commonly used herbal product for chronic liver disease, yet whether silymarin protects against liver disease progression remains unclear.
Aim
To assess the effects of silymarin use on subsequent liver disease progression in 1049 patients of the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis (HALT-C) trial who had advanced fibrosis or cirrhosis and had failed prior peginterferon plus ribavirin treatment.
Methods
Patients recorded their use of similymarin at baseline and were followed for liver disease progression (two point increase in Ishak fibrosis score across baseline, year 1.5, and year 3.5 biopsies) and over 8.65 years for clinical outcomes.
Results
At baseline, 34% of patients had ever taken silymarin, half of whom were current users. Use of silymarin was associated (p <0.05) with male sex; esophageal varices; higher ALT and albumin; and lower AST/ALT ratio, among other features. Baseline users had less hepatic collagen content on study biopsies and had less histologic progression (HR: 0.57, 95%CI: 0.33–1.00; p-trend for longer duration of use=0.026). No effect was seen for clinical outcomes.
Conclusion
Silymarin use among patients with advanced hepatitis C-related liver disease is associated with reduced progression from fibrosis to cirrhosis, but has no impact on clinical outcomes. The HALT-C Trial was registered with Clinicaltrials.gov (#NCT00006164).
Keywords: hepatitis C, cirrhosis, disease progression
Introduction
An estimated 130–170 million individuals are chronically infected with hepatitis C virus worldwide.1 Pegylated interferon and ribavirin treatment results in an approximate sustained virologic response rate of 55%.2,3 But individuals who fail to respond or who are unable to tolerate treatment have few additional options. As such, many patients have turned to complementary and alternative medications (CAM) instead of, or in addition to, standard therapy.4,5
An extract of the milk thistle plant, silymarin (Silybum marianum), has been used to treat chronic liver disease since the time of the ancient Greeks.6 Silymarin is the most commonly used herbal product by individuals with chronic liver disease, and a recent publication from the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis (HALT-C) trial indicated that nearly 1/3 of patients in the trial were former or current users.7 Though the exact chemical composition of preparations vary, silymarin consists of a mixture of flavonoids termed flavonolignans.8 Results from laboratory, animal, and clinical studies suggest that silymarin may have anti-inflammatory,9–11 anti-viral,11–14 anti-oxidant,10,15 and anti-fibrotic effects in the liver.10,16,17 However, clinical efficacy, particularly in the context of chronic hepatitis C is unproven and results from most previous studies, including randomized trials, are inconsistent.5,6
As an a priori hypothesis, information on baseline silymarin use was collected as part of the HALT-C trial. In the current report, we examined the association of baseline silymarin use with subsequent liver disease progression in 1049 patients with advanced chronic hepatitis C.
Patients and Methods
The HALT-C trial was designed to evaluate the efficacy of long-term treatment with low dose peginterferon alfa-2a for patients with hepatitis C-related bridging fibrosis and cirrhosis who had failed standard of care peginterferon plus ribavirin therapy.18 Patients were recruited from ten US medical centers and met the following criteria: detectable HCV RNA; non-response to prior peginterferon/ ribavirin therapy; hepatic bridging fibrosis or cirrhosis on liver biopsy (Ishak fibrosis stage ≥ 2); and the absence of defined exclusion criteria (such as liver disease other than hepatitis C or history of hepatic decompensation or HCC).
Study design
A detailed description of the design of the HALT-C Trial is published.18 Patients whose previous failed treatment had not included peginterferon plus ribavirin were treated with this therapeutic combination as part of the “lead-in” phase of the trial. If patients had detectable HCV RNA at 20 weeks of treatment, they were considered non-responders and included in the randomized phase of the trial. Responders, who had undetectable HCV RNA at 20 weeks of treatment, received peginterferon plus ribavirin treatment for 48 weeks. Patients experiencing breakthrough, defined by detectable HCV RNA between 20 and 48 weeks of treatment, and patients who relapsed after completion of 48 weeks of therapy could also enroll in the randomized phase of the trial. Finally, patients who upon recruitment had already failed peginterferon plus ribavirin therapy were immediately entered into the randomized phase of the trial (express patients).
During the randomized phase of the trial, patients were randomized to peginterferon alfa-2a 90 mcg weekly or no treatment. Liver biopsies were repeated 1.5 and 3.5 years after randomization. A panel of twelve hepatic pathologists reviewed all biopsies and scored inflammation (0–18) and fibrosis (0–6) using the Ishak scoring system.19 As peginterferon therapy did not affect clinical outcome or histologic progression,18 treated and untreated participant data from the randomized phase of the trial were combined.
Assessment of silymarin use
At baseline, trained study coordinators obtained patient medication use by way of an in- person interview. In addition to assessing prescription and non-prescription drugs, interviewers assessed CAM (herbal medications, dietary supplements, and botanical products). Ever users had used CAM at least once a week for one month or longer in their lives. Duration of use was also recorded. To facilitate recall, patients were shown a card indicating 37 examples of herbal products in alphabetical order. Silymarin was one of these products. Patients also had the opportunity to indicate use of herbal products not listed on the card. Every three months after baseline, and as often as every two weeks during the lead-in phase, patients were asked whether they had stopped using any herbal products since their last visit or whether they were taking any new herbal products. Of 1,050 randomized participants, we excluded one patient who lacked a medication record. We considered current users to be those using silymarin on the day of study randomization. Former users could have used silymarin at any time prior to randomization, including the lead-in phase. Duration of silymarin use included all months of use up to the day of randomization. To analyze duration, we created a three level variable which included never users as the reference category. Months of use were then split at the median (16.6 months).
Morphometric image analysis of hepatic collagen content
Collagen in liver biopsy sections was stained with Sirius red and the degree of staining, which is proportional to the collagen content, was assessed by Image Pro Plus 6.0 imaging software (Media Cybernetics, Silver Spring, MD) as previously described.20,21 Sirius red values are expressed in arbitrary units but reflect collagen content on a continuous scale. Analyses of collagen content were restricted to unfragmented biopsies with more than 10 mm2 of liver tissue in the section in order to avoid artifact and to reduce sampling variability. In total, 558 patients had morphometric image analysis performed on baseline biopsies, 550 patients had year 1.5 biopsies assessed by morphometry and 409 patients had year 3.5 biopsies analyzed in this way. Of these patients, 183 had all three biopsies read.
Assessment of fibrosis and clinical outcomes
Patients were seen every three months in the randomized phase, at which point complete blood counts, a liver chemistry panel, and alpha-fetoprotein were tested at each clinical site. Patients also had at least one abdominal ultrasound examination every 12 months. Clinical outcomes included ascites, Child-Turcotte-Pugh score of ≥7 22 on two consecutive study visits, liver disease related death, hepatic encephalopathy, hepatocellular carcinoma (HCC), spontaneous bacterial peritonitis, or variceal hemorrhage. Outcome reports were reviewed by an Outcomes Review Panel consisting of three investigators from the participating clinical centers. Participants with bridging fibrosis at baseline and a ≥ 2 point increase in Ishak fibrosis score (TPI) on either of the follow-up biopsies were considered to have fibrosis progression. Results are presented separately for clinical outcomes and for a two point increase in Ishak fibrosis score as well as for the two endpoints combined. HCC was diagnosed via ultrasound, AFP, and histologic confirmation as previously described.23
All details of this study were approved by the local Institutional Review Board at each participating institution and all participants gave written informed consent.
Statistical Analyses
We performed analyses with SAS release 9.1 (SAS Institute, Cary, NC). All tests were two-sided and an alpha level of <0.05 was considered statistically significant.
We tabulated baseline demographic, behavioral, and clinical factors by categories of silymarin use (never, former, and current). Statistically significant variation across categories of silymarin use was assessed for categorical variables by the Mantel-Haenszel test for trend and for continuous variables with the Jonckheere-Terpstra test of trend.
Relative risks and 95% confidence intervals for the association of silymarin use with disease progression were calculated by use of Cox proportional hazards regression.24 Person-time was calculated from baseline to first outcome, end of study, or date of patient withdrawal. For clinical outcomes and the combined endpoint, follow-up time was for up to 8.65 years. For TPI, follow-up was until first (1.5 years) or second biopsy (3.5 years). For clinical outcomes, Kaplan-Meier curves were generated for never, former, and current silymarin users and were compared with the log-rank test.
Linear trend tests across increasing categories of silymarin use were performed by creating an ordinal variable for each category and entering the term as a continuous variable into the regression model. We tested the proportional hazards assumption by modeling interaction terms of time with trend variables for silymarin use. No significant deviations were found for silymarin use with either TPI or clinical outcomes; though a significant deviation was found for HCC (p=0.011).
Relative risks for liver disease progression were estimated from crude models and two different multivariate adjusted models. The first multivariate model included continuous age, lifetime alcohol use, and coffee intake along with categorical variables for education (high school or less, some post high school, completed college), race/ethnicity (Caucasian, African American, Hispanic, and other), sex, diabetes, and ever use of other herbal products besides silymarin, such as green tea, garlic, ginseng, or echinacea. A second multivariate model was additionally adjusted for continuous mental and physical short-form (SF)-36 summary quality of life scores, 25 and serological predictors of liver disease progression26-- AST/ALT ratio, albumin, platelets, bilirubin, as well as categorical variables for cirrhosis status at baseline and presence or absence of esophageal varices. For analyses of silymarin use during the trial, we updated silymarin use at the time of each follow-up biopsy. As for the main analyses, patients with a TPI at biopsy one (year 1.5) were censored at this time.
We assessed possible effect modification (interaction) by randomization group, cirrhosis at baseline, median mental, and physical SF-36 quality of life scores, and sex using stratification. Risk estimates did not vary by stratification group (p>0.23 for all). Results stratified by cirrhosis at baseline are presented in the results section.
Finally, we analyzed changes in morphometric collagen content across study biopsies using repeated analysis of variance, assuming an autoregressive covariance structure with the PROC MIXED function of SAS 9.1. Again, analyses were restricted to patients who had not had an outcome prior to a particular scheduled study biopsy. Time (baseline, biopsy one, and biopsy two) and silymarin use (former and current) were included in the model as fixed effects. Adjustment for age and sex did not alter risk estimates and so were not included in the final models. Possible differences between the collagen content of biopsies taken from former or current silymarin users were compared with the collagen content of biopsies taken from never silymarin users by the Mann-Whitney test.
Results
At baseline, 17.0% (178/1049) of patients were former users of silymarin and 16.2% (170/1049) of patients were current users compared to 66.8% (701/1049) who reported never using silymarin (Table 1). The median duration of use for current users up to study entry was 35 months, whereas the median duration of use for former users was 6 months. Baseline silymarin use was associated with Caucasian race, completing college, male sex, lower prevalence of diabetes mellitus, higher lifetime alcohol and coffee consumption, and higher physical quality of life score. Silymarin was also modestly associated with a lower AST/ALT ratio and alkaline phosphatase levels and higher ALT, albumin levels, and prevalence of esophageal varices (p <0.05 for all). No association was observed for age, treatment group, patient cohort, body mass index, mental summary SF-36 score, serum AST, bilirubin, platelets, prothrombin time, HCV genotype or log RNA level, hepatic cirrhosis, collagen content, steatosis grade, or Ishak inflammation score.
Table 1.
Baseline demographic, clinical, and lab features of 1049 participants of the HALT-C trial by category of silymarin use.
| Variables | ||||
|---|---|---|---|---|
|
| ||||
| Never | Former | Current | P for trend* | |
|
| ||||
| Number in cohort, No. (%) | 701 (66.8) | 178 (17.0) | 170 (16.2) | |
| Duration of silymarin use, months, Median (IQR) | 0 | 6 (3–12) | 35 (20–53) | |
| Age, years, Median (IQR) | 50 (46–54) | 48 (45–53) | 50 (47–53) | 0.52 |
| Gender, female, No. (%) | 216 (70.8) | 52 (17.1) | 37 (12.1) | 0.027 |
| Race/ethnicity, Caucasian, No. (%) | 474 (63.1) | 134 (17.8) | 143 (19.0) | 0.001 |
| Treatment group, No. (%) | 337 (65.3) | 93 (18.0) | 86 (16.7) | 0.41 |
| Source of patient randomized | ||||
| Lead-in non-responder | 431 (65.1) | 122 (18.4) | 109 (16.5) | 0.11 |
| Lead-in breakthrough or relapse | 97 (64.2) | 27 (17.9) | 27 (17.9) | |
| Express | 173 (73.3) | 29 (12.3) | 34 (14.4) | |
| Education†, Completed college, No. (%) | 164 (60.1) | 51 (18.7) | 58 (21.3) | <.0001 |
| Lifetime alcohol consumption, # of drinks, Median (IQR) | 6758 (972–21,168) | 7622 (1850–20,398) | 9580 (1644–27,512) | 0.035 |
| Coffee intake,† drinks/day, Median (IQR) | 1 (0.03–2) | 1 (0.3–2) | 1 (0.3–2) | 0.023 |
| Body Mass Index, Median (IQR) | 29.3 (26.3–32.8) | 29.4 (26.3–32.8) | 28.5 (25.8–31.8) | 0.23 |
| Diabetes, Glucose ≥ 126 mg/dl, No. (%) | 186 (73.2) | 36 (14.2) | 32 (12.6) | 0.016 |
| Mental summary score from SF-36,† Median (IQR) | 53 (46–57) | 51 (45–56) | 53 (47–57) | 0.25 |
| Physical summary score from SF-36,† Median (IQR) | 47 (35–54) | 47 (37–54) | 50 (41–54) | 0.038 |
|
| ||||
| AST, U/L, Median (IQR) | 70 (50–102) | 72 (54–124) | 71 (49–113) | 0.24 |
| ALT, U/L, Median (IQR) | 82 (57–123) | 94 (67–140) | 90 (60–139) | 0.009 |
| AST/ALT Ratio, Median (IQR) | 0.82 (0.69–1.04) | 0.79 (0.64–1.00) | 0.80 (0.64–0.94) | 0.004 |
| Albumin, g/dL, Median (IQR) | 3.9 (3.6–4.1) | 3.9 (3.6–4.1) | 4.0 (3.7–4.2) | 0.018 |
| Alk. Phos. U/L, Median (IQR) | 90 (71–118) | 89 (71–113) | 84 (68–106) | 0.012 |
| Bilirubin, mg/dL, Median (IQR) | 0.7 (0.5–0.9) | 0.7 (0.5–0.9) | 0.8 (0.5–1.0) | 0.40 |
| Platelets, 1000/mL, Median (IQR) | 159 (116–208) | 161 (114–205) | 158 (119–197) | 0.42 |
| Prothrombin time, INR, Median (IQR) | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 0.49 |
| Log10 HCV RNA level, Median (IQR) | 6.5 (6.1–6.8) | 6.5 (6.2–6.8) | 6.6 (6.2–6.8) | 0.58 |
| HCV genotype 1, No. (%) | 649 (66.3) | 167 (17.1) | 163 (16.7) | 0.12 |
|
| ||||
| Cirrhosis on biopsy, No. (%) | 280 (65.4) | 74 (17.3) | 74 (17.3) | 0.38 |
| Hepatic steatosis, ≥ Grade 2 | 279 (64.0) | 88 (20.2) | 69 (15.8) | 0.38 |
| Ishak inflammation score, Median (IQR) | 7 (6–9) | 7 (6–9) | 8 (6–9) | 0.67 |
| Esophageal varices†, No. (%) | 158 (60.5) | 52 (19.9) | 51 (19.5) | 0.021 |
| Collagen content†, Median (IQR) | 0.04 (0.02–0.08) | 0.04 (0.02–0.09) | 0.04 (0.02–0.07) | 0.36 |
Abbreviations: No: Number; IQR: Interquartile range; AST, aspartate aminotransferase; ALT, alanine aminotransferase; Alk. Phos., Alkaline phosphatase; HCV, hepatitis C.
Mantel-Haenszel test for trend for categorical variables. Jonckheere-Terpstra test for trend for continuous variables.
Data not available for all participants: Coffee was available for 791 participants; Collagen content for 558 patients; Education available for 1045 participants; Esophageal varices for 1016 participants; SF-36 scores for 1043 participants.
At baseline, 621 patients had fibrosis and 428 patients had cirrhosis. During 4,758 person-years of follow-up (median: 5.5 years per patient, interquartile range: 3.0–6.6 years), 384 patients had a two point increase in fibrosis score (TPI) from baseline or had a clinical outcome for liver disease. Combining these endpoints, we observed an inverse association between baseline silymarin use and liver disease progression (Table 2). In crude models, the relative risk (RR) associated with former use of silymarin was 0.87 (95% CI: 0.66–1.15) whereas the RR for current use was 0.73 (95% CI: 0.54–0.98; p-trend across categories= 0.029). Upon stratification by outcome, current silymarin use was associated with less TPI (RR for current vs. never use of silymarin, 0.54, 95% CI: 0.32–0.93; p-trend= 0.015), but had no association with clinical outcomes (RR for current vs. never, 0.86, 0.61–1.20; p-trend= 0.42). Multivariate adjustment for age, education, race/ethnicity, sex, lifetime alcohol use, diabetes, coffee intake, ever use of other herbal products besides silymarin, mental and physical quality of life scores, baseline cirrhosis, AST/ALT ratio, albumin, platelets, bilirubin, and esophageal varices only modestly affected risk estimates. After multivariate adjustment, the RR for current versus never use of silymarin was 0.57 (95% CI: 0.33–1.00; p-trend=0.042) for TPI and 1.09 (95% CI: 0.77–1.56; p-trend=0.89) for clinical outcomes.
Table 2.
Association of silymain use with liver disease progression in 1049 participants of the HALT-C trial.
| Categories | Non-users | Former users | Current users | p-trend | |
|---|---|---|---|---|---|
|
| |||||
| TPI + clinical outcomes* | Cohort, No. (%) | 661 (66.6) | 165 (16.6) | 167 (16.8) | |
| Case, No. (%) | 271 (70.6) | 60 (15.6) | 53 (13.8) | ||
| Crude, RR (95% CI) | 1.00 (Ref) | 0.87 (0.66–1.15) | 0.73 (0.54–0.98) | 0.029 | |
| Multivariate #1,† RR (95% CI) | 1.00 (Ref) | 0.89 (0.67–1.18) | 0.73 (0.54–0.99) | 0.038 | |
| Multivariate #2,‡ RR (95% CI) | 1.00 (Ref) | 0.74 (0.56–0.99) | 0.84 (0.62–1.13) | 0.089 | |
|
| |||||
| TPI | Cohort, No. (%) | 368 (67.3) | 91 (16.6) | 88 (16.1) | |
| Case, No. (%) | 116 (76.3) | 21 (13.8) | 15 (9.9) | ||
| Crude, RR (95% CI) | 1.00 (Ref) | 0.75 (0.47–1.19) | 0.54 (0.32–0.93) | 0.015 | |
| Multivariate #1,† RR (95% CI) | 1.00 (Ref) | 0.75 (0.47–1.20) | 0.56 (0.32–0.97) | 0.025 | |
| Multivariate #2,‡ RR (95% CI) | 1.00 (Ref) | 0.82 (0.51–1.32) | 0.57 (0.33–1.00) | 0.042 | |
|
| |||||
| Clinical outcomes | Cohort, No. (%) | 701 (66.8) | 178 (17.0) | 170 (16.2) | |
| Case, No. (%) | 187 (68.3) | 46 (16.8) | 41 (15.0) | ||
| Crude, RR (95% CI) | 1.00 (Ref) | 1.00 (0.73–1.38) | 0.86 (0.61–1.20) | 0.42 | |
| Multivariate #1,† RR (95% CI) | 1.00 (Ref) | 1.07 (0.77–1.48) | 0.89 (0.63–1.26) | 0.65 | |
| Multivariate #2,‡ RR (95% CI) | 1.00 (Ref) | 0.77 (0.54–1.08) | 1.09 (0.77–1.56) | 0.89 | |
Abbreviations: TPI, two point increase in Ishak fibrosis score; No, Number; RR, relative risk; CI, confidence interval.
Excluding 56 participants with fibrosis at baseline who did not receive follow-up biopsies or have a clinical outcome.
Adjusted for continuous age, lifetime alcohol use, and coffee intake along with categorical variables for education (high school or less, some post high school, completed college), race/ethnicity (Caucasian, African American, Hispanic, and other), sex, diabetes, and ever use of other herbal products besides silymarin, such as green tea, garlic, ginsing, and Echinacea.
Additionally adjusted for continuous mental and physical SF-36 quality of life scores, AST/ALT ratio, albumin, platelets, bilirubin, as well as categorical variables for cirrhosis status at baseline and presence or absence of esophageal varices.
Duration of silymarin use, prior to baseline, was also assessed. Relative to never users, patients who used silymarin for up to the median duration (16.6 months) had a RR for TPI of 0.93 (0.58–1.51), whereas patients who used silymarin for greater than the median duration had a RR of 0.51 (95% CI: 0.30–0.90; p-trend=0.026). The RRs for clinical outcomes for the same two categories of silymarin use were 0.86 (95%CI: 0.61–1.21) and 0.94 (95% CI: 0.66–1.35; p-trend=0.57) respectively (data not shown in table).
In addition to silymarin use at baseline, we examined silymarin use over the course of the study. At the time of the second biopsy, three and a half years after randomization, 69% of baseline users continued to use silymarin (88/128), whereas only 3% of baseline non-users (15/477) had started use. The risk of TPI among patients with fibrosis who continued to use silymarin throughout the study was 0.55 (95%CI: 0.29–1.03; 68 patients, 11 events), whereas the risk of TPI among patients who stopped using silymarin during follow-up was 0.66 (95%CI: 0.23–1.87; 20 patients, 4 events) (data not shown in table).
Silymarin was the most commonly used herbal product in the HALT-C trial. Fourteen percent of patients used an herbal product other than silymarin (n=142). Use of a non-silymarin herbal product had no association with either TPI (0.92, 95% CI: 0.57–1.48) or clinical outcomes (0.87, 95% CI: 0.60–1.25) (data not shown in table).
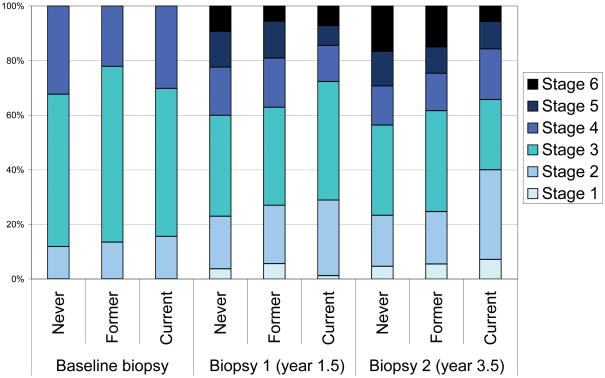
Among those with fibrosis, comparing current users of silymarin to never users, the RR for TPI was 0.19 (95%CI: 0.02–2.05; 16 events) for patients with an Ishak score of 2 at baseline, 0.48 (95%CI: 0.22–1.04; 87 events) for patients with an Ishak score of 3 at baseline, and 1.04 (95% CI: 0.37–2.90; 49 events) for patients with an Ishak score of 4 at baseline (data not shown in table). For those with fibrosis at baseline, we also examined the distribution of Ishak scores at year 1.5 and year 3.5 protocol biopsies. The distribution of Ishak scores was similar between former and never silymarin users for both biopsies (p>0.30). For current silymarin users vs. never silymarin users, p-values for differences in the distribution of Ishak scores were 0.097 and 0.0059, for year 1.5 and year 3.5 biopsies, respectively (Figure 1).
Figure 1.
Distribution at each biopsy for 621 patients without cirrhosis at baseline (Ishak score of 2, 3, or 4). Repeat protocol biopsies were performed 1.5 years (Biopsy 1, 524 patients) and 3.5 years after randomization (Biopsy 2, 443 patients). The p-values for the distribution of Ishak scores of former vs. never silymarin users were 0.086, 0.30, and 0.47 for baseline, biopsy 1, and biopsy 2, respectively. For current silymarin users vs. never silymarin users, p-values for the distribution of Ishak scores at each biopsy were 0.42, 0.097, and 0.0059, respectively.
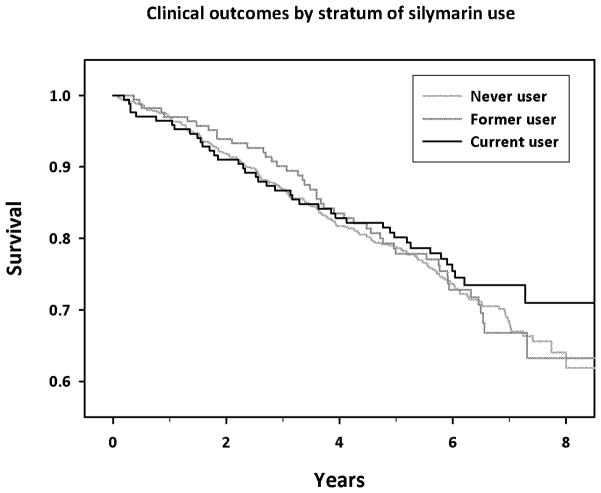
No association for clinical outcomes was found for either those with fibrosis or cirrhosis at baseline (RR for current use vs. never use, 1.36, 95% CI: 0.74–2.50, p-trend=0.32, 98 events and 0.97, 95% CI: 0.61–1.53, p-trend=0.41, 176 events, for fibrosis and cirrhosis respectively). The association between silymarin and clinical outcomes was also similar for outcomes occurring during years zero through four (RR for current vs. never use, 1.20, 95% CI: 0.78–1.86; p-trend = 0.85, 173 events) and five through eight of follow-up (0.88, 95% CI: 0.47–1.63; p-trend=0.88, 101 events) (data not shown in table). Kaplan-Meier curves for clinical outcomes among current, former, and never users of silymarin were similar (Figure 2; p=0.657). In a secondary analysis of 88 incident cases of HCC, relative to never use, the RR for former and current users was 1.15 (95%CI: 0.62–2.13) and 1.60 (95%CI: 0.93–2.76) respectively. This possible effect was restricted to events occurring during the first four years (HR: 1.96, 95%CI: 0.95–4.05; 47 events), but not years five-eight of follow-up (HR: 1.26, 95%CI: 0.54–2.94; 41 events).
Figure 2.
Kaplan-Meier survival analysis of time to first clinical outcome by stratum of baseline silymarin use (never, former, and current; P=0.657).
Finally, we examined the association between silymarin use and biopsy collagen content as measured by morphometric image analysis (Table 3). The collagen content of each study biopsy appeared generally similar in former and never users of silymarin. But, the study biopsies of baseline silymarin users tended to have lower collagen content than study biopsies of never users. For example, the mean collagen content on the year 3.5 biopsy was 0.071 (standard deviation=0.069) among current silymarin users and 0.090 (standard deviation=0.085) among never users, p-value=0.061. The overall p-value comparing the change in collagen content across repeated biopsies in baseline silymarin users relative to never users was 0.037. After stratification by baseline cirrhosis status, the association between silymarin use with change in collagen content across repeated study biopsies persisted in both patients with fibrosis (overall p-value=0.034) and cirrhosis (overall p-value=0.011) at baseline.
Table 3.
Morphometric collagen content at each biopsy by stratum of silymarin use
| Baseline Biopsy | Biopsy 1 (year 1.5) | Biopsy 2 (year 3.5) | Overall P-value across repeated biopsies‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Silymarin use | Mean (SD) | No. | P-value relative to never users† | Mean (SD) | No. | P-value relative to never users† | Mean (SD) | No. | P-value relative to never users† | |
| All patients | ||||||||||
| Never (No.=701) | 0.061 (0.061) | 361 | Referent group | 0.088 (0.079) | 358 | Referent group | 0.090 (0.085) | 271 | Referent group | Referent group |
| Former (No.=178) | 0.062 (0.058) | 113 | 0.82 | 0.089 (0.092) | 100 | 0.81 | 0.077 (0.087) | 69 | 0.15 | 0.93 |
| Current (No.=170) | 0.054 (0.056) | 84 | 0.17 | 0.068 (0.053) | 92 | 0.10 | 0.071 (0.069) | 69 | 0.061 | 0.037 |
|
| ||||||||||
| Fibrosis at baseline | ||||||||||
| Never (No.=421) | 0.039 (0.036) | 231 | Referent group | 0.070 (0.064) | 239 | Referent group | 0.069 (0.065) | 182 | Referent group | Referent group |
| Former (No.=104) | 0.037 (0.028) | 71 | 0.95 | 0.070 (0.088) | 64 | 0.38 | 0.064 (0.072) | 48 | 0.41 | 0.53 |
| Current (No.=96) | 0.031 (0.027) | 46 | 0.15 | 0.063 (0.057) | 58 | 0.25 | 0.043 (0.036) | 42 | 0.018 | 0.034 |
|
| ||||||||||
| Cirrhosis at baseline | ||||||||||
| Never (No.=280) | 0.101 (0.075) | 130 | Referent group | 0.124 (0.092) | 119 | Referent group | 0.134 (0.103) | 89 | Referent group | Referent group |
| Former (No.=74) | 0.105 (0.070) | 42 | 0.60 | 0.122 (0.089) | 36 | 0.97 | 0.107 (0.110) | 21 | 0.14 | 0.98 |
| Current (No.=74) | 0.081 (0.069) | 38 | 0.052 | 0.077 (0.045) | 34 | 0.023 | 0.115 (0.086) | 27 | 0.48 | 0.011 |
Abbreviations: SD, standard deviation; No, number.
Assessment of morphometric collagen content was limited to biopsies with at least 10 mm2 of tissue, including 558 biopsies at baseline, 550 biopsies at year 1.5, and 409 biopsies at year 3.5.
Mann-Whitney test for the difference between mean collagen content in former or current silymarin users relative to never users.
P-value for the overall effect of silymarin use on morphometric collagen content across repeated biopsies was assessed by repeated-measures analysis of variance assuming an autoregressive covariance structure. Time (baseline, biopsy 1, and biopsy 2) and silymarin use were included in the models as fixed effects.
Discussion
In a large prospective cohort of individuals with advanced hepatitis C- related chronic liver disease, no clinical benefit was found for current silymarin use at baseline. In addition, we observed a non-significant increase in HCC risk among current silymarin users, which was present only in the first four years of follow-up. Baseline silymarin use was associated with less liver disease progression as measured by a two-point increase in Ishak fibrosis score as well as in the distribution of fibrosis scores in follow-up biopsies. A dose-response with duration of use was observed. Current use of silymarin at baseline, but not former use prior to baseline, was associated inversely with biopsy collagen content, regardless of whether patients had fibrosis or cirrhosis.
Silymarin has been used to treat liver disease for thousands of years.6,27 Furthermore, results from animal, in vitro, and clinical studies suggest that silymarin has possible anti-inflammatory,9–11 anti-viral,11–14 anti-oxidant,10,15 and anti-fibrotic effects.10,16,17 Yet, few clinical and observational studies have evaluated the effect of silymarin on liver disease progression and clinical outcomes in humans. Prior studies had small size, limited power to detect associations, and yielded mixed results.6,27 For example, one trial of 170 patients with alcohol-related liver disease showed an effect of silymarin on survival,28 whereas a second trial of 200 patients showed similar survival rates in the randomized and control arms.29 Even less data are available for hepatitis C-related liver disease. Data from an Egyptian randomized trial of 141 patients showed no effect for silymarin on outcomes.30,31
It is not clear why silymarin was associated with a reduction in rate of fibrosis progression, but not with clinical outcomes in our study. One possibility is that in order to exert an effect, silymarin must be used early in the disease progression process. In support of this hypothesis, silymarin seemed to have an effect on histologic progression if patients had an Ishak score of 2 or 3 at baseline, but no effect on individuals with a score of 4 at baseline. On the other hand, silymarin had no effect on clinical outcomes for either individuals with cirrhosis or fibrosis at baseline, or for outcomes occurring during the first four years, or years five-eight of follow-up. It remains possible, however, that follow-up was too short to see an effect on clinical outcomes.
Fibrosis progression is not the sole determinant of subsequent decompensation or complications of portal hypertension. As such, it is also possible that silymarin does not have a beneficial effect on other determinants of clinical outcomes.32 Alternatively, differences between histologic progression and clinical outcomes could simply reflect chance.
Strengths of our study include assessment of silymarin use before disease progression, the large number of patients with advanced hepatitis C-related liver disease, comprehensive assessment of clinical, demographic, and lifestyle information, and careful assessment of clinical and histologic outcomes. The major limitation was a complete lack of information on the amount of silymarin patients used per day. We also lacked information on how silymarin was prepared. Patients in the HALT-C trial likely used many different dosages and formulations of silymarin and even for individual patients, preparations likely varied day by day and week by week. Furthermore, it is unlikely that patients would have ingested pharmacologic doses of silymarin as have shown effect in vitro, clinical, and animal studies. For example, a recent study of 36 patients observed an effect of intravenously (IV) administered silymarin (as silibinin) on hepatitis C viral level,14 though a study with similar dosing of orally administered silymarin showed no effect.33 Most likely, patients in HALT-C used less silymarin than in the IV study. Further complicating interpretation, the pharmacokinetics of silymarin may be altered by fibrosis. A recent study administered a standard silymarin dose to cirrhotics and healthy volunteers. In response to silymarin treatment, serum flavonolignans were higher in the cirrhotic volunteers.34 Finally, not all study biopsies were large enough to have morphometric analysis performed, a potential source of selection bias. Indeed, patients with cirrhosis were less likely to receive all biopsies.21 Yet, since we observed an apparent inverse association between silymarin use and collagen content, such a bias, if anything, would likely attenuate the observed association between silymarin use and collagen content.
In the HALT-C trial, use of silymarin was associated with Caucasian race, completing college, and a higher SF-36 physical quality of life score, suggesting that silymarin use might be a marker for a large number of other lifestyle factors. We adjusted our risk estimates for these and other possible confounders. After adjustment, risk estimates were only modestly altered. In addition, the observed effect of silymarin does not simply reflect a propensity to use herbal products. Using herbal products, other than silymarin, had no association with either histologic progression or clinical outcomes in our study. Nevertheless, as an observational study, the inverse association observed between silymarin use and histologic progression could reflect another exposure or chance. We did not have any information on brand or dosage of silymarin. However, this limitation is reflective of the difficulty in detailing patient behavior outside of controlled studies, Many, if not most, patients with currently incurable liver disease seek alternative, unapproved therapies that can not be easily quantified, yet deserve evaluation.
In summary, among individuals with advanced hepatitis-C associated liver disease, we observed an inverse association between silymarin use and the progression of liver disease from fibrosis to cirrhosis, but no evidence for an effect on clinical outcomes. As our results are from an observational study, it is possible that the observed beneficial effect on liver disease progression is due to chance. Future studies with comprehensive assessment of silymarin dose are needed to replicate these findings. Nevertheless our results provide support for conducting additional studies of silymarin, including intervention trials with defined dosage regimens and standard silymarin product. Such studies would be most appropriate for patients who have not responded or are not candidates for antiviral therapy and have limited other treatment options. Importantly, our results do not support the use of ad hoc dosing of silymarin by patients with chronic liver disease.
Acknowledgments
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
University of Massachusetts Medical Center, Worcester, MA: (Contract N01-DK-9-2326) Gyongyi Szabo, MD, Barbara F. Banner, MD, Maureen Cormier, RN, Donna Giansiracusa, RN
University of Connecticut Health Center, Farmington, CT: (Grant M01RR-06192) Herbert L. Bonkovsky, MD, Gloria Borders, RN, Michelle Kelley, RN, ANP
Saint Louis University School of Medicine, St Louis, MO: (Contract N01-DK-9-2324) Adrian M. Di Bisceglie, MD, Bruce Bacon, MD, Brent Neuschwander-Tetri, MD, Elizabeth M. Brunt, MD, Debra King, RN
Massachusetts General Hospital, Boston, MA: (Contract N01-DK-9-2319, Grant M01RR-01066; Grant 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center) Jules L. Dienstag, MD, Raymond T. Chung, MD, Andrea E. Reid, MD, Atul K. Bhan, MD, Wallis A. Molchen, David P. Lundmark
University of Colorado Denver, School of Medicine, Aurora, CO: (Contract N01-DK-9-2327, Grant M01RR-00051, Grant 1 UL1 RR 025780-01), Gregory T. Everson, MD, Thomas Trouillot, MD, Marcelo Kugelmas, MD, S. Russell Nash, MD, Jennifer DeSanto, RN, Carol McKinley, RN
University of California - Irvine, Irvine, CA: (Contract N01-DK-9-2320, Grant M01RR-00827) Timothy R. Morgan, MD, John C. Hoefs, MD, John R. Craig, MD, M. Mazen Jamal, MD, MPH, Muhammad Sheikh, MD, Choon Park, RN
University of Texas Southwestern Medical Center, Dallas, TX: (Contract N01-DK-9-2321, Grant M01RR-00633, Grant 1 UL1 RR024982-01, North and Central Texas Clinical and Translational Science Initiative) William M. Lee, MD, Thomas E. Rogers, MD, Peter F. Malet, MD, Janel Shelton, Nicole Crowder, LVN, Rivka Elbein, RN, BSN, Nancy Liston, MPH
University of Southern California, Los Angeles, CA: (Contract N01-DK-9-2325, Grant M01RR-00043) Karen L. Lindsay, MD, MMM, Sugantha Govindarajan, MD, Carol B. Jones, RN, Susan L. Milstein, RN
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042, Grant 1 UL1 RR024986, Michigan Center for Clinical and Health Research) Anna S. Lok, MD, Robert J. Fontana, MD, Joel K. Greenson, MD, Pamela A. Richtmyer, LPN, CCRC, R. Tess Bonham, BS
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Mitchell L. Shiffman, MD, Richard K. Sterling, MD, MSc, Melissa J. Contos, MD, A. Scott Mills, MD, Charlotte Hofmann, RN, Paula Smith, RN
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD: Marc G. Ghany, MD, T. Jake Liang, MD, David Kleiner, MD, PhD, Yoon Park, RN, Elenita Rivera, RN, Vanessa Haynes-Williams, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: Patricia R. Robuck, PhD, Jay H. Hoofnagle, MD
University of Washington, Seattle, WA: (Contract N01-DK-9-2318) David R. Gretch, MD, PhD, Minjun Chung Apodaca, BS, ASCP, Rohit Shankar, BC, ASCP, Natalia Antonov, M. Ed.
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Kristin K. Snow, MSc, ScD, Anne M. Stoddard, ScD, Margaret C. Bell, MS, MPH
Armed Forces Institute of Pathology, Washington, DC: Fanny Monge, Michelle Parks
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia-Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perrillo, MD
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute, the National Center for Minority Health and Health Disparities and by General Clinical Research Center and Clinical and Translational Science Center grants from the National Center for Research Resources, National Institutes of Health (grant numbers are listed in the Acknowledgement). This research was also supported in part by the Intramural Research Program of the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Additional funding to conduct this study was supplied by Hoffmann-La Roche, Inc., through a Cooperative Research and Development Agreement (CRADA) with the National Institutes of Health.
List of abbreviations
- HALT-C
Hepatitis C Antiviral Long-Term Treatment against Cirrhosis
- AFP
alpha-fetoprotein
- RR
relative risk
- CI
confidence interval
- HCC
hepatocellular carcinoma
- TPI
two point increase in Ishak fibrosis score
Footnotes
This is publication #55 of the HALT-C Trial.
Statement of Interests:
Authors with no financial relationships related to this project are: Neal D. Freedman, Teresa M. Curto, Chihiro Morishima, Leonard B. Seeff, Zachary D. Goodman, Elizabeth C. Wright, Rashmi Sinha, James E. Everhart.
References
- 1.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29 (Suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 2.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 3.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 4.Stickel F, Schuppan D. Herbal medicine in the treatment of liver diseases. Dig Liver Dis. 2007;39:293–304. doi: 10.1016/j.dld.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, Thuluvath PJ. Complementary and alternative medicine in hepatology: review of the evidence of efficacy. Clin Gastroenterol Hepatol. 2007;5:408–416. doi: 10.1016/j.cgh.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Rambaldi A, Jacobs BP, Iaquinto G, Gluud C. Milk thistle for alcoholic and/or hepatitis B or C liver diseases--a systematic cochrane hepato-biliary group review with meta-analyses of randomized clinical trials. Am J Gastroenterol. 2005;100:2583–2591. doi: 10.1111/j.1572-0241.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- 7.Seeff LB, Curto TM, Szabo G, et al. Herbal product use by persons enrolled in the hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) Trial. Hepatology. 2008;47:605–612. doi: 10.1002/hep.22044. [DOI] [PubMed] [Google Scholar]
- 8.Shibano M, Lin AS, Itokawa H, Lee KH. Separation and characterization of active flavonolignans of Silybum marianum by liquid chromatography connected with hybrid ion-trap and time-of-flight mass spectrometry (LC-MS/IT-TOF) J Nat Prod. 2007;70:1424–1428. doi: 10.1021/np070136b. [DOI] [PubMed] [Google Scholar]
- 9.Morishima C, Shuhart MC, Wang CC, et al. Silymarin Inhibits In Vitro T-Cell Proliferation and Cytokine Production in Hepatitis C Virus Infection. Gastroenterology. 2010;138:671–681. doi: 10.1053/j.gastro.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trappoliere M, Caligiuri A, Schmid M, et al. Silybin, a component of sylimarin, exerts anti-inflammatory and anti-fibrogenic effects on human hepatic stellate cells. J Hepatol. 2009;50:1102–1111. doi: 10.1016/j.jhep.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Polyak SJ, Morishima C, Shuhart MC, et al. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin. Gastroenterology. 2007;132:1925–1936. doi: 10.1053/j.gastro.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed-Belkacem A, Ahnou N, Barbotte L, et al. Silibinin and Related Compounds Are Direct Inhibitors of Hepatitis C Virus RNA-Dependent RNA Polymerase. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 13.Bonifaz V, Shan Y, Lambrecht RW, et al. Effects of silymarin on hepatitis C virus and haem oxygenase-1 gene expression in human hepatoma cells. Liver Int. 2009;29:366–373. doi: 10.1111/j.1478-3231.2008.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferenci P, Scherzer TM, Kerschner H, et al. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology. 2008;135:1561–1567. doi: 10.1053/j.gastro.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 15.Pietrangelo A, Borella F, Casalgrandi G, et al. Antioxidant activity of silybin in vivo during long-term iron overload in rats. Gastroenterology. 1995;109:1941–1949. doi: 10.1016/0016-5085(95)90762-9. [DOI] [PubMed] [Google Scholar]
- 16.Boigk G, Stroedter L, Herbst H, et al. Silymarin retards collagen accumulation in early and advanced biliary fibrosis secondary to complete bile duct obliteration in rats. Hepatology. 1997;26:643–649. doi: 10.1002/hep.510260316. [DOI] [PubMed] [Google Scholar]
- 17.Dehmlow C, Erhard J, de GH. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23:749–754. doi: 10.1053/jhep.1996.v23.pm0008666328. [DOI] [PubMed] [Google Scholar]
- 18.Di Bisceglie AM, Shiffman ML, Everson GT, et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429–2441. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 20.Goodman ZD, Becker RL, Jr, Pockros PJ, Afdhal NH. Progression of fibrosis in advanced chronic hepatitis C: evaluation by morphometric image analysis. Hepatology. 2007;45:886–894. doi: 10.1002/hep.21595. [DOI] [PubMed] [Google Scholar]
- 21.Goodman ZD, Stoddard AM, Bonkovsky HL, et al. Fibrosis progression in chronic hepatitis C: morphometric image analysis in the HALT-C trial. Hepatology. 2009;50:1738–1749. doi: 10.1002/hep.23211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 23.Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–148. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society Series B-Statistical Methodology. 1972;34:187. [Google Scholar]
- 25.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 26.Ghany MG, Lok AS, Everhart JE, et al. Predicting clinical and histologic outcomes based on standard laboratory tests in advanced chronic hepatitis C. Gastroenterology. 2010;138:136–146. doi: 10.1053/j.gastro.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer KE, Myers RP, Lee SS. Silymarin treatment of viral hepatitis: a systematic review. J Viral Hepat. 2005;12:559–567. doi: 10.1111/j.1365-2893.2005.00636.x. [DOI] [PubMed] [Google Scholar]
- 28.Ferenci P, Dragosics B, Dittrich H, et al. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9:105–113. doi: 10.1016/0168-8278(89)90083-4. [DOI] [PubMed] [Google Scholar]
- 29.Pares A, Planas R, Torres M, et al. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J Hepatol. 1998;28:615–621. doi: 10.1016/s0168-8278(98)80285-7. [DOI] [PubMed] [Google Scholar]
- 30.Strickland GT, Tanamly MD, Tadros F, et al. Two-year results of a randomised double-blinded trial evaluating silymarin for chronic hepatitis C. Dig Liver Dis. 2005;37:542–543. doi: 10.1016/j.dld.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Tanamly MD, Tadros F, Labeeb S, et al. Randomised double-blinded trial evaluating silymarin for chronic hepatitis C in an Egyptian village: study description and 12-month results. Dig Liver Dis. 2004;36:752–759. doi: 10.1016/j.dld.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Cho YK, Yun JW, Park JH, et al. Deleterious effects of silymarin on the expression of genes controlling endothelial nitric oxide synthase activity in carbon tetrachloride-treated rat livers. Life Sci. 2009;85:281–290. doi: 10.1016/j.lfs.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Gordon A, Hobbs DA, Bowden DS, et al. Effects of Silybum marianum on serum hepatitis C virus RNA, alanine aminotransferase levels and well-being in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2006;21:275–280. doi: 10.1111/j.1440-1746.2006.04138.x. [DOI] [PubMed] [Google Scholar]
- 34.Schrieber SJ, Wen Z, Vourvahis M, et al. The pharmacokinetics of silymarin is altered in patients with hepatitis C virus and nonalcoholic Fatty liver disease and correlates with plasma caspase-3/7 activity. Drug Metab Dispos. 2008;36:1909–1916. doi: 10.1124/dmd.107.019604. [DOI] [PubMed] [Google Scholar]