Abstract
Background/Objectives
En coup de sabre (ECDS) and Parry-Romberg syndrome (PRS) are variants of linear morphea on the head and neck that can be associated with neurologic manifestations. Intracranial abnormalities on computed tomography (CT) and magnetic resonance imaging (MRI) can be present in a significant proportion of patients.
Methods
We describe 32 pediatric patients from our institution with ECDS or PRS, in whom neuroimaging was performed in 21 cases. We also review 51 additional patients from the literature.
Results
Nineteen percent of the children at our institution had intracranial abnormalities on MRI, half of whom were asymptomatic. Hyperintensities on T2-weighted sequences were the most common finding, present in all patients who had intracranial abnormalities on MRI. Seizures and headaches were the most common neurologic symptom, affecting 13% and 9% of our population, respectively. The presence of neurologic symptoms was not correlated with neuroimaging abnormalities as 2 asymptomatic patients had marked MRI findings, while the MRI was abnormal in only 2/9 symptomatic patients. Similarly, the severity of the superficial disease did not predict neurologic involvement; a patient with subtle skin involvement had striking MRI findings and seizures while another patient with a bony defect had no brain parenchymal involvement.
Conclusions
Neurologic symptoms and neuroimaging abnormalities are found in a surprisingly substantial percentage of children with ECDS and PRS. Early recognition of neurologic involvement is necessary as it affects treatment choices. As clinical predictors of intracranial abnormalities are poor, strong consideration should be given to obtaining an MRI prior to treatment initiation to assist in management decisions and establish a baseline examination.
Keywords: morphea, scleroderma, en coup de sabre, Parry-Romberg, progressive hemifacial atrophy, neurology, imaging, neuroimaging, brain, magnetic resonance imaging, computed tomography
Introduction
Morphea, also known as localized scleroderma, is a rare disease seen in both adults and children. Most pediatric patients have the linear subtype, which can extend deeply into the subcutaneous tissue, muscle, and bone. Linear morphea on the head and neck, called en coup de sabre (ECDS), and Parry-Romberg syndrome (PRS), also called progressive hemifacial atrophy, are felt to be related variants within the morphea spectrum of disease (1). Both ECDS and PRS may be associated with cerebral inflammation and neurologic abnormalities. A variety of neurologic symptoms have been reported, most commonly seizures and headaches (2). In addition, computed tomography (CT) and magnetic resonance imaging (MRI) can reveal calvarial and intracranial abnormalities, even in asymptomatic patients (3). The use of neuroimaging in evaluating ECDS and PRS patients is not standardized and varies between providers and institutions. Yet, early recognition of neurologic involvement in these children is important so that appropriate treatment with systemic medications may be initiated. In this paper, we describe the neurologic symptoms and neuroimaging abnormalities seen in a significant portion of children with ECDS and/or PRS based on an institutional retrospective review, and performed a systematic review of the literature to illustrate the range of neurologic involvement that can be seen in this population.
Materials and Methods
Institutional review board approval was obtained to conduct a retrospective chart review of patients with morphea seen at the Children’s Hospital and Health System (CHHS) in Milwaukee, Wisconsin. Children seen between 2000 and 2011 were identified from a search of the hospital database using International Classification of Diseases, Ninth Revision (ICD-9) discharge diagnoses of 701.0 (circumscribed scleroderma) and 710.1 (systemic sclerosis). Each chart was reviewed to identify those patients diagnosed by the treating clinician as ECDS and/or PRS and exclude those with lichen sclerosus, systemic sclerosis, and other diagnoses. For the purposes of this study, ECDS was defined as linear plaques affecting the head and neck while PRS was defined as hemifacial atrophy, though we consider the two diagnoses to be on the same spectrum of disease. The ECDS and PRS charts were then reviewed to extract cutaneous manifestations and associated neurological symptoms. CT and MRI scans were reviewed by a pediatric neuroradiologist (M.M.).
Ovid Medline was used to search the medical literature from 1946 to October 2011 to identify pediatric cases of ECDS and PRS where neuroimaging was performed. Search terms used were localized scleroderma, morphea, scleroderma en coup de sabre, Parry Romberg, facial hemiatrophy, progressive facial hemiatroph*, progressive hemifacial atroph*, neuro*, X-ray computed tomography, CT scan, magnetic resonance imaging, magnetic resonance spectroscopy, MRI, neuroimag*, and tomography. Reports were excluded if the publication was not written in English, the imaging was performed when the subject was 18-years or older, or there was insufficient detail about the imaging results. The publications were reviewed to collect details about cutaneous manifestations, associated neurological symptoms, and CT and MRI results.
Results
Retrospective chart review
Thirty-two patients with ECDS and PRS were identified from the chart review (Table 1). Three of the patients (patients 13, 24, 29) were included in a previous report from our institution (4). Twenty-one were female (66%) and 11 were male (34%), and the average onset of disease was at 6.9 years (range 1-15 years). ECDS alone was present in 24, and an overlap of ECDS and PRS was present in 8. Of these patients, 21 had MRI and/or CT of the head performed, and 18 radiographic exams were available for review by the authors. Nine of these patients had imaging performed because of neurologic complaints while 12 of the patients were asymptomatic. Three of the radiographic examinations were performed at another facility and could not be reviewed by the authors; the radiology reports of these examinations were all normal. The average age at the time of first imaging was 9.3 years (range 3-17 years).
Table 1.
Thirty-Two Individuals with ECDS and PRS at CHHS
| Name | Sex | Age of onset |
Cutaneous involvement |
Neurologic involvement | Neuroimaging findings |
|---|---|---|---|---|---|
| Patient 1 | F | 9y | Left ECDS | None | CT: ipsilateral focal scalp atrophy. MRI: N/A |
| Patient 2 | M | 4y | Right ECDS | Seizures Headaches Impaired cognition and memory Arm and leg pain and spasms (Patient has a history of Arnold- Chiari I malformation s/p craniectomy though many symptoms could not be attributed to prior abnormality or surgery.) |
CT: changes of suboccipital decompression for Chiari I malformation; ipsilateral focal scalp and calvarial atrophy MRI: changes of suboccipital decompression for Chiari I malformation; no brain parenchymal abnormality; ipsilateral focal scalp atrophy |
| Patient 3 | F | 1y | Right ECDS | None | N/A |
| Patient 4 | M | 11y | Right ECDS | None | CT: N/A MRI: no intracranial abnormality; ipsilateral focal scalp atrophy |
| Patient 5 | F | 12y | Right ECDS | None | CT: N/A MRI: no intracranial abnormality; ipsilateral focal scalp atrophy |
| Patient 6 | F | 10y | Right ECDS | None | N/A |
| Patient 7 | F | 10y | Left ECDS, right PRS |
None | N/A |
| Patient 8 | F | 3y | Left ECDS | None | N/A |
| Patient 9 | F | 2y | Left ECDS | Headaches | CT: ipsilateral focal scalp atrophy MRI: no intracranial abnormality; ipsilateral focal scalp atrophy |
| Patient 10 | F | 9y | Left ECDS | None | N/A |
| Patient 11 | M | 8y | LEFT ECDS and PRS |
None | CT: N/A MRI: ipsilateral caudate nucleus heterogenous T2 signal (calcification or chronic blood products); ipsilateral focal scalp and calvarial atrophy. |
| Patient 12 | F | 6y | Left ECDS | Behavioral issues | CT: N/A MRI: normal* |
| Patient 13^ | F | 5y | Left ECDS | Seizures | CT: N/A MRI: ipsilateral frontal T2 HI with patchy enhancement |
| Patient 14 | M | 4y | Right ECDS | None | CT: N/A MRI: normal* |
| Patient 15 | F | Unkno wn |
Left ECDS and PRS |
None | N/A |
| Patient 16 | F | 10y | Right ECDS and PRS |
None | N/A |
| Patient 17 | M | 6y | Left ECDS | None | N/A |
| Patient 18 | F | 5y | Left ECDS | None | CT: N/A MRI: no intracranial abnormality; ipsilateral focal scalp atrophy |
| Patient 19 | F | 3y | Left ECDS | Headaches | CT: N/A MRI: no intracranial abnormality; |
| Patient 20 | M | 5y | Right ECDS | None | N/A |
| Patient 21 | F | 9y | Left ECDS | None | N/A |
| Patient 22 | M | 13y | Right ECDS and PRS |
None | CT: N/A MRI: no intracranial abnormality; hypoplastic right maxilla |
| Patient 23 | M | 2y | Left ECDS | None | CT: N/A MRI: ipsilateral frontoparietal T2 HI; mild effacement of sulci; ipsilateral focal scalp atrophy |
| Patient 24^ | F | 4y | Left ECDS | Seizures | CT: N/A MRI: no intracranial abnormality; ipsilateral focal scalp and calvarial atrophy |
| Patient 25 | M | 14y | Right ECDS and PRS |
None | CT: N/A MRI: normal* |
| Patient 26 | F | 14y | Left ECDS | None | N/A |
| Patient 27 | F | 5y | Left ECDS | None | CT: N/A MRI: no intracranial abnormality |
| Patient 28 | F | 4y | Right ECDS and PRS; left ECDS |
Tongue deviation | CT: no intracranial abnormality; ipsilateral scalp atrophy MRI: N/A |
| Patient 29^ | M | 5y | Right ECDS | Strabismus (attributed to lateral rectus muscle involvement) Slurred speech |
CT: N/A MRI: no intracranial abnormality; focal scalp and soft tissue atrophy; ipsilateral lateral rectus muscle T2 HI |
| Patient 30 | M | 1y | Left ECDS and PRS |
None | CT: ipsilateral lytic lesion with soft tissue swelling MRI: soft tissue thickening with underlying calvarial defect; mild effacement of sulci from mass effect of the soft tissue lesion |
| Patient 31 | F | 15y | Left ECDS | None | CT: N/A MRI: no intracranial abnormality; ipsilateral focal scalp atrophy |
| Patient 32 | F | 5y | Right and left ECDS |
Bell’s palsy Facial weakness Sensory defects Dysmetria C7-8 radiculopathy |
CT: N/A MRI: bilateral cerebellar T2 HI; right thalamic T2 HI; right focal scalp atrophy |
HI = hyperintensity/hyperintensities
Previously reported by Holland et al.
MRI images not available for review.
Of the 21 patients with imaging, 2 patients had CT head alone, 16 had MRI brain alone, and 3 had both. Thirteen patients (62%) had evidence of scalp or calvarial atrophy. Four patients (19%) who were imaged had brain parenchymal abnormalities ipsilateral to their cutaneous lesion on MRI (Figure 1). No intracranial abnormalities were seen on CT, although the 4 patients with intracranial MRI findings did not have a CT scan performed. All 4 had T2 hyperintensities, 1 had chronic blood products or calcification, and 1 had mild mass effect with effacement of the cortical sulci. Two of these patients, representing 10% of those imaged, had neuroradiologic abnormalities without neurologic symptoms.
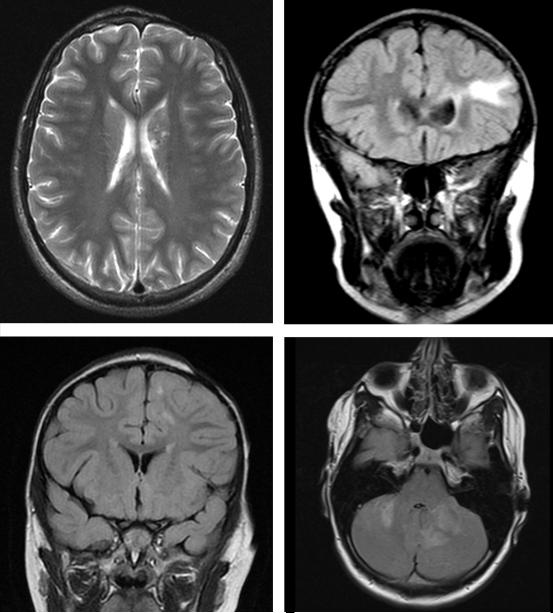
Figure 1.
A: Patient 11, Axial T2 weighted image demonstrating left scalp atrophy with T2 hyperintensities and chronic blood products or calcification in the head of left caudate nucleus; B: Patient 13, Coronal FLAIR image demonstrating T2 hyperintensity in the left frontal white matter without associated scalp or calvarial atrophy; C: Patient 23, Coronal FLAIR image demonstrates small multifocal T2 hyperintensities in the left frontoparietal white matter and ipsilateral scalp atrophy; D: Patient 32, Axial FLAIR image demonstrates ill-defined T2 hyperintensities in the cerebellum.
Of the 32 patients, 9 (28%) had neurologic symptoms attributed to the ECDS or PRS. The most common neurologic abnormality was seizures reported in 4 patients (13%), followed by headache in 3 patients (9%). Abnormalities of the cranial nerves and motor and sensory defects in the extremities were reported by 2 patients (6%) each. Additional complaints included cognitive problems, behavioral issues, dysmetria, and slurred speech in one patient each. All 9 of the symptomatic patients had neuroimaging performed but only 2 (22%) of these symptomatic patients had intracranial abnormalities on MRI.
Onset of neurologic symptoms was concurrent or within 1 year of onset of the cutaneous disease in 6 patients. Two patients developed neurologic symptoms 4 and 11 years after cutaneous lesions were first noticed, although both had ongoing active cutaneous disease at the time. The ninth patient had tongue deviation that was noted incidentally on examination 3 years after initial onset of her continued cutaneous disease.
In our retrospective review, cutaneous severity, neuroimaging findings, and neurologic symptoms did not appear to correlate. For example, a 5-year-old girl (patient 13) had no evidence of soft tissue or cranial atrophy on clinical exam or MRI, yet she had a large ipsilateral T2 hyperintensity (Figure 1B) and unremitting complex partial seizures (previously reported by Holland et al) (4). Another 5-year-old boy (patient 30) had severe soft tissue thickening and complete absence of bone underlying his ECDS but no brain parenchymal involvement. All 4 patients with neuroimaging findings and 8 of the 9 patients with neurologic symptoms had ECDS alone without PRS.
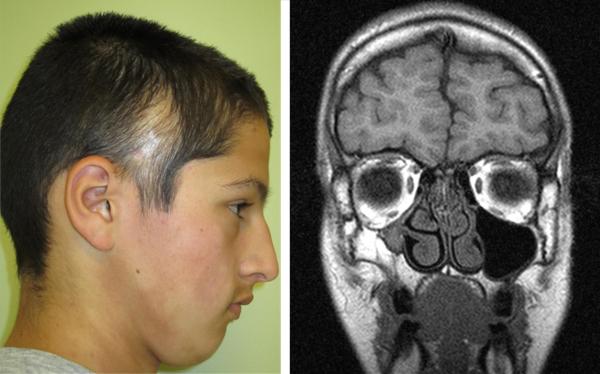
The treatment plan for one patient was altered by the MRI findings. A 13-year-old asymptomatic boy (patient 22) with ECDS of the right temple was found to have hypoplasia of the ipsilateral maxillary sinus without overlying skin changes; there was no other clear etiology for the sinus hypoplasia other than the morphea en coup de sabre (Figure 2). Treatment with systemic immunosuppressants was started in place of phototherapy.
Figure 2.
A: Patient 22, Morphea en coup de sabre of the right temple; B, Coronal T1 weighted MRI shows right maxillary sinus hypoplasia.
Literature review
Fifty-one ECDS and PRS patients with neuroimaging results were identified from the literature search (Table 2). Six patients were evaluated by CT, 23 patients were evaluated by MRI, and 22 patients were evaluated by both. Of these 51 patients, 37 (73%) had intracranial abnormalities; soft tissue and cranial atrophy were not consistently reported. Five patients had MRI abnormalities not detected by CT scan. The most common findings were T2 hyperintensities (n=25, 49%), calcifications (n=13, 25%), and ipsilateral cerebral atrophy (n=9, 17%). Nine patients (17%) had MRI abnormalities without neurologic symptoms.
Table 2.
Fifty-two Individuals with ECDS and PRS Reported in the Literature
| Reference | Cutaneous involvement |
Neurologic involvement |
Neuroimaging findings† |
|---|---|---|---|
| Asher 1982 (27) |
Right PRS | Migraine headaches Sensory defects Nerve palsies Abnormal deep tendon reflexes Vision loss |
CT: normal MRI: N/A |
| Asher 1982(27) |
Left PRS | None | CT: contralateral parietal non-enhancing area of increased density MRI: N/A |
| David 1990(28) |
Left ECDS | Headaches Seizures |
CT: ipsilateral retrobulbar soft tissue mass with proptosis; ipsilateralparieto-occipital calcification with enhancement MRI: N/A |
| Fry 1992(29) | Right PRS | Hemiparesis | CT: normal MRI: N/A |
| Fry 1992(29) | Right PRS | Headaches | CT: ipsilateral frontal calcification MRI: ipsilateral frontal T2 HI |
| Fry 1992(29) | Right PRS | Behavioral disorder Seizures |
CT: ipsilateral frontal calcification MRI: ipsilateral frontal T2 HI |
| Fry 1992(29) | Right PRS | None | CT: normal MRI: ipsilateral frontal T2 HI |
| Fry 1992(29) | Left PRS | None | CT: ipsilateral frontal calcification MRI: ipsilateral hemispheric multifocal T2 HI |
| Fry 1992(29) | Left PRS | None | CT: N/A MRI: normal |
| Liu 1994(30) | Left ECDS | None | CT: ipsilateral frontal calcification MRI: ipsilateral frontal neuromigrational abnormality; ipsilateral frontal calcification and T2 HI |
| Liu 1994(30) | Left ECDS | None | CT: N/A MRI: normal |
| Liu 1994(30) | Left PRS | N/A | CT: normal MRI: normal |
| Liu 1994(30) | N/A | None | CT: ipsilateral frontal calcification MRI: ipsilateral frontal neuromigrational abnormality; ipsilateral frontal calcification and T2 HI |
| Liu 1994(30) | N/A | None | CT: ipsilateral parietal hypoattenuating lesion MRI: N/A |
| Liu 1994(30) | N/A | None | CT: N/A MRI: normal |
| Cory 1995(31) | Left PRS | Seizures | CT: ipsilateral cingulate gyrus calcification; ipsilateral frontal and parietal subcortical white matter flow densities MRI: ipsilateral cingulate gyrus T1 and T2 calcification; ipsilateral corpus callosal genu, subcortical white matter of cingulate gyrus, deep frontal white matter, frontoparietal subcortical white matter T2 HI; encephalomalacia; ipsilateral frontoparietal pial enhancement |
| Derex 1995(32) |
Left PRS | Seizures Mental retardation |
CT: ipsilateral parietal porencephalic cyst; left frontal and parietal calcification MRI: ipsilateral parietal porencephalic cyst |
| Schievink 1996(33) |
Left PRS | Vision loss Nerve palsies Sensory defects |
CT: ipsilateral cerebral atrophy; ipsilateral basal ganglia, lateral ventricle, and parietal calcifications; contralateral cavernous sinus aneurysm MRI: N/A |
| Goldberg- Stern 1997(19) |
Left PRS | Abnormal deep tendon reflex Seizures |
CT: ipsilateral frontal densities MRI: ipsilateral frontal T2 HI; ipsilateral frontoparietal gyral or sulcal enhancement |
| Menni 1997(34) |
Right ECDS and PRS |
Hemiparesis | CT: normal MRI: ipsilateral hemispheric T2 HI |
| Taylor 1997(35) |
Right ECDS and PRS |
Seizures Vision loss |
CT: ipsilateral temporal calcification MRI: ipsilateral temporal mixed signal abnormality |
| Higashi 2000(36) |
Left ECDS | Headaches Seizures Dizziness |
CT: N/A MRI: ipsilateral frontal T2 HI |
| Yano 2000(37) | Left ECDS and PR |
Seizures | CT: ipsilateral frontal and parietal calcifications; ipsilateral white matter hypodensity MRI: ipsilateral frontal and parietal T2 HI |
| Aynaci 2001(38) |
Left PRS | Adie’s pupil | CT: ipsilateral parietal calcification MRI: ipsilateral parietal subcortical T2 HI MRA: normal |
| Moko 2003(39) |
Left PRS | Migraine headaches | CT: N/A MRI: ipsilateral cerebral T2 HI |
| Moko 2003(39) | Left PRS | None | CT: N/A MRI: normal |
| Shah 2003(13) | Right PRS | Migraine headaches Seizures Hemiparesis Abnormal deep tendon reflexes |
CT: N/A MRI: ipsilateral frontoparietal atrophy; ipsilateral frontal T2 HI MRA: normal |
| Appenzeller 2004(3) |
Right ECDS | None | CT: normal MRI: abnormal gyral pattern; blurring of gray-white matter |
| Appenzeller 2004(3) |
Right ECDS | None | CT: normal MRI: abnormal gyral pattern; blurring of gray-white matter; calcification |
| Finley 2004(40) |
Right PRS | None | CT: N/A MRI: ipsilateral orbital atrophy MRA: normal |
| Hulzebos 2004(41) |
Right PRS | None | CT: N/A MRI: ipsilateral orbital atrophy |
| Sandhu 2004(42) |
Left PRS | Seizures | CT: N/A MRI: ipsilateral frontal subdural hygroma |
| Sathornsumete e 2005(43) |
Right ECDS and PRS |
Seizures Dysarthria Dysphagia Nerve palsies Abnormal deep tendon reflexes |
CT: N/A MRI: ipsilateral cerebral atrophy; hippocampal asymmetry; ipsilateral subdural fluid collection; ipsilateral frontal T2 HI |
| Holl-Weiden 2006(16) |
Left ECDS | Headaches | CT: N/A MRI: ipsilateral frontoparietal and occipital T2 HI |
| Okumura 2006(44) |
Left PRS | None | CT: ipsilateral cerebral and contralateral parieto-occipital hypoattenuating lesions MRI: ipsilateral cerebral and contralateral parieto-occipital T2 HI |
| Paprocka 2006(15) |
Left PRS | Seizures Hemiparesis Mental retardation |
CT: N/A MRI: ipsilateral frontoparietal atrophy; ipsilateral parietal T2 hyperintensity; contralateral occipital T2 hyperintensity MRA: normal MRS: decreased NAA and creatine; presence of lipid and lactate (ipsilateral> contralateral) SPECT: ipsilateral frontoparietal decreased radiopharmaceutical uptake |
| Sommer 2006(1) |
Right PRS | Seizures Migraine headaches |
CT: N/A MRI: normal |
| Sommer 2006(1) |
Right PRS | None | CT: N/A MRI: normal |
| Sommer 2006(1) |
Left ECDS and PRS |
Seizures Migraine headaches |
CT: N/A MRI: normal |
| Sommer 2006(1) |
Right PRS | None | CT: N/A MRI: normal |
| Sommer 2006(1) |
Right PRS | Developmental delay | CT: N/A MRI: normal |
| Carreno 2007(11) |
Left PRS | Seizures | CT: N/A MRI: ipsilateral cerebral atrophy |
| Verhels 2008(45) |
Right ECDS and PRS |
Seizures Developmental regression |
CT: ipsilateral cortical and subcortical calcifications MRI: ipsilateral hippocampal atrophy |
| Chiang 2009(46) |
Right ECDS | Seizures | CT: normal MRI: ipsilateral cerebral atrophy; ipsilateral temporo-occipital T2 HI |
| Menascu 2009(21) |
Left ECDS and PRS |
Migraine headaches Abnormal deep tendon reflexes Fine motor impairment |
CT: N/A MRI: ipsilateral fronto-parietal and temporal T2 HI MRA: normal MRS: normal |
| Sartori 2009(47) |
Left ECDS and PRS |
Seizures Behavioral disorder |
CT: ipsilateral basifrontal and temporal calcification MRI: ipsilateral basifrontal cortical and subcortical T2 and FLAIR HI |
| Qureshi 2010(48) |
Left PRS | Vision loss Seizures Hemiparesis |
CT: narrowing of ipsilateral carotid canal MRI: ipsilateral cerebral atrophy; ipsilateral orbital atrophy MRA: diffuse narrowing of ipsilateral ICA and PCA |
| Takenouchi 2010(49) |
Right PRS | Hemiparesis Migraine headaches Movement disorder |
CT: N/A MRI: ipsilateral cerebral and thalamic atrophy and T2 and FLAIR HI MRA: normal |
| Fain 2011(18) | Right ECDS and PRS |
Seizures Headaches Hemiparesis Sensory defects |
CT: N/A MRI: ipsilateral sulci effacement; ipsilateral cerebral T2 and FLAIR HI; ipsilateral punctate hypointensities (may represent micro hemorrhage or calcification, possibly a cavernous malformation) |
| Fain 2011(18) | Right ECDS | Seizures Sensory defects |
CT: ipsilateral parietal high density lesion MRI: ipsilateral parietal GRE hypointensity suggestive of blood products or calcification, possibly a cavernous malformation |
| Longo 2011(20) |
Left PRS | Seizures | CT: N/A MRI: ipsilateral T2 HI and T1 hypointensity |
HI = hyperintensity/hyperintensities
Soft tissue and calvarial changes were not uniformly reported by all authors and are not included in this table.
Thirty-three of the 51 reported patients (65%) had neurologic symptoms. The most common symptoms were seizures (n=22, 43%), headaches (n=12, 24%), hemiparesis (n=7, 14%), abnormal deep tendon reflexes (n=5, 10%), sensory defects (n=4, 8%), and vision loss (n=4, 8%). Six patients had symptoms without radiographic abnormalities, though 3 were evaluated by only CT while the other 3 had MRI alone.
Discussion
This study demonstrates that a substantial portion of patients with ECDS and PRS have neurologic involvement, either in the form of neuroimaging abnormalities or neurologic symptoms. Given the bias inherent in reporting positive findings, it is not surprising that the literature review found a high rate of neuroimaging abnormalities (73%) and neurologic symptoms (65%). Yet, the results of the institutional retrospective review also revealed a substantial rate of neurologic involvement in the form of abnormal imaging or symptoms in 34% overall.
At our institution, 19% of the imaged patients had brain parenchymal abnormalities found on MRI; 17% of the asymptomatic patients and 22% of the symptomatic patients had abnormalities detected on MRI. The most common imaging abnormalities were hyperintense lesions seen on T2 sequences, consistent with our review of the literature. A prior review of adults with ECDS and PRS also found T2 hyperintensities to be the most common, although another series of 9 adult patients with ECDS and PRS found that all 9 patients with ECDS and PRS had evidence of cerebral edema while only 3 had T2 hyperintensities (2, 3). The retrospective chart review also confirms prior reports that 19-39% of children with ECDS and PRS have neurologic symptoms (5-7). In our study, the most common neurologic symptom was seizure, again in line with prior studies (5, 6).
The association of neurologic symptoms and neuroimaging abnormalities with the ECDS and PRS cannot be determined with complete certainty. The severity of the cutaneous manifestations did not predict neurologic symptoms or imaging findings as demonstrated by patients 13 and 30. On the other hand, the temporal relationship, with 6 of the 9 patients developing neurologic symptoms at approximately the same time as their cutaneous disease, suggests parallel disease courses.
The pathogenesis of morphea is unknown, and the pathogenesis of the neurologic involvement is even more unclear; an inflammatory process, particularly a vasculitis, is suspected. Brain biopsies have been performed in some patients and show a perivascular lymphocytic infiltrate with features of vasculitis (4, 8-13). Gliosis (proliferation of astrocytes usually leading to scar) and sclerosis of the leptomeninges have also been reported (8, 14). Intraparenchymal and intravascular calcifications can be seen (4). Abnormal and ectatic blood vessels are seen on both biopsies and angiographic studies, supporting the theory that vasculopathy or vasculitis are at the root of the cerebral changes (4, 15-17).
There are no published prospective studies on the effects of treatment on neurologic involvement; however, systemic anti-inflammatory and immunosuppressive medications are routinely used to treat other vasculitides. Additionally, data from case reports suggest that disease-modifying treatment improves the neurologic symptoms and abnormal radiologic findings (16, 18-21). Instances where neurologic symptoms were refractory to systemic therapy may represent late sequelae such as calcifications and fibrosis, similar to the cutaneous scarring of superficial disease, and immunosuppressive therapy is unlikely to alter the course in such cases.
The data from our institution show that neurologic symptoms did not necessarily predict neuroimaging abnormalities and vice versa. The MRI was abnormal in only 2 out of the 9 symptomatic patients, and 2 of the asymptomatic patients had marked MRI findings. Further research is necessary to determine the significance of MRI abnormalities in the absence of clinical symptoms and whether treatment alters the course of the neurologic disease. As understanding of the neurologic disease of ECDS and PRS is still limited, caution is advised in the treatment of these children.
We recommend that all symptomatic children with ECDS or PRS have an MRI of the brain performed at initial diagnosis with further imaging dictated by the initial findings and disease course. An MRI is preferred because it is more sensitive than CT at detecting the brain parenchymal abnormalities seen in ECDS and PRS.
Even when children are asymptomatic, brain imaging should be strongly considered to guide therapeutic decisions. Clinical predictors of intracranial disease are poor and the presence of intracranial involvement cannot be determined by history and physical examination. Waiting until symptom development to obtain an MRI and start systemic therapy may not be sufficient to completely prevent neurologic sequelae. A study looking at prescribing patterns found that dermatologists preferentially prescribed topical therapy and phototherapy for linear morphea over methotrexate and systemic corticosteroids, which were favored by rheumatologists (22). In fact, dermatologists prescribed methotrexate and systemic corticosteroids for less than 5% of patients with linear morphea, which many argue routinely requires systemic therapy given the potential for prominent disfigurement when on the face or functional compromise when on a limb (22). While topical therapies and phototherapy have been reported to be successful in treating the superficial manifestations of ECDS, these therapies are not thought to penetrate beyond the dermis and this modality should not be used for deeper disease (23, 24). The presence of intracranial or bony abnormalities on MRI should prompt consideration of early systemic therapy rather than topical therapy or phototherapy. If systemic immunosuppressive treatment will be used to treat the child, neuroimaging may still be helpful as a baseline examination to determine the presence or absence of intracranial abnormalities before starting therapy.
As ECDS and PRS are rare diseases, patient accrual for prospective studies is difficult and retrospective reviews are limited by small patient numbers. Although this study of pediatric ECDS and PRS patients evaluated by neuroimaging is the largest to date, it is limited by the small numbers and retrospective nature. Not all of the patients underwent neuroimaging, introducing the possibility of bias in that symptomatic patients were preferentially referred for radiologic scans; however, a substantial number of asymptomatic patients underwent imaging and rates of abnormal scans were not considerably different between asymptomatic (17%) and symptomatic patients (22%). Not all MRI images could be reviewed by the authors, though the unavailable MRIs were reported to be normal and any abnormalities would have increased the detection rate. The lack of findings in patients who are symptomatic suggests that MRI is not sensitive enough to pick up all neurologic involvement. All MRIs at our institution were performed using a 1.5 Tesla MRI scanner. Future studies may be needed to determine whether 3 Tesla scanners offer improved sensitivity, as has been shown in other diseases such as multiple sclerosis, or if other modalities are necessary (25, 26).
Despite the small numbers, this study suggests that a substantial fraction of children with ECDS and PRS have neurologic involvement with their disease. Children may not have neurologic symptoms and still have bony or intracranial manifestations of disease. Additionally, there is poor correlation between the severity of the soft tissue disease and neuroimaging findings. Before treatment initiation with topical therapy or phototherapy, screening MRIs should be considered and systemic immunosuppressive therapy should be strongly advised for those children found to have calvarial or intracranial abnormalities.
Acknowledgment
We are indebted to Ariel Rosen, research coordinator, for help with the institutional review board application and coordination of the project. We are indebted to Rita Sieracki, librarian, for assistance with the literature search. This publication was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1RR031973. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
References
- 1.Sommer A, Gambichler T, Bacharach-Buhles M, von Rothenburg T, Altmeyer P, Kreuter A. Clinical and serological characteristics of progressive facial hemiatrophy: a case series of 12 patients. J Am Acad Dermatol. 2006;54:227–233. doi: 10.1016/j.jaad.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Kister I, Inglese M, Laxer RM, Herbert J. Neurologic manifestations of localized scleroderma: a case report and literature review. Neurology. 2008;71:1538–1545. doi: 10.1212/01.wnl.0000334474.88923.e3. [DOI] [PubMed] [Google Scholar]
- 3.Appenzeller S, Montenegro MA, Dertkigil SS, Sampaio-Barros PD, Marques-Neto JF, Samara AM, et al. Neuroimaging findings in scleroderma en coup de sabre. Neurology. 2004;62:1585–1589. doi: 10.1212/01.wnl.0000124518.25087.18. [DOI] [PubMed] [Google Scholar]
- 4.Holland KE, Steffes B, Nocton JJ, Schwabe MJ, Jacobson RD, Drolet BA. Linear scleroderma en coup de sabre with associated neurologic abnormalities. Pediatrics. 2006;117:e132–136. doi: 10.1542/peds.2005-0470. [DOI] [PubMed] [Google Scholar]
- 5.Christen-Zaech S, Hakim MD, Afsar FS, Paller AS. Pediatric morphea (localized scleroderma): review of 136 patients. J Am Acad Dermatol. 2008;59:385–396. doi: 10.1016/j.jaad.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Zulian F, Athreya BH, Laxer R, Nelson AM, Feitosa de Oliveira SK, Punaro MG, et al. Juvenile localized scleroderma: clinical and epidemiological features in 750 children. An international study. Rheumatology (Oxford) 2006;45:614–620. doi: 10.1093/rheumatology/kei251. [DOI] [PubMed] [Google Scholar]
- 7.Zulian F, Vallongo C, Woo P, Russo R, Ruperto N, Harper J, et al. Localized scleroderma in childhood is not just a skin disease. Arthritis Rheum. 2005;52:2873–2881. doi: 10.1002/art.21264. [DOI] [PubMed] [Google Scholar]
- 8.Obermoser G, Pfausler BE, Linder DM, Sepp NT. Scleroderma en coup de sabre with central nervous system and ophthalmologic involvement: treatment of ocular symptoms with interferon gamma. J Am Acad Dermatol. 2003;49:543–546. doi: 10.1067/s0190-9622(03)00901-0. [DOI] [PubMed] [Google Scholar]
- 9.Stone J, Franks AJ, Guthrie JA, Johnson MH. Scleroderma “en coup de sabre”: pathological evidence of intracerebral inflammation. J Neurol Neurosurg Psychiatry. 2001;70:382–385. doi: 10.1136/jnnp.70.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moseley BD, Burrus TM, Mason TG, Shin C. Neurological picture. Contralateral cutaneous and MRI findings in a patient with Parry-Romberg syndrome. J Neurol Neurosurg Psychiatry. 2010;81:1400–1401. doi: 10.1136/jnnp.2009.202044. [DOI] [PubMed] [Google Scholar]
- 11.Carreno M, Donaire A, Barcelo MI, Rumia J, Falip M, Agudo R, et al. Parry Romberg syndrome and linear scleroderma in coup de sabre mimicking Rasmussen encephalitis. Neurology. 2007;68:1308–1310. doi: 10.1212/01.wnl.0000259523.09001.7a. [DOI] [PubMed] [Google Scholar]
- 12.Pupillo G, Andermann F, Dubeau F. Linear scleroderma and intractable epilepsy: neuropathologic evidence for a chronic inflammatory process. Ann Neurol. 1996;39:277–278. doi: 10.1002/ana.410390223. [DOI] [PubMed] [Google Scholar]
- 13.Shah JR, Juhasz C, Kupsky WJ, Asano E, Sood S, Fain D, et al. Rasmussen encephalitis associated with Parry-Romberg syndrome. Neurology. 2003;61:395–397. doi: 10.1212/wnl.61.3.395. [DOI] [PubMed] [Google Scholar]
- 14.Chung MH, Sum J, Morrell MJ, Horoupian DS. Intracerebral involvement in scleroderma en coup de sabre: report of a case with neuropathologic findings. Ann Neurol. 1995;37:679–681. doi: 10.1002/ana.410370519. [DOI] [PubMed] [Google Scholar]
- 15.Paprocka J, Jamroz E, Adamek D, Marszal E, Mandera M. Difficulties in differentiation of Parry-Romberg syndrome, unilateral facial sclerodermia, and Rasmussen syndrome. Childs Nerv Syst. 2006;22:409–415. doi: 10.1007/s00381-005-1262-x. [DOI] [PubMed] [Google Scholar]
- 16.Holl-Wieden A, Klink T, Klink J, Warmuth-Metz M, Girschick HJ. Linear scleroderma ‘en coup de sabre’ associated with cerebral and ocular vasculitis. Scand J Rheumatol. 2006;35:402–404. doi: 10.1080/03009740600556126. [DOI] [PubMed] [Google Scholar]
- 17.Sakai M, Aoki S, Inoue Y, Ashida R, Yamada H, Kiryu S, et al. Silent white matter lesion in linear scleroderma en coup de sabre. J Comput Assist Tomogr. 2008;32:822–824. doi: 10.1097/RCT.0b013e318153fd60. [DOI] [PubMed] [Google Scholar]
- 18.Fain ET, Mannion M, Pope E, Young DW, Laxer RM, Cron RQ. Brain cavernomas associated with en coup de sabre linear scleroderma: Two case reports. Pediatr Rheumatol Online J. 2011;9:18. doi: 10.1186/1546-0096-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg-Stern H, deGrauw T, Passo M, Ball WS., Jr Parry-Romberg syndrome: follow-up imaging during suppressive therapy. Neuroradiology. 1997;39:873–876. doi: 10.1007/s002340050525. [DOI] [PubMed] [Google Scholar]
- 20.Longo D, Paonessa A, Specchio N, Delfino LN, Claps D, Fusco L, et al. Parry-Romberg syndrome and Rasmussen encephalitis: possible association. Clinical and neuroimaging features. J Neuroimaging. 2011;21:188–193. doi: 10.1111/j.1552-6569.2009.00398.x. [DOI] [PubMed] [Google Scholar]
- 21.Menascu S, Padeh S, Hoffman C, Ben-Zeev B. Parry-Romberg syndrome presenting as status migrainosus. Pediatr Neurol. 2009;40:321–323. doi: 10.1016/j.pediatrneurol.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Johnson W, Jacobe H. Morphea in adults and children cohort II: Patients with morphea experience delay in diagnosis and large variation in treatment. J Am Acad Dermatol. 2012 doi: 10.1016/j.jaad.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Zwischenberger BA, Jacobe HT. A systematic review of morphea treatments and therapeutic algorithm. J Am Acad Dermatol. 2011;65:925–941. doi: 10.1016/j.jaad.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Walker D, Jacobe H. Phototherapy in the age of biologics. Semin Cutan Med Surg. 2011;30:190–198. doi: 10.1016/j.sder.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Wattjes MP, Harzheim M, Kuhl CK, Gieseke J, Schmidt S, Klotz L, et al. Does high-field MR imaging have an influence on the classification of patients with clinically isolated syndromes according to current diagnostic mr imaging criteria for multiple sclerosis? AJNR Am J Neuroradiol. 2006;27:1794–1798. [PMC free article] [PubMed] [Google Scholar]
- 26.Stankiewicz JM, Glanz BI, Healy BC, Arora A, Neema M, Benedict RH, et al. Brain MRI lesion load at 1.5T and 3T versus clinical status in multiple sclerosis. J Neuroimaging. 2011;21:e50–56. doi: 10.1111/j.1552-6569.2009.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asher SW, Berg BO. Progressive hemifacial atrophy: report of three cases, including one observed over 43 years, and computed tomographic findings. Arch Neurol. 1982;39:44–46. doi: 10.1001/archneur.1982.00510130046011. [DOI] [PubMed] [Google Scholar]
- 28.David J, Wilson J, Woo P. Scleroderma ‘en coup de sabre’. Ann Rheum Dis. 1991;50:260–262. doi: 10.1136/ard.50.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fry JA, Alvarellos A, Fink CW, Blaw ME, Roach ES. Intracranial findings in progressive facial hemiatrophy. J Rheumatol. 1992;19:956–958. [PubMed] [Google Scholar]
- 30.Liu P, Uziel Y, Chuang S, Silverman E, Krafchik B, Laxer R. Localized scleroderma: imaging features. Pediatr Radiol. 1994;24:207–209. doi: 10.1007/BF02012193. [DOI] [PubMed] [Google Scholar]
- 31.Cory RC, Clayman DA, Faillace WJ, McKee SW, Gama CH. Clinical and radiologic findings in progressive facial hemiatrophy (Parry-Romberg syndrome) AJNR Am J Neuroradiol. 1997;18:751–757. [PMC free article] [PubMed] [Google Scholar]
- 32.Derex L, Isnard H, Revol M. Progressive facial hemiatrophy with multiple benign tumors and hamartomas. Neuropediatrics. 1995;26:306–309. doi: 10.1055/s-2007-979779. [DOI] [PubMed] [Google Scholar]
- 33.Schievink WI, Mellinger JF, Atkinson JL. Progressive intracranial aneurysmal disease in a child with progressive hemifacial atrophy (Parry-Romberg disease): case report. Neurosurgery. 1996;38:1237–1241. doi: 10.1097/00006123-199606000-00038. [DOI] [PubMed] [Google Scholar]
- 34.Menni S, Marzano AV, Passoni E. Neurologic abnormalities in two patients with facial hemiatrophy and sclerosis coexisting with morphea. Pediatr Dermatol. 1997;14:113–116. doi: 10.1111/j.1525-1470.1997.tb00216.x. [DOI] [PubMed] [Google Scholar]
- 35.Taylor HM, Robinson R, Cox T. Progressive facial hemiatrophy: MRI appearances. Dev Med Child Neurol. 1997;39:484–486. doi: 10.1111/j.1469-8749.1997.tb07469.x. [DOI] [PubMed] [Google Scholar]
- 36.Higashi Y, Kanekura T, Fukumaru K, Kanzaki T. Scleroderma en coup de sabre with central nervous system involvement. J Dermatol. 2000;27:486–488. [PubMed] [Google Scholar]
- 37.Yano T, Sawaishi Y, Toyono M, Takaku I, Takada G. Progressive facial hemiatrophy after epileptic seizures. Pediatr Neurol. 2000;23:164–166. doi: 10.1016/s0887-8994(00)00168-5. [DOI] [PubMed] [Google Scholar]
- 38.Aynaci FM, Sen Y, Erdol H, Ahmetoglu A, Elmas R. Parry-Romberg syndrome associated with Adie’s pupil and radiologic findings. Pediatr Neurol. 2001;25:416–418. doi: 10.1016/s0887-8994(01)00333-2. [DOI] [PubMed] [Google Scholar]
- 39.Moko SB, Mistry Y, Blandin de Chalain TM. Parry-Romberg syndrome: intracranial MRI appearances. J Craniomaxillofac Surg. 2003;31:321–324. doi: 10.1016/s1010-5182(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 40.Finley TA, Siatkowski RM. Progressive visual loss in a child with Parry-Romberg syndrome. Semin Ophthalmol. 2004;19:91–94. doi: 10.1080/08820530490891139. [DOI] [PubMed] [Google Scholar]
- 41.Hulzebos CV, de Vries TW, Armbrust W, Sauer PJ, Kerstjens-Frederikse WS. Progressive facial hemiatrophy: a complex disorder not only affecting the face. A report in a monozygotic male twin pair. Acta Paediatr. 2004;93:1665–1669. [PubMed] [Google Scholar]
- 42.Sandhu K, Handa S. Subdural hygroma in a patient with Parry-Romberg syndrome. Pediatr Dermatol. 2004;21:48–50. doi: 10.1111/j.0736-8046.2004.21109.x. [DOI] [PubMed] [Google Scholar]
- 43.Sathornsumetee S, Schanberg L, Rabinovich E, Lewis D, Jr., Weisleder P. Parry-Romberg syndrome with fatal brain stem involvement. J Pediatr. 2005;146:429–431. doi: 10.1016/j.jpeds.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 44.Okumura A, Ikuta T, Tsuji T, Kato T, Fukatsu H, Naganawa S, et al. Parry-Romberg syndrome with a clinically silent white matter lesion. AJNR Am J Neuroradiol. 2006;27:1729–1731. [PMC free article] [PubMed] [Google Scholar]
- 45.Verhelst HE, Beele H, Joos R, Vanneuville B, Van Coster RN. Hippocampal atrophy and developmental regression as first sign of linear scleroderma “en coup de sabre”. Eur J Paediatr Neurol. 2008;12:508–511. doi: 10.1016/j.ejpn.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Chiang KL, Chang KP, Wong TT, Hsu TR. Linear scleroderma “en coup de sabre”: initial presentation as intractable partial seizures in a child. Pediatr Neonatol. 2009;50:294–298. doi: 10.1016/S1875-9572(09)60081-4. [DOI] [PubMed] [Google Scholar]
- 47.Sartori S, Martini G, Calderone M, Patrizi A, Gobbi G, Zulian F. Severe epilepsy preceding by four months the onset of scleroderma en coup de sabre. Clin Exp Rheumatol. 2009;27:64–67. [PubMed] [Google Scholar]
- 48.Qureshi UA, Wani NA, Altaf U. Parry-Romberg syndrome associated with unusual intracranial vascular malformations and Phthisis bulbi. J Neurol Sci. 2010;291:107–109. doi: 10.1016/j.jns.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Takenouchi T, Solomon GE. Alien hand syndrome in Parry-Romberg syndrome. Pediatr Neurol. 2010;42:280–282. doi: 10.1016/j.pediatrneurol.2009.11.010. [DOI] [PubMed] [Google Scholar]