Abstract
Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial orsystematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply ordistribution in any form to anyone is expressly forbidden
The publisher does not give any warranty express or implied or make any representation that the contentswill be complete or accurate or up to date. The accuracy of any instructions, formulae and drug dosesshould be independently verified with primary sources. The publisher shall not be liable for any loss,actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directlyor indirectly in connection with or arising out of the use of this material.
Previous observational studies suggest that vitamin C may reduce risk of colorectal cancer. Vitamin C transport is facilitated by membrane bound sodium-dependent transporters, SVCT1 (encoded by SLC23A1) and SVCT2 (encoded by SLC23A2). To investigate if common genetic variants in these two genes are associated with risk of colorectal tumor development, we conducted a case-control study of 656 Caucasian advanced distal colorectal adenoma cases and 665 Caucasian sigmoidoscopy-negative controls nested within the screening arm of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. The analysis of common single nucleotide polymorphisms in SLC23A1 revealed no association. For SLC23A2, overall, there was no association with haplotypes, but two SNPs located inintron 8 and exon 11 could be associated (odds ratio = 0.49, 95% confidence interval = 0.25–0.95 for haplotype G-C vs. haplotype C-C). The findings should be confirmed in follow-up studies, and further investigation is required to probe the functional basis of this finding.
Introduction
Vitamin C is a potent water-soluble antioxidant, which protects against damage from free radicals. Vitamin C is distributed throughout the body and protects cell membranes and DNA from oxidative damage. Furthermore, vitamin C functions as an electron donor for enzymatic reactions that control key pathways in the metabolism and synthesis of amino acids as well as essential building blocks of intracellular and extracellular matrices and signaling (1).
Colon carcinogenesis is a multistep process in which oxygen radicals can enhance carcinogenesis at each stage of initiation, promotion, and progression (2). The level of nonenzymatic antioxidants such as glutathione, vitamin C, and vitamin E has been shown to be significantly decreased in colon cancer tissue (2). Because lipid peroxidation is enhanced during carcinogenesis, the role of an antioxidant, such as vitamin C, could be important in protection against damage due to oxidative stress and lipid peroxidation (2). There is considerable evidence that vitamin C intake protects against aerodigestive cancers, namely, oral, esophageal, and stomach (3); but for colorectal adenoma and cancer, the published evidence is inconclusive. The results from some but not all observational studies and clinical trials have associated decreased risk for colorectal adenomas and tumor formation with increased vitamin C intake (4). Moreover, the interpretation of these results is daunting because vitamin C in take is highly correlated with fruit and vegetables consumption containing levels of other micronutrients that could be protective. Intervention trials using specified amounts of antioxidative supplements have found a risk reduction for adenoma recurrence (5,6). However, in familial polyposis, 4 trials that have studied recurrent adenoma or occurrence of polyps found no risk reduction (7–10). These trials have used combinations of antioxidants (carotenoids, vitamin C and E, selenium), which does not allow to distinguish between the effects of the single antioxidants. A Cochrane review, “Antioxidant supplements for preventing gastrointestinal cancers” (The Cochrane Library, 2005), found no effect of vitamin C in preventing colon cancer. However, a recent study found a protective dosage-dependent effect of vitamin C on colon cancer (11). Overall, the evidence leans toward a possible protective effect of vitamin C for colorectal adenoma and cancer, but further epidemiological studies are needed to clarify whether there is no protective effect of vitamin C for colorectal adenoma or cancer (4).
In order to examine the possible contribution of host genetic variation in the major transport system for vitamin C, we con ducted an association study in a nested case control study of SNPs in the sodium-dependent vitamin C transporters SVCT1 (encoded by SLC23A1) and SVCT2 (encoded by SLC23A2) and colorectal adenoma, an early precursor for colorectal cancer (12,13). SVCT1 controls bulk absorption and reabsorption of vitamin C and is primarily expressed as an epithelial trans-membrane transporter localized to intestine, liver, and kidney. The transporter responsible for generalized vitamin C accumulation is SVCT2, which is widely distributed across most tissues including colon tissue (14). There is a second mechanism for absorption of vitamin C via the glucose transporters (15–18), which transport dehydroascorbic acid, the oxidized form of vitamin C that is reduced back to ascorbic acid intracellularly (19,20). The dominant transport mechanism in most tissues, including colonic epithelium, is sodium-dependent transport based on the evidence that dehydroascorbic acid is not found in blood (21) and that vitamin C accumulation is virtually eliminatedin most tissues in the slc23a2 (svct2) knockout mouse (22).
Materials and Methods
Study Design
This case-control study was nested within the screening arm of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening (PLCO) Trial (23,24). The trial was conducted at 10 centers throughout the United States (Birmingham, AL; Denver, CO; Detroit, MI; Honolulu, HI; Marshfield, WI; Minneapolis, MN; Pittsburgh, PA; Salt Lake City, UT; St. Louis, MO; and Washington, DC) and enrolled men and women between 55 and 74 yr of age. Participants randomly assigned to the screening arm of the trial were offered a flexible sigmoidoscopy examination of the distal colon (60 cm) at study entry. If polyps or other suspect lesions were identified, participants were referred for further colonoscopy and surgery, if indicated. All available medical and pathologic reports on follow-up obtained within 12 mo were coded by trained medical record abstractors. The institutional review boards of the U.S. National Cancer Institute and the 10 screening centers approved the study, and all participants provided informed consent.
Study Population
Cases and controls for this study were drawn from the participants randomly assigned to the screening arm of the PLCO Trial between September 1993 and September 1999 who filled out the risk factor questionnaire, had a successful sigmoidoscopy (insertion to at least 50 cm with >90% of mucosa visible or a suspect lesion identified), and provided a blood sample for use in etiologic studies (n = 42,037). Of these participants, we excluded 4,834 with a self-reported history of ulcerative colitis, Crohn's disease, familial polyposis, colorectal polyps, Gardner's syndrome, or cancer, except basal-cell skin cancer. We randomly selected 772 of 1,234 cases with at least one distal advanced colorectal adenoma (≥1 cm, high-grade dysplasia, or villous elements including tubulovillous adenoma) and 777 of 26,651 control participants with a negative sigmoidoscopy screening (i.e., no polyp or other suspect lesion) matched to the cases by gender and ethnicity.
Genes and SNP Selection
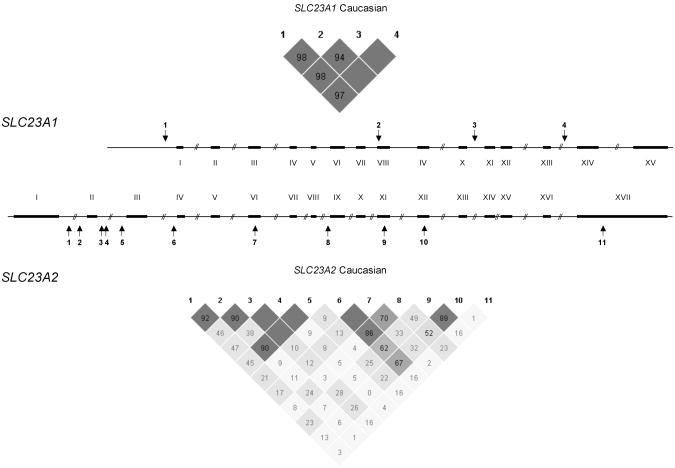
SLC23A1 and SLC23A2 are the two sodium-dependent transporter genes in humans and differ by 10-fold in size (approximately16 kb vs. 160 kb, SLC23A1 and SLC23A2, respectively) yet have homologous coding regions and intron/exon junctions. Extensive analysis of the pattern of common genetic variation (i.e., SNPs and haplotypes) has been performed for SLC23A1 but not for SLC23A2 (25). Because of the size difference and the different patterns of linkage disequilibrium (LD), the approach to the association study differed for the two genes. For SLC23A1, a haplotype-based approach was implemented be cause prior work had defined haplotype tagging SNPs across the gene based on extensive resequence analysis of the gene (including exons and promoter regions) (25). For the larger gene, SLC23A2, 11 tag SNPs with high minor allele frequencies (MAF; i.e., MAF greater than 5%) were selected for analysis from a resequence analysis of nearly 16 kb across the gene (including all exons and promoter regions) (25). The SNPs assayed in both genes are listed in Table 1 and pictured in Fig. 1.
Table 1. SNP Positions and Allele Frequencies in Caucasian Individualsa.
| SNP500 ID | dbSNP ID | Position | Allele | Minor Allele Frequency |
|---|---|---|---|---|
| SLC23A1 | ||||
| SLC23A1-18 | rs10063949 | 5′/-584 | A/G | G = 0.33 |
| SLC23A1-05 | Pending | Ex8/3441 | G/A | A = 0.03 |
| SLC23A1-09 | rs4257763 | In10/4784 | T/C | C = 0.34 |
| SLC23A1-21 | rs6596473 | In13/8367 | C/G | G = 0.31 |
| SLC23A2 | ||||
| SLC23A2-31 | rs12479919 | In1/-67652 | G/A | A = 0.39 |
| SLC23A2-32 | rs2681118 | In1/-54795 | A/C | C = 0.16 |
| SLC23A2-08 | rs6139591 | In2/-38152 | C/T | T = 0.43 |
| SLC23A2-09 | rs2681116 | In2/-38124 | G/A | A = 0.42 |
| SLC23A2-10 | Pending | In2/-38008 | G/T | T = 0.44 |
| SLC23A2-33 | rs4813725 | In2/-4777 | G/A | A = 0.36 |
| SLC23A2-26 | rs1715365 | In3/14121 | G/A | A = 0.48 |
| SLC23A2-03 | rs1776964 | Ex6/32901 | C/T | T = 0.48 |
| SLC23A2-05 | rs4987219 | In8/48263 | G/C | C = 0.39 |
| SLC23A2-01 | rs1110277 | Ex11/58527 | T/C | C = 0.32 |
| SLC23A2-02 | Pending | 3′/78315 | C/T | T = 0.11 |
Abbreviations are as follows: SNP, single nucleotide poly morphism; dbSNP, the single nucleotide polymorphism database; SLC23A1, solute carrier family 23 (nucleobase transporters), member 1; SLC23A2, SLC23A member 2; Ex, exon; In, intron. Table shows minor allele frequency in the Caucasian control population. The exonic SNP in exon 8, SLC23A1-05 are nonsynonymous (i.e., changes the amino-acid from valine to methionine at position 264). The exonic SNPs in SLC23A2 exon 6 and 11 are synonymous. All variants are in Hardy-Weinberg equilibrium (HWE).
Fig. 1.
The two genes SLC23A1 and SLC23A2 differ 10-fold in size (16 kb vs. 160 kb, respectively) but have similar exon/intron architecture. Arrows indicate the positions of SNPs used for the association study. The degree of linkage disequilibrium (LD) in the two genes is represented in the triangular figures. Red indicates a high degree of LD, whereas blue indicates uncertain results. D' values are given in percentage.
Genotyping
Genomic DNA was extracted from buffy coat or whole blood samples by standard methods. Genotype analysis was performed by TaqMan assays (Applied Biosystems, Foster City, CA), and assay conditions for each optimized assay are publicly available on the public Web site, SNP500cancer (http://snp500cancer.nci.nih.gov) (26). A small number of samples were excluded from the analysis because of insufficient DNA yields, inability to amplify, or failure of unambiguous genotyping (n = 73 cases and 6 controls); therefore, genotype results were available for 669 cases and 708 controls. The primary analysis was restricted to the Caucasians, which represented 93.8% of the cases (n = 656) and 93.9% of the controls (n = 665). Forty individuals (blinded quality control samples) were assayed between 2 and 4 times, which resulted in a total of 136 reads per SNP assay. The concordance of genotype results was 99% for SLC23A1-2 and 100% for the 14 other genotype assays. The average sample completion for the 15 assays was greater than 95% per assay.
Risk Factor Questionnaire
A questionnaire designed to investigate risk factors was ad ministered at enrollment and assessed demographic factors, personal and family medical history, physical activity, weight, height, and smoking. Dietary intake over the last 12 mo be fore enrollment was assessed using a 137-item food frequency questionnaire that also included 14 additional questions on supplement use. To calculate daily nutrient intake, such as vitamin C from the diet, we multiplied the daily frequency of each food item by the nutrient value of the gender-specific portion size (27) using the U.S. Department of Agriculture nutrient database (28). To calculate total vitamin C intake, we combined dietary and supplemental vitamin C intake.
Statistical Analysis
We used logistic regression analysis to estimate odds ratios (ORs) for colorectal adenoma and 95% confidence intervals (CIs) for the association between genotypes and colorectal adenoma risk adjusted for age, screening center, gender, and ethnicity. The homozygote common allele was used as reference group. Pair-wise LD between SNPs and haplotype block structure was estimated using Haploview version 2.05 (29) (http://www.broad.mit.edu/personal/jcbarret/haploview/). Haplotype blocks were defined according to Gabriel et al. (30). For SNPs within the same block, we estimated haplotype frequencies with the expectation-maximization (EM) algorithm (31) and tested the overall differences in haplotype frequencies between cases and controls with the permutation omnibus test available in SAS/Genetics. We also used logistic regression to estimate the associations between each haplotype and colorectal adenoma using haplotype frequency (estimated by EM algorithm), with the most common haplotype as the reference group (32,33). Total vitamin C (dietary + supplemental) intake was adjusted for educational attainment, smoking, alcohol in take, aspirin use, ibuprofen use, physical activity, body mass index, energy intake, fiber intake, red meat intake, calcium in take, and folate intake. Interaction effects for disease between vitamin C intake and genetic variants in SLC23A1 and SLC23A2 were examined by stratified analyses and tests for multiplicative interaction, with inclusion of cross-product terms in the logistic regression models. All statistical analyses were performed using SAS genetics version 9.1 (SAS Institute, Cary, NC).
Results
Cases tended to be slightly older and less educated (Table 2). The distribution of sex and race/ethnicity was very similar in cases and controls, as they were matched on both factors. The MAFs of the 4 SNPs in SLC23A1 were between 3% and 34% and of the 11 SNPs in SLC23A2 between 11% and 48% among controls (Table 3). All SNPs were in Hardy-Weinberg equilibrium (P > 0.05) among Caucasian controls. There were no significant associations between single genotypes in either gene and advanced distal colorectal adenoma. The 4 SNPs in SLC23A1 captured greater than 90% of common haplotypes within the single block (Fig. 1) (30). Notably, haplotype frequencies did not differ between cases and controls (Table 3).
Table 2. Characteristics of the Study Population (Limited to Caucasian Only).
| Characteristics | Cases | Controls |
|---|---|---|
| N | 656 | 665 |
| Age, yr mean | 63.1 | 62.1 |
| Female, n (%) | 190 (29.0%) | 207 (31.1%) |
| Education, n (%) | ||
| <12 yr of school | 60 (9.2%) | 38 (5.7%) |
| 12 yr of school or high school equivalent | 156 (23.8%) | 161 (24.2%) |
| Some college | 236 (36.0%) | 216 (32.5%) |
| College and above | 204 (31.1%) | 250 (37.6%) |
Table 3. Association Between Polymorphisms in SLC23A1 and SLC23A2 and Advanced Distal Colorectal Adenoma Risk in Caucasiansa.
| SNP/Genotype | Controls | Cases | OR | 95% CI | PTrend |
|---|---|---|---|---|---|
| SLC23A1 | |||||
| SLC23A1-18 | |||||
| GG | 299 | 295 | 1.00 | ref | |
| GA | 267 | 274 | 1.01 | 0.79–1.28 | |
| AA | 83 | 71 | 0.79 | 0.55–1.14 | 0.34 |
| SLC23A1-05 | |||||
| GG | 625 | 605 | 1.00 | ref | |
| GA | 35 | 44 | 1.26 | 0.79–2.01 | |
| AA | 2 | 0 | — | — | 0.58 |
| SLC23A1-09 | |||||
| TT | 300 | 291 | 1.00 | ref | |
| TC | 272 | 277 | 1.01 | 0.80–1.29 | |
| CC | 86 | 72 | 0.79 | 0.55–1.14 | 0.36 |
| SLC23A1-21 | |||||
| 323 | 321 | 1.00 | ref | ||
| GG/GC | 261 | 269 | 0.99 | 0.78–1.25 | |
| GG/CC | 70 | 54 | 0.71 | 0.48–1.06 | 0.21 |
| SLC23A2 | |||||
| SLC23A2-31 | |||||
| AA | 237 | 230 | 1.00 | ref | |
| GA | 318 | 314 | 1.02 | 0.71–1.41 | |
| GG | 98 | 94 | 1.00 | 0.80–1.30 | 0.97 |
| SLC23A2-32 | |||||
| CC | 453 | 444 | 1.00 | ref | |
| CA | 187 | 176 | 0.94 | 0.73–1.21 | |
| AA | 13 | 22 | 1.83 | 0.90–3.74 | 0.60 |
| SLC23A2-08 | |||||
| TT | 201 | 211 | 1.00 | ref | |
| TC | 344 | 314 | 0.89 | 0.69–1.14 | |
| CC | 113 | 122 | 1.07 | 0.77–1.48 | 0.89 |
| SLC23A2-09 | |||||
| GG | 215 | 211 | 1.00 | ref | |
| GA | 330 | 311 | 0.98 | 0.76–1.26 | |
| AA | 110 | 123 | 1.11 | 0.80–1.54 | 0.63 |
| SLC23A2-10 | |||||
| GG | 188 | 209 | 1.00 | ref | |
| GT | 342 | 307 | 0.83 | 0.64–1.07 | |
| TT | 115 | 124 | 1.00 | 0.72–1.39 | 0.78 |
| SLC23A2-33 | |||||
| AA | 264 | 268 | 1.00 | ref | |
| AG | 299 | 284 | 0.94 | 0.74–1.43 | |
| GG | 86 | 87 | 1.01 | 0.71–1.43 | 0.86 |
| SLC23A2-26 | |||||
| AA | 168 | 175 | 1.00 | ref | |
| GA | 270 | 261 | 0.91 | 0.69–1.21 | |
| GG | 141 | 134 | 0.90 | 0.65–1.24 | 0.50 |
| SLC23A2-03 | |||||
| CC | 182 | 176 | 1.00 | ref | |
| CT | 329 | 313 | 0.95 | 0.73–1.24 | |
| TT | 150 | 163 | 1.10 | 0.80–1.50 | 0.59 |
| SLC23A2-05 | |||||
| CC | 233 | 258 | 1.00 | ref | |
| CG | 325 | 298 | 0.81 | 0.64–1.03 | |
| GG | 88 | 82 | 0.82 | 0.57–1.17 | 0.13 |
| SLC23A2-01 | |||||
| TT | 295 | 300 | 1.00 | ref | |
| TC | 301 | 275 | 0.90 | 0.71–1.14 | |
| CC | 63 | 74 | 1.12 | 0.76–1.63 | 0.98 |
| SLC23A2-02 | |||||
| TT | 512 | 500 | 1.00 | ref | |
| TC | 138 | 138 | 1.01 | 0.77–1.33 | |
| CC | 3 | 6 | 2.29 | 0.56–9.32 | 0.62 |
Abbreviations are as follows: SLC23A1, solute carrier family 23 (nucleobase transporters), member 1; SLC23A2, SLC23A member 2; SNP, single nucleotide polymorphism; OR, odds ratio; CI, confidence interval. Distal defined as descending, sigmoid colon or rectum; advanced adenoma defined as large (>1 cm) or high-grade dysplasia, or villous elements (including tubulo-villous adenomas).
In the larger gene, SLC23A2, our analysis included partial assessment of common variation across the gene, which appears to have at least 3 distinct haplotype blocks based on the analysis of the controls (Fig. 1). Overall, the distribution of haplotypes did not differ between cases and controls (Table 4), but the region containing SLC23A2-05 and SLC23A2-01 (+48263 and +58527), despite the nonsignificant finding (P for exact test = 0.12), is of interest. The two variants reside 10 kb apart in intron 8 and a synonymous SNP in exon 11 and are in strong LD (D′ = 0.89; 95% CI = 0.86–0.92). The diplotype associated with a decreased risk was G-C (OR = 0.49, 95% CI = 0.25–0.95; Table 5). The individual genotype analysis was unremarkable for the heterozygous state of SLC23A2-05 (OR = 0.81, 95% CI = 0.64–1.03) and SLC23A2-01 (OR = 0.90, 95% CI = 0.71– 1.14; Table 3).
Table 4. Association Between Haplotypes of SLC23A1 and SLC23A2 and Advanced Colorectal Adenomaa.
| Haplotype | SNPs | Exact P Value b |
|---|---|---|
| SLC23A1c | ||
| Block 1 | SLC23A1-18, -05, -09, and -21 | 0.92 |
| SLC23A2c | ||
| Region 1a | SLC23A2-31, -32, 08, -09, and -10 | 0.71 |
| Region 1b | SLC23A2-08, -09, and -10 | 0.67 |
| Region 2a | SLC23A2-33, -26, and -03 | 0.43 |
| Region 2b | SLC23A2-33 and -26 | 0.46 |
| Region 3 | SLC23A2-05 and -01 | 0.12 |
Abbreviations are as follows: SLC23A1, solute carrier family 23 (nucleobase transporters), member 1; SLC23A2, SLC23A member 2; SNP, single nucleotide polymorphism.
Global test for association between haplotypes and colorectal adenoma.
In SLC23A1, analysis by block structure as defined by Gabriel et al. (29). In SLC23A2, no haplotype blocks as defined by Gabriel et al. were identified. A sliding window of regions displaying high degree of linkage disequilibrium were utilized for exploratory analyses.
Table 5. Association Between Haplotype of SLC23A2 (SLC23A2-05 and SLC23A2-01) and Advanced Colorectal Adenomasa.
| Haplotype | Cases (%) | Controls (%) | ORb | 95% CI | Pc |
|---|---|---|---|---|---|
| C-C | 61.4 | 59.3 | 1.00 | Ref | |
| G-T | 2.1 | 1.7 | 1.84 | 0.54–6.28 | 0.33 |
| G-C | 6.0 | 8.3 | 0.49 | 0.25–0.95 | 0.04 |
| C-T | 30.5 | 30.7 | 0.88 | 0.62–1.25 | 0.48 |
Abbreviations are as follows: SLC23A1, solute carrier family 23 (nucleobase transporters), member 1; SLC23A2, SLC23A member 2; OR, odds ratio; CI, confidence interval.
Adjusted for age, sex, clinical center, and ethnicity.
Overall haplotype test likelihood ratio test P = 0.11 (exact test).
We observed no association between vitamin C intake and risk of advanced colorectal adenoma (Table 6).
Table 6. Association Between Vitamin C Intake and Risk of Advanced Distal Colorectal Adenomasa.
| Vitamin C (Quintiles) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1b | 2 | 3 | 4 | 5 | PTrend | |
| Mean (ng/ml) | <125 | 125–198 | >198–324 | >324–739 | >739 | |
| Overall | ||||||
| No. of cases | 150 | 163 | 138 | 105 | 104 | |
| OR | 1.00 | 0.78 | 0.93 | 1.26 | 1.16 | 0.10 |
| (95% CI)c | — | 0.54–1.12 | 0.63–1.39 | 0.83–1.90 | 0.72–1.85 | |
Abbreviations are as follows: OR, odds ratio; CI, confidence interval.
Reference category.
Adjusted for age; center; gender; energy intake; ethnic origin; educational attainment; tobacco use; alcohol intake; aspirin and ibuprofen use; physical activity; body mass index; and intake of red meat, folate, calcium, and dietary fiber.
Discussion
This is the first study, to our knowledge, which has investigated the association between colorectal adenomas and common genetic variants in the genes encoding the major transporters of vitamin C, SLC23A1 and SLC23A2. We observed that common genetic variation in the more ubiquitously expressed transporter, SLC23A2, could be associated with a reduction in the risk of colorectal adenoma. Notably, a common haplotype, G-C (8.3% in Caucasian controls) in SLC23A2 is possibly associated with reduced risk of distal colorectal adenoma. Our results for the SLC23A1 gene included common genetic variation across the gene, whereas our analysis of SLC23A2 analyzed only a proportion of common genetic variation in the larger gene characterized by a more complex pattern of LD. We did not observe an association between colorectal adenomas and common genetic variation in SLC23A1, the transporter responsible for uptake and reabsorption in the gastrointestinal tract, kidney, and liver.
Because our strategy for SLC23A1 comprehensively captured common genetic variation, it is unlikely that there is an association with colorectal adenoma. We used an incomplete set of markers for surveying common genetic variation across SLC23A2; but with the recent completion of a haplotype map of the human genome by the International HapMap project (http://www.hapmap.org/), it will be possible to investigate across the entire gene (34). Moreover, it will be possible to conduct saturation genotype analysis across the region in which SLC23A2-01 and SLC23A2-05 reside to determine markers in this region that should be studied in follow-up studies to dissect the region and determine functional correlations.
The functional consequences of the variants, SLC23A2-01 and SLC23A2-05, have not yet been characterized. Although it is notable that 1 is an intronic SNP and the other a synonymous SNP, it is plausible that these variants could be in LD with the functional variant(s) elsewhere in the gene or in contiguous regions. Additional studies will be required to fully characterize the LD pattern as well as determine the functional implications of the SNPs identified in this study. Because this is a pilot study of SNPs from two genes with related function and in which the variants are not independent because of LD, we report our initial findings without correction.
Because of the study design of PLCO, it has been possible to randomly select cases and matched controls from the same study population. All subjects were screened and enrolled following a standard procedure, which would eliminate some of the bias associated with case selection based on clinical presentation or symptoms. Furthermore, we chose to analyze a critical inter mediate outcome, colorectal adenoma. Previously, others have reported differences in the risk factors for left-sided and right-sided adenomas, which underscore the importance of exercising caution in extrapolating to all adenomas. Nonetheless, colorectal adenomas are an important intermediate outcome, and therefore, it is possible to consider the implications of dietary recommendations that could be linked to genetic variation in the major transporters of vitamin C.
In conclusion, we did not identify any association between common haplotypes in SLC23A1, or single genotypes in either gene, and risk of colorectal adenoma. We observed an association between common genetic variants, specifically a diplotype, in the ubiquitously expressed SLC23A2 gene and lower risk for advanced colorectal adenomas. It is notable that the diplotype of interest is relatively rare and will require follow-up studies de signed to replicate the observation. Our findings will need to be validated in subsequent follow-up studies that can also provide an opportunity to investigate whether there could be an interaction between dietary vitamin C and SLC23A2. Moreover, it will be important to conduct the study with a denser set of common genetic variants to adequately capture the common haplotype structure across this region of the gene. Laboratory investigation will be required to investigate the functional consequences of the variants studied or those in LD that could be the causal variant(s). Last, our observations suggest that one or both of the sodium-dependent vitamin C transporters could be suitable candidate genes for study in other types of cancer previously associated with vitamin C intake such as oral, esophageal, and gastric cancer (3,35).
Contributor Information
Hans Christian Erichsen, Section on Genomic Variation, Pediatric Oncology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland, USA.
Ulrike Peters, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, Maryland; Fred Hutchinson Cancer Research Center, Seattle, Washington; and University of Washington, Seattle, Washington, USA.
Peter Eck, Molecular and Clinical Nutrition Section, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland, USA.
Robert Welch, Core Genotyping Facility, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland, USA.
Robert E. Schoen, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
Meredith Yeager, Core Genotyping Facility, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland, USA.
Mark Levine, Molecular and Clinical Nutrition Section, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland, USA.
Richard B. Hayes, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, Maryland, USA
Stephen Chanock, Section on Genomic Variation, Pediatric Oncology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland and Core Genotyping Facility, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland, USA.
References
- 1.Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415–1423. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- 2.Skrzydlewska E, Stankiewicz A, Sulkowska M, Sulkowski S, Kasacka I. Antioxidant status and lipid peroxidation in colorectal cancer. J Toxicol Environ Health A. 2001;64:213–222. doi: 10.1080/15287390152543690. [DOI] [PubMed] [Google Scholar]
- 3.Byers T, Guerrero N. Epidemiologic evidence for vitamin C and vitamin E in cancer prevention. Am J Clin Nutr. 1995;62:1385S–1392S. doi: 10.1093/ajcn/62.6.1385S. [DOI] [PubMed] [Google Scholar]
- 4.Food, Nutrition and the Prevention of Cancer: a global perspective. Washington, DC: The World Cancer Research Fund/American Institute for Cancer Research Expert Report; p. 1997. [Google Scholar]
- 5.Roncucci L, Di Donato P, Carati L, Ferrari A, Perini M, et al. Antioxidant vitamins or lactulose for the prevention of the recurrence of colorectal adenomas. Colorectal Cancer Study Group of the University of Modena and the Health Care District 16. Dis Colon Rectum. 1993;36:227–234. doi: 10.1007/BF02053502. [DOI] [PubMed] [Google Scholar]
- 6.Hofstad B, Almendingen K, Vatn M, Andersen SN, Owen RW, et al. Growth and recurrence of colorectal polyps: a double-blind 3-year intervention with calcium and antioxidants. Digestion. 1998;59:148–156. doi: 10.1159/000007480. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg ER, Baron JA, Tosteson TD, Freeman DH, Jr, Beck GJ, et al. A clinical trial of antioxidant vitamins to prevent colorectal adenoma. Polyp Prevention Study Group. N Engl J Med. 1994;331:141–147. doi: 10.1056/NEJM199407213310301. [DOI] [PubMed] [Google Scholar]
- 8.McKeown-Eyssen G, Holloway C, Jazmaji V, Bright-See E, Dion P, et al. A randomized trial of vitamins C and E in the prevention of recurrence of colorectal polyps. Cancer Res. 1988;48:4701–4705. [PubMed] [Google Scholar]
- 9.DeCosse JJ, Miller HH, Lesser ML. Effect of wheat fiber and vitamins C and E on rectal polyps in patients with familial adenomatous polyposis. J Natl Cancer Inst. 1989;81:1290–1297. doi: 10.1093/jnci/81.17.1290. [DOI] [PubMed] [Google Scholar]
- 10.Bussey HJ, DeCosse JJ, Deschner EE, Eyers AA, Lesser ML, et al. A randomized trial of ascorbic acid in polyposis coli. Cancer. 1982;50:1434–1439. doi: 10.1002/1097-0142(19821001)50:7<1434::aid-cncr2820500733>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 11.Kune G, Watson L. Colorectal cancer protective effects and the dietary micronutrients folate, methionine, vitamins B6, B12, C, E, selenium, and lycopene. Nutr Cancer. 2006;56:11–21. doi: 10.1207/s15327914nc5601_3. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Dutta B, Huang W, Devoe LD, Leibach FH, et al. Human Na+-dependent vitamin C transporter 1 (hSVCT1): primary structure, functional characteristics and evidence for a non-functional splice variant. Biochim Biophys Acta. 1999;1461:1–9. doi: 10.1016/s0005-2736(99)00182-0. [DOI] [PubMed] [Google Scholar]
- 13.Stratakis CA, Taymans SE, Daruwala R, Song J, Levine M. Mapping of the human genes (SLC23A2 and SLC23A1) coding for vitamin C transporters 1 and 2 (SVCT1 and SVCT2) to 5q23 and 20p12, respectively. J Med Genet. 2000;37:E20. doi: 10.1136/jmg.37.9.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 15.Rumsey SC, Kwon O, Xu GW, Burant CF, Simpson I, et al. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem. 1997;272:18982–18989. doi: 10.1074/jbc.272.30.18982. [DOI] [PubMed] [Google Scholar]
- 16.Rumsey SC, Welch RW, Garraffo HM, Ge P, Lu SF, et al. Specificity of ascorbate analogs for ascorbate transport. Synthesis and detection of [(125)I]6-deoxy-6-iodo-L-ascorbic acid and characterization of its ascorbate-specific transport properties. J Biol Chem. 1999;274:23215–23222. doi: 10.1074/jbc.274.33.23215. [DOI] [PubMed] [Google Scholar]
- 17.Rumsey SC, Daruwala R, Al-Hasani H, Zarnowski MJ, Simpson IA, et al. Dehydroascorbic acid transport by GLUT4 in Xenopus oocytes and isolated rat adipocytes. J Biol Chem. 2000;275:28246–28253. doi: 10.1074/jbc.M000988200. [DOI] [PubMed] [Google Scholar]
- 18.Corpe CP, Lee JH, Kwon O, Eck P, Narayanan J, et al. 6-Bromo-6-deoxy-L-ascorbic acid: an ascorbate analog specific for Na+-dependent vitamin C transporter but not glucose transporter pathways. J Biol Chem. 2005;280:5211–5220. doi: 10.1074/jbc.M412925200. [DOI] [PubMed] [Google Scholar]
- 19.Washko PW, Wang Y, Levine M. Ascorbic acid recycling in human neutrophils. J Biol Chem. 1993;268:15531–15535. [PubMed] [Google Scholar]
- 20.Wang Y, Russo TA, Kwon O, Chanock S, Rumsey SC, et al. Ascorbate recycling in human neutrophils: induction by bacteria. Proc Natl Acad Sci USA. 1997;94:13816–13819. doi: 10.1073/pnas.94.25.13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhariwal KR, Hartzell WO, Levine M. Ascorbic acid and dehy-droascorbic acid measurements in human plasma and serum. Am J Clin Nutr. 1991;54:712–716. doi: 10.1093/ajcn/54.4.712. [DOI] [PubMed] [Google Scholar]
- 22.Sotiriou S, Gispert S, Cheng J, Wang Y, Chen A, Hoogstraten-Miller S, et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med. 2002;8:514–517. doi: 10.1038/0502-514. [DOI] [PubMed] [Google Scholar]
- 23.Gohagan JK, Prorok PC, Hayes RB, Kramer BS. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin Trials. 2000;21:251S–272S. doi: 10.1016/s0197-2456(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 24.Hayes RB, Reding D, Kopp W, Subar AF, Bhat N, et al. Etiologic and early marker studies in the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21:349S–355S. doi: 10.1016/s0197-2456(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 25.Eck P, Erichsen HC, Taylor JG, Yeager M, Hughes AL, et al. Comparison of the genomic structure and variation in the two human sodium-dependent vitamin C transporters, SLC23A1 and SLC23A2. Hum Genet. 2004;115:285–294. doi: 10.1007/s00439-004-1167-x. [DOI] [PubMed] [Google Scholar]
- 26.Packer BR, Yeager M, Staats B, Welch R, Crenshaw A, et al. SNP500Cancer: a public resource for sequence validation and assay development for genetic variation in candidate genes. Nucleic Acids Res. 2004;32:D528–D532. doi: 10.1093/nar/gkh005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subar AF, Ziegler RG, Thompson FE, Johnson CC, Weissfeld JL, et al. Is shorter always better? Relative importance of questionnaire length and cognitive ease on response rates and data quality for two dietary questionnaires. Am J Epidemiol. 2001;153:404–409. doi: 10.1093/aje/153.4.404. [DOI] [PubMed] [Google Scholar]
- 28.Cypel YS, Guenther PM, Petot GJ. Validity of portion-size measurement aids: a review. Am Diet Assoc. 1997;97:289–292. doi: 10.1016/S0002-8223(97)00074-6. [DOI] [PubMed] [Google Scholar]
- 29.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 30.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 31.Excoffier L, Slatkin M. Maximum-likelihood estimation of molecular haplotype frequencies in a diploid population. Mol Biol Evol. 1995;12:921–927. doi: 10.1093/oxfordjournals.molbev.a040269. [DOI] [PubMed] [Google Scholar]
- 32.Schaid DJ. Relative efficiency of ambiguous vs. directly measured haplo-type frequencies. Genet Epidemiol. 2002;23:426–443. doi: 10.1002/gepi.10184. [DOI] [PubMed] [Google Scholar]
- 33.Zaykin DV, Westfall PH, Young SS, Karnoub MA, Wagner MJ, et al. Testing association of statistically inferred haplotypes with discrete and continuous traits in samples of unrelated individuals. Hum Hered. 2002;53:79–91. doi: 10.1159/000057986. [DOI] [PubMed] [Google Scholar]
- 34.The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 35.airfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults: scientific review. JAMA. 2002;287:3116–3126. doi: 10.1001/jama.287.23.3116. [DOI] [PubMed] [Google Scholar]