Abstract
Objective
Examine the relation of Alzheimer's disease (AD) pathology, cerebral infarcts, and Lewy body (LB) pathology to cognition in persons without cognitive impairment.
Methods
Persons without dementia from two cohort studies of aging, the Religious Orders Study and the Memory and Aging Project, agreed to annual clinical evaluation and brain donation. The studies had 19 neuropsychological performance tests in common that assessed five cognitive domains. Of 296 persons without cognitive impairment who died and underwent post-mortem assessment, we quantified AD pathology as a global pathology score, and as amyloid load and PHFtau tangle density, cerebral infarcts and LB pathology. Linear regression was used to examine the relation of neuropathology to cognitive abilities controlling for demographics.
Results
Nearly all persons had AD pathology with more than three quarters exhibiting amyloid; 22% macroscopic and 24% microscopic infarctions, and 13% had LB pathology. The global measure of AD pathology was related to global cognition (p=0.008) whereas infarcts and Lewy bodies were not. Amyloid load was related to global cognition (p<0.05) with only a trend for tangles (p=0.08). In analyses of cognitive domains, AD pathology (p=0.006), PHFtau tangles (p=0.03), and macroscopic infarctions (p=0.02) were related to episodic memory with a trend for amyloid load (p=0.06); and AD pathology (p=0.02) and amyloid load (p=0.03) were related to working memory. Findings for global cognition and episodic memory were stronger in additional analyses with neocortical amyloid and mesial temporal tangles.
Interpretation
AD pathology and macroscopic infarctions are common in older persons without cognitive impairment and related to episodic and working memory.
Keywords: clinical-pathology, no cognitive impairment
Introduction
Understanding the preclinical phase of AD is an urgent public health priority [1,2]. It has long been recognized that older persons without dementia can accumulate neuropathologic changes of AD, cerebral infarctions, and Lewy bodies. However, this pathology often is related to the presence of mild cognitive impairment (MCI) [3-5]. Much less is known about the relation of common neuropathologic indices to cognitive function in persons without cognitive impairment (i.e., persons without dementia or MCI). Recent in vivo imaging data suggests that fibrillar amyloid and hippocampal volume are both related to episodic memory in persons without cognitive impairment [6]. We previously examined the relation of the pathologic diagnoses of AD to cognition in older persons without cognitive impairment [7]. We found that about a third of persons met intermediate or high likelihood National Institute on Aging-Reagan (NIA-Reagan) neuropathologic criteria for AD and that persons meeting such criteria exhibited lower scores on tests of episodic memory. In this paper, we quantify a global index of AD pathology, amyloid load and paired helical filament tau-positive (PHFtau) tangles, macro- and microscopic cerebral infarcts and LB pathology. We then examine their relation to global cognitive function, episodic memory and four other cognitive domains assessed proximate to death in nearly 300 persons without cognitive impairment proximate to death from two community based cohort studies of aging.
Methods
Participants
Subjects are participants from two, clinical-pathologic cohort studies of aging and dementia: the Religious Orders Study and the Memory and Aging Project. Both studies were approved by the Institutional Review Board of Rush University Medical Center.
Religious Orders Study participants were older Catholic nuns, priests and brothers without known dementia who agreed to annual clinical evaluations and signed an informed consent and an Anatomical Gift Act for brain donation at the time of death. Subjects come from about 40 groups across the United States. The study has a rolling admission and 1,162 persons completed a uniform structured baseline clinical evaluation between January 1994 and November 2011. Follow-up evaluations, identical in all essential details, were performed annually by examiners blinded to previously collected data. Participation in the annual follow-up evaluations exceeds 95% of survivors. The autopsy rate exceeds 90% with 543 autopsies of 578 deaths, including 163 autopsies on persons without cognitive impairment.
Memory and Aging Project participants were older community-dwelling persons without known dementia who agreed to annual clinical evaluations and signed an informed consent and an Anatomical Gift Act for donation of brain, spinal cord, and selected nerves and muscles at the time of death. Subjects come from about 40 retirement communities and senior subsidized housing facilities across northeastern Illinois. The study has a rolling admission and 1,490 persons completed a uniform structured baseline clinical evaluation between October 1997 and November 2011. Follow-up evaluations, identical in all essential details, were performed annually by examiners blinded to previously collected data. Participation in the annual follow-up evaluations exceeds 90% of survivors. The autopsy rate is exceeds 80% with 425 autopsies of 521 deaths, including 126 autopsies of persons without cognitive impairment.
Clinical evaluation procedures
Subjects in both studies underwent uniform, structured, clinical evaluations that included an identical evaluation for dementia, AD, and MCI as previously reported [8-10]. Briefly, the evaluation included a medical history, neurologic examination, and neuropsychological performance testing. Diagnostic classification of dementia and AD proceeded in a multi-step process. The diagnoses of dementia and AD were based on the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association. MCI referred to those persons rated as impaired on cognitive testing by the neuropsychologist but did not meet criteria for dementia. At the time of death, all available clinical data were reviewed by a neurologist and a summary diagnostic opinion was rendered regarding the most likely clinical diagnosis at the time of death.
The two studies had 19 cognitive performance tests in common. The Mini-Mental State Examination (MMSE) was only used to describe the cohort and complex ideational material was only used for diagnostic classification. The remaining 17 tests were used to assess five domains of cognition. As previously described, there were seven tests of episodic memory, three of semantic memory, three of working memory, two of perceptual speed, and two of visuospatial ability [7]. The tests were converted to z scores, using the mean and standard deviation from the baseline evaluation of all participants in both studies, and averaged to yield summary measures of global cognition and five cognitive domains. The summary measures have the advantage of minimizing floor and ceiling effects and other sources of random variability.
Brain autopsy procedures
Brains of deceased subjects were removed, weighed, cut into 1cm coronal slabs and underwent complete macroscopic evaluation as described previously [7]. The slabs were then immersion fixed in 4% paraformaldehyde for 72 hours, blocked, and embedded in paraffin. The neuropathologic evaluation was completed on the first 296 consecutive deceased and autopsied participants without dementia or MCI (165 from the Religious Orders Study and 131 from the Memory and Aging Project).
Paraffin-embedded tissue blocks were sectioned at 6μm. One block was dissected from five regions (mid-frontal cortex, middle/superior temporal cortex, inferior parietal cortex, hippocampus and entorhinal cortex) and stained with modified Bielschowsky to assess for AD pathology. These blocks (except the hippocampus) and the midbrain, anterior cingulate cortex were also stained with antibodies to alpha-synuclein to assess for Lewy bodies. These seven regions plus two others (anterior basal ganglia, and thalamus) were stained with hemotoxylyn and eosin to assess for microscopic infarctions.We also dissected blocks for histologival confirmation of macroscopic infarctions. Separately, tissue blocks from each 1cm slab from two regions (entorhinal cortex, CA1/subiculum of the hippocampus) and two tissue blocks from five regions (superior frontal cortex, dorsolateral prefrontal cortex, inferior temporal cortex, angular gyrus, and anterior cingulate cortex) were cut into 20μm sections to assess amyloid load and PHFtau positive tangles. Multiple blocks were taken to reduce random variability.
Neuritic and diffuse plaques, and neurofibrillary tangles were counted in the region which appeared to have the maximum density of each pathology as previously described [11]. A standardized score was created for each neuropathology in each region by dividing the raw count by the standard deviation of the mean for the same neuropathology in the same region. This standardization procedure puts the pathologic indices on a relatively common scale. They were averaged to create a composite measure supported by factor analysis (the principal component accounted for 36.9% of the variance) and Cronbach coefficient alpha (0.87). Because the distribution was skewed, the square root of the global AD pathology measure was used in analyses. This mathematical transformation was needed to meet the assumptions of multi-variable normality for linear regression and to be consistent with prior work from these cohorts [11,12].
Amyloid-β was labeled with an N-terminus directed monoclonal antibody (10D5, Elan Pharm.; 1:1000). Immunohistochemistry was performed as previously described using diaminobenzidine as the reporter with 2.5% nickel sulfate to enhance immunoreaction product contrast [12,13]. Video images of amyloid-β stained sections were captured for a random sample for quantitative analysis of amyloid deposition. Briefly, the region of interest was outlined at low power using StereoInvestigator software version 9 (MicroBrightfield Inc., Colchester, VT.) and an Olympus BX-51 microscope with an attached motorized stage. A grid was randomly placed over the outlined area and approximately 25 to 50% of the region of interest was sampled. Following camera and illumination calibration, the magnification was raised to the 20X objective and images at each sampling site were obtained with a motorized stage. Quantification of amyloid-β load was accomplished by image processing in an automated, multistage computational image analysis protocol. Mean fraction (% area) per region and per subject was computed. The value included intracortical vascular amyloid. The overall measure of amyloid load was supported by factor analysis (the principal component accounted for 85.8%% of the variance) and Cronbach coefficient alpha (0.97). Secondary analyses examined mesial temporal lobe and neocortical amyloid separately.
PHFtau was labeled with an antibody specific for phosphorylated tau (AT8, Innogenetics, San Ramon, CA, 1:1000). Quantification of tangle density was accomplished with the stereological mapping station as described previously [12,13]. Briefly, after the region was delimited at low power, a grid was randomly placed over the entire region by the software program. Magnification was raised to the 40X objective and the program was engaged to direct the motorized stage to stop at each intersection point of the grid for sampling. The operator focused on each field visualized on the video monitor within the superimposed counting frame. All objects within the 150×150μm counting frame that did not touch the exclusion lines of the box (bottom and left sides) were counted. Over 15 % of the area is sampled for tangles. The overall measure of PHFtau tangles was supported by factor analysis (the principal component accounted for 54.4% of the variance) and Cronbach coefficient alpha (0.82). Secondary analyses examined mesial temporal lobe and neocortical PHFtau tangles separately.
For each brain we identified the age, volume (in mm3), side, and anatomic location of all chronic macroscopic cerebral infarctions [7,14]. Locations of all chronic microinfarcts, including cavitated or incomplete infarcts, with few remaining macrophages and fibrillary gliosis, [15]. Macroscopic and microscopic infarctions were analyzed as dichotomous variables.
Lewy bodies were identified and recorded as nigral predominant, limbic type, or neocortical type as as previously described with an antibody to α-synuclein (Zymed, LB509, 1:100) [7,14]. Lewy body pathology was analyzed as a dichotomous variable.
Statistical analyses
We first describe the demographic, clinical, and pathologic characteristics of the participants. Multiple linear regression analyses were used to examine the relation between neuropathologic indices and global cognition and five cognitive domains proximate to death. For each measure of cognitive function, we first developed a core model examining cognition as a function of age, sex and education. We then added a term for the global measure of AD pathology. Finally, we added terms for macroscopic and microscopic infarctions, and Lewy bodies. We determined the adjusted R2 for each model. The next set of analyses replaced the global measure of AD pathology with separate measures of amyloid load and PHFtau in the fully adjusted model. Additional analyses evaluated regional differences in amyloid load and PHFtau tangle pathology with separate measures of mesial temporal lobe (hippocampal and entorhinal cortex) and neocortical pathology (middle frontal, superior frontal, inferior temporal, and angular gyrus). This was supported conceptually and empirically by factor analysis. Varimax rotation for tangle pathology resulted in two factors (Spearman σ = 0.57, p<.0001). For conceptual reasons, we also examined neocortical and mesial temporal lobe amyloid separately despite high inter-correlations (Spearman σ = 0.87, p<.0001). Analyses were performed in SAS® [16].
Results
Subjects were approximately age 85 at death, more than a third were male, nearly all were non-Hispanic white, and they had an average of more than 16 years of education (table 1). The MMSE score, obtained an average 7 months prior to death, was greater than 28. Mean levels of cognitive function proximate to death, expressed in standard units were positive demonstrating that these persons scored above the average for the entire cohort at baseline. The mean, range, and percent positive for the global AD pathology score, amyloid, and PHFtau tangles are included in table 1, as is the distribution of macroscopic and microscopic cerebral infarctions and Lewy bodies.
Table 1.
Selected demographic, clinical and pathologic characteristics of subjects without cognitive impairment from the Religious Orders Study and Memory and Aging Project.
| Characteristics | |
|---|---|
| N | 296 |
| Age at death, mean (SD) | 85.0 (6.6) |
| Male, N (%) | 112 (37.8%) |
| Non-Hispanic White, N (%) | 281 (94.9) |
| Education, mean (SD) | 16.5 (3.8) |
| Interval (cognition to death in month), mean (SD) | 7.1 (4.4) |
| Cognitive function tests proximate to death, mean (SD) | |
| MMSE | 28.2 (1.8) |
| Global Cognition | 0.15 (0.42) |
| Episodic Memory | 0.36 (0.53) |
| Working Memory | 0.06 (0.67) |
| Semantic Memory | 0.09 (0.58) |
| Perceptual Speed | -0.24 (0.84) |
| Visuospatial Ability | 0.09 (0.69) |
| AD Pathologic characteristics; mean (SD; range), % non-zero* | |
| Global AD pathology score | 0.41 (0.40; 0-1.9), 98.3 |
| Amyloid load | 2.32 (3.09; 0-22.9), 76.4 |
| Mesial Temporal Lobe amyloid | 1.51 (2.23; 0-15.5), 57.7 |
| Neocortical amyloid | 2.62 (3.40; 0-25.3), 76.0 |
| PHFtau neurofibrillary tangles | 2.63 (3.32; 0-24.0), 99.3 |
| Mesial Temporal Lobe tangles | 9.42 (10.36; 0.01-63.3), 100 |
| Neocortical score | 0.58 (1.63; 0-16.8), 91.6 |
| Infarctions and Lewy bodies, N (%) | |
| Macroscopic Infarcts | 66 (22.3%) |
| Microscopic Infarcts | 71 (24.0%) |
| Lewy bodies | 39 (13.2%) |
| Nigral predominant | 13 (4.4%) |
| Limbic predominant | 14 (4.7%) |
| Neocortical | 12 (4.1%) |
amyloid load and PHFtau tangles missing for 12 and 18 respectively
Relation of neuropathology to global cognition
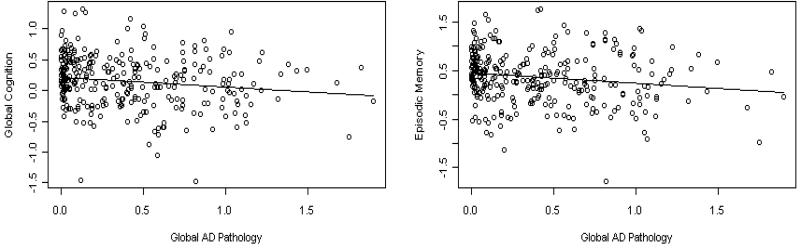
The core model examined cognition as a function of demographics (Table 2). We next examined global cognition as a function of the global AD pathology score. In these analyses, each unit of AD pathology was associated with a nearly 0.2 standard unit lower global cognition score. We then added terms for macroscopic and microscopic infarctions, and Lewy bodies. The association of AD pathology with cognition was unchanged and the additional neuropathologies were not related to cognition. Finally, we substituted separate measures of amyloid load and PHFtau tangles for the global AD pathology score. In this analysis, each percent increase in amyloid was associated with nearly a 0.02 standard unit lower global cognition score. Further, each PHFtau tangle was associated with more than a 0.01 standard unit lower global cognition score, but this failed to reach statistical significance. Infarctions and Lewy bodies were not related to global cognition in these models. We used the adjusted R2 to convey the magnitude of the association. The core model with age, sex, and education explained 7% of the variance of global cognition. Measures of AD pathology increased the R2 to about 11%. The scatter plot and model-derived regression line for the relation of global pathology with global cognition is shown in Figure 1A.
Table 2.
Linear regression models examining level of global cognitive function proximate to death as a function of neuropathology. The Core Model shows the R2 from the model with age, sex, and education. Model 1 includes a global measure of AD pathology based on silver stain. Model 2 adds terms for macroscopic and microscopic infarctions, and Lewy bodies. Model 3 includes separate measures of amyloid load and PHFtau positive tangles, in addition to macroscopic and microscopic infarctions, and Lewy bodies. Estimate (Standard Error, p Value). All models controlled for age, sex, and education.
| Term | Core Model | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Adjusted R2 | 0.070 | 0.095 | 0.107 | 0.106 |
| Global AD pathology | -0.177 (0.059,0.000) | -0.159 (0.059, 0.008) | ||
| Amyloid load | -0.017 (0.008, 0.048) | |||
| PHFtau tangles | -0.014 (0.008, 0.076) | |||
| Macroscopic infarcts | -0.107 (0.058, 0.06) | -0.094 (0.058, 0.10) | ||
| Microscopic infarcts | -0.083 (0.055, 0.14) | -0.061 (0.057, 0.28) | ||
| Lewy bodies | -0.021 (0.069, 0.76) | -0.020 (0.069, 0.78) |
Figure 1.
Relation of global pathology to global cognition (A, left) and to episodic memory (B, right) from regression models controlling for age, sex, education, macroscopic and microscopic infarctions, and Lewy bodies.
Relation of neuropathology to episodic memory and other cognitive domains
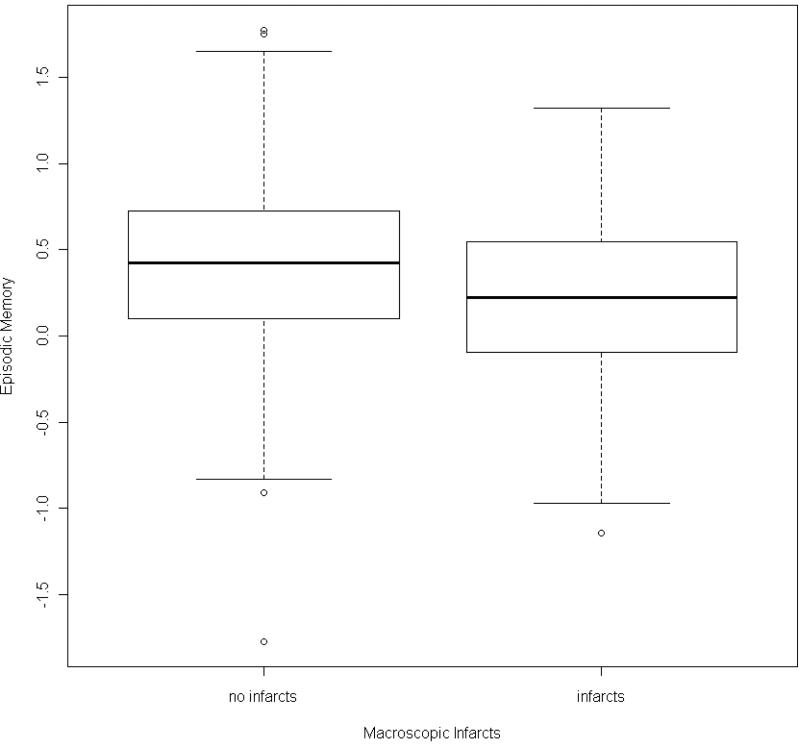
The global cognitive measure is comprised of measures of five related but relatively dissociable cognitive systems. Therefore, we next conducted a series of analyses examining the relation of neuropathology to level of function in each domain (Table 3). In the first set of models, both the global measure of AD pathology and macroscopic infarcts were related to episodic memory (Table 3. A.). The core model explained approximately 7% of the variance of global cognition whereas the model with the AD pathology and macroscopic infarcts increased the R2 to more than 11% with AD pathology being associated with about a quarter standard unit lower episodic memory score. The scatter plot and model-derived regression line for the relation of global pathology to episodic memory is shown in Figure 1B. Box plots showing the relation of infarcts to episodic memory is shown in Figure 2. The global measure of AD pathology was also related to about a quarter standard unit lower working memory score. However, the variance explained was quite small increasing from less than 1% in the core model to only 2.6% with AD pathology (Table 3. B.). Neuropathologic indices were not related to semantic memory, perceptual speed, or visuospatial ability.
Table 3.
Linear regression models examining level of five cognitive abilities proximate to death as a function of neuropathology. The Core Model shows the R2 from the model with age, sex, and education. Model 1 includes a global measure of AD pathology based on silver stain. Model 2 adds terms for macroscopic and microscopic infarctions, and Lewy bodies. Estimate (Standard Error, p Value). All models controlled for age, sex, and education.
| A. Episodic Memory | |||
|---|---|---|---|
| Term | Core Model | Model 1 | Model 2 |
| Adjusted R2 | 0.072 | 0.098 | 0.111 |
| Global AD pathology | -0.228 (0.075,0.002) | -0.208 (0.075,0.006) | |
| Macroscopic infarcts | -0.178 (0.073,0.015) | ||
| Microscopic infarcts | -0.048 (0.070,0.491) | ||
| Lewy bodies | -0.027 (0.087,0.756) | ||
| B. Working Memory | |||
|---|---|---|---|
| Term | Core Model | Model 1 | Model 2 |
| Adjusted R2 | 0.009 | 0.026 | 0.026 |
| Global AD pathology | -0.244 (0.098,0.013) | -0.240 (0.099,0.016) | |
| Macroscopic infarcts | -0.117 (0.096,0.224) | ||
| Microscopic infarcts | 0.023 (0.092,0.804) | ||
| Lewy bodies | 0.126 (0.115,0.274) | ||
| C. Semantic Memory | |||
|---|---|---|---|
| Term | Core Model | Model 1 | Model 2 |
| Adjusted R2 | 0.052 | 0.049 | 0.061 |
| Global AD pathology | -0.045 (0.084,0.594) | -0.019 (0.084,0.820) | |
| Macroscopic infarcts | -0.139 (0.081,0.090) | ||
| Microscopic infarcts | -0.119 (0.079,0.131) | ||
| Lewy bodies | -0.062 (0.097,0.523) | ||
| D. Perceptual Speed | |||
|---|---|---|---|
| Term | Core Model | Model 1 | Model 2 |
| Adjusted R2 | 0.094 | 0.092 | 0.091 |
| Global AD pathology | -0.070 (0.119,0.555) | -0.056 (0.120,0.641) | |
| Macroscopic infarcts | -0.096 (0.116,0.409) | ||
| Microscopic infarcts | -0.021 (0.112,0.854) | ||
| Lewy bodies | -0.195 (0.138,0.160) | ||
| E. Visuospatial Ability | |||
|---|---|---|---|
| Term | Core Model | Model 1 | Model 2 |
| Adjusted R2 | 0.048 | 0.046 | 0.040 |
| Global AD pathology | -0.051 (0.099,0.607) | -0.046 (0.100,0.649) | |
| Macroscopic infarcts | -0.029 (0.097,0.768) | ||
| Microscopic infarcts | -0.005 (0.094,0.962) | ||
| Lewy bodies | -0.121 (0.116,0.300) | ||
Figure 2.
Box plots showing the relation of macroscopic infarctions to episodic memory.
In the final set of analyses, we substituted separate measures of amyloid load and PHFtau tangles for the global AD pathology score and examined their associations with each domain (Table 4). In this analysis, each percent increase in amyloid was associated with a 0.02 standard unit lower episodic memory score (p=0.059) and each PHFtau tangle was associated with a 0.02 standard unit lower episodic memory score (p=0.034). Macroscopic infarctions were also related to episodic memory. Together, AD pathology and macroscopic infarctions explained more than 11% of the variance in episodic memory. Amyloid load was also associated with working memory, but the effect was small. Neither amyloid load nor PHFtau tangles were related to semantic memory, perceptual speed, or visuospatial ability.
Table 4.
Linear regression models examining level of five cognitive abilities proximate to death as a function of neuropathologic indices. Estimate (Standard Error, p Value). All models controlled for age, sex, and education.
| Term | Episodic Memory | Working Memory | Semantic Memory | Perceptual Speed | Visuospatial Ability |
|---|---|---|---|---|---|
| Adjusted R2 | 0.116 | 0.022 | 0.044 | 0.073 | 0.039 |
| Amyloid load | -0.020 (0.011,0.059) | -0.030 (0.014,0.034) | 0.003 (0.012,0.793) | -0.019 (0.017,0.260) | -0.002 (0.015,0.894) |
| PHFtau tangles | -0.021 (0.010,0.034) | -0.011 (0.013,0.404) | 0.001 (0.011,0.905) | -0.006 (0.016,0.720) | 0.022 (0.013,0.096) |
| Macroscopic infarcts | -0.169 (0.073,0.022) | -0.101 (0.097,0.297) | -0.100 (0.084,0.235) | -0.053 (0.118,0.652) | -0.009 (0.100,0.927) |
| Microscopic infarcts | -0.040 (0.073,0.580) | 0.035 (0.096,0.719) | -0.116 (0.084,0.167) | -0.011 (0.117,0.924) | -0.054 (0.100,0.587) |
| Lewy bodies | -0.020 (0.088,0.817) | 0.130 (0.117,0.267) | -0.050 (0.101,0.621) | -0.207 (0.142,0.145) | -0.148 (0.121,0.220) |
Relation of mesial temporal lobe and neocortical amyloid and tangles to cognition
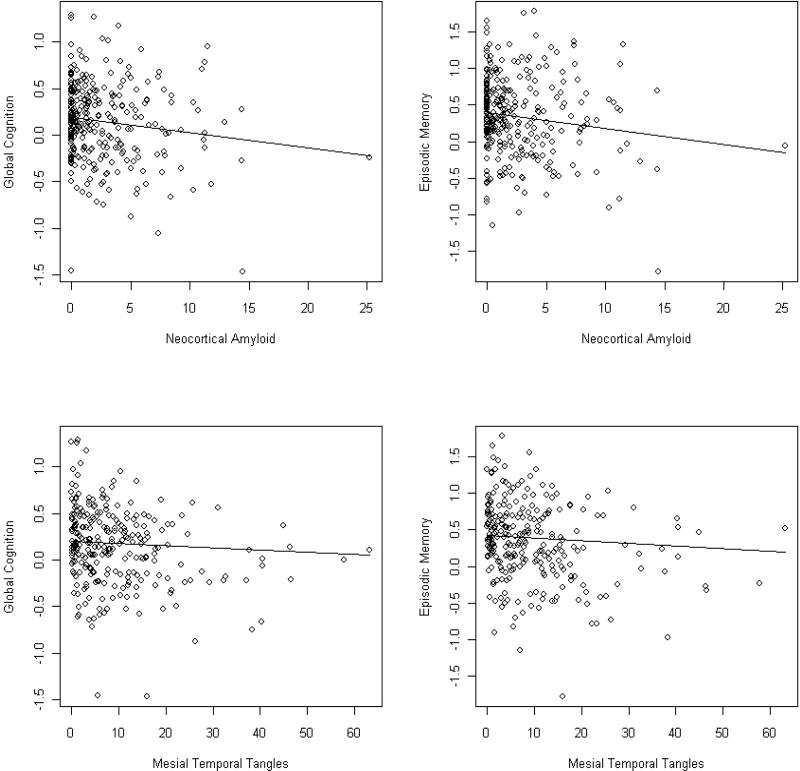
It is possible that regionally specific measures of AD pathology would explain more variance in cognition than global measures. Therefore, we conducted additional analyses of amyloid load and PHFtau tangles in the mesial temporal lobe and neocortex. In these models, neocortical amyloid and mesial temporal PHFtau tangles were both related to global cognition whereas mesial temporal amyloid and neocortical tangles were not. Together, they explained an additional 1% of the variance compared to the model with overall amyloid load and PHFtau tangles. Similarly, neocortical amyloid and mesial temporal PHFtau tangles were both related to episodic memory. Together, they explained an additional 2% of the variance relative to the model with overall amyloid load and PHFtau tangles. Working memory was also associated with neocortical amyloid (p=0.039) with a trend for mesial temporal lobe amyloid (p=0.064). However, with less regional specificity, the model did not explain additional variance. The scatter plot and model-derived regression lines for the relation of neocortical amyloid and mesial temporal tangles to global cognition and episodic memory is shown in Figure 3.
Figure 3.
Relation of neocortical amyloid to global cognition (A, top left) and to episodic memory (B, top right), and of mesial temporal lobe PHFtau tangles to global cognition (C, bottom left) and to episodic memory (D, bottom right), from regression models controlling for age, sex, education, macroscopic and microscopic infarctions, and Lewy bodies.
Discussion
We examined the relation of AD pathology, cerebral infarctions, and Lewy bodies to level of global cognition, episodic memory and four other cognitive abilities in nearly 300 persons without cognitive impairment from two community-based cohort studies. We found that AD pathology, especially neocortical amyloid and mesial temporal lobe tangles, was robustly related to global cognitive function, and to episodic memory, accounting for up to five percent of the variance in cognition. The variance explained by the pathologic indices approached that explained by age, sex, and education combined, but overall is relatively small. While it's possible that more precise measures of pathology would improve the prediction, we think it is likely that other as yet unknown processes contribute to cognition among persons without dementia or MCI. Macroscopic infarctions also contributed to episodic memory performance, but not to other cognitive abilities. We did not find associations between microscopic infarctions or Lewy bodies with cognition in this group of very high functioning individuals. Amyloid was also weakly associated with working memory. Together, these findings suggest that AD pathology and, to some extent, macroscopic infarctions are associated with decrements in cognitive functioning, particularly episodic memory and working memory to a lesser extent, even among older persons without cognitive impairment.
There is great interest in the preclinical phase of AD, especially the pathophysiologic process that precedes MCI [1,2]. Several studies have reported the presence of AD pathology in persons without dementia [4,5,17-24]. However, little data are available regarding AD and other pathology among persons without cognitive impairment, and even less on the relation of pathology to cognition in this group [3,7,25-29]. In our prior study of persons without cognitive impairment, the presence of pathologic AD, defined as intermediate or highly likelihood AD by NIA-Reagan criteria, was associated with about a quarter standard unit lower episodic memory score [7]. The present study expanded on our prior work in four important ways. First, we used quantitative measures of AD pathology, including molecular-specific measures to assess AD pathology. Second, we examined regional differences in the relation of AD pathology to cognition. Third, we explicitly examined the associations of macro- and microscopic infarctions and Lewy body pathology with cognition. Finally, the sample size is more than double. We found associations between AD pathology and working memory, in addition to episodic memory. Of note, at least one other study also found associations between AD pathology and multiple cognitive domains among persons without dementia or MCI [28]. Further, in this study, we also found that macroscopic infarctions were also related to episodic memory but not to other cognitive domains. Some data suggest that cerebral infarctions have a relatively selective effect on measures of executive function, such as working memory and perceptual speed [30]. However, increasing information suggests that episodic memory depends on a distributed network making it vulnerable to other pathologies including infarctions, in addition to AD pathology which has a predilection for mesial temporal lobe structures [31]. Recent evidence also suggests that AD pathology in persons without cognitive impairment assessed with PET imaging is related to attention and other domains of cognition in addition to episodic memory [32].
We also examined the relation of amyloid load and PHFtau tangles separately using protein-specific immunostaining and unbiased quantitative techniques. We first examined overall amyloid load and PHFtau tangles, and subsequently, region-specific indices. We found that neocortical amyloid was related to gobal cognition, episodic memory, and working memory. We also found that mesial temporal lobe tangles were related to global cognition and episodic memory. This is an intriguing finding and supports the hypothesis that mesial temporal lobe neurofibrillary pathology may result from two or more age-related processes, one of which is AD [33]. Considerable evidence suggests that amyloid is an early event eventually leading to tangle formation in the development of AD [34]. However, tangles can be seen in other disease processes [35]. Amyloid deposition appears to begin in the neocortex [36,37]. This could account for the associations with working memory in addition to episodic memory. By contrast, tangle formation appears to begin in the mesial temporal lobe [38]. In this study, nearly every brain had mesial temporal lobe neurofibrillary pathology whereas just over 70% had amyloid. The recently published NIA-Alzheimer's Association guidelines for the neuropathologic assessment of AD require amyloid for its pathologic diagnosis [39].
The study has strengths that lend confidence in the findings. All subjects were recruited from the community and underwent a annual detailed clinical evaluation and were determined to be free of dementia and MCI proximate to death. Annual follow-up and autopsy rates were very high. Uniform, structured procedures were followed that enhanced stability of diagnoses across time, space, and examiners. All post-mortem evaluations were performed by experienced and trained examiners shielded to all clinical data. The study also had limitations. AT8 identified pretangles in addition to mature tangles and evidence suggests that one obtains different associations when examining different tau epitopes [40]. There were not an exhaustive search for infarcts and it is likely that some were missed. Nor did we have measures of white matter changes [41]. Similarly, our search for Lewy body pathology was not exhaustive, and the cognitive tests administered were not necessarily the most sensitive to Lewy body pathology. Thus, it is possible that we underestimated the effect of cerebrovascular disease and Lewy body pathology on cognition. Further, Lewy body pathology in these persons may be associated with parkinsonian or other non-cognitive signs such as odor identification, even in persons without Parkinson's disease [42,43].
Table 5.
Linear regression models examining level of global cognitive function and episodic memory proximate to death as a function of neuropathology. All models include terms for macroscopic and microscopic infarctions, and Lewy bodies. Model 1 includes a term for mesial temporal lobe amyloid load. Model 2 includes a term for neocortical amyloid load. Model 3 includes a term for mesial temporal lobe PHFtau tangles. Model 4 includes a term for neocortical PHFtau tangles. Model 5 includes terms for both neocortical amyloid load and mesial temporal lobe PHFtauu tangles. Estimate (Standard Error, p Value). All models controlled for age, sex, and education, macroscopic infarctions, microscopic infarctions and Lewy bodies.
| A. Global Cognition | |||||
|---|---|---|---|---|---|
| Term | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
| Adjusted R2 | 0.076 | 0.093 | 0.104 | 0.079 | 0.116 |
| Mesial temporal amyloid | -0.013 (0.012,0.257) | ||||
| Neocortical amyloid | -0.016 (0.007,0.028) | -0.016 (0.007,0.030) | |||
| Mesial temporal tangles | -0.007 (0.003,0.004) | -0.006 (0.003,0.013) | |||
| Neocortical tangles | -0.010 (0.015,0.496) | ||||
| B. Episodic Memory | |||||
|---|---|---|---|---|---|
| Term | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
| Adjusted R2 | 0.085 | 0.101 | 0.122 | 0.089 | 0.136 |
| Mesial temporal amyloid | -0.008 (0.015,0.589) | ||||
| Neocortical amyloid | -0.019 (0.009,0.039) | -0.022 (0.010,0.023) | |||
| Mesial temporal tangles | -0.011 (0.003,0.001) | -0.010 (0.003,0.003) | |||
| Neocortical tangles | -0.016 (0.019,0.409) | ||||
Acknowledgment
We thank the participants in the Religious Orders Study and the Memory and Aging Project, and the staff of the Rush Alzheimer's Disease Center. This study was supported by National Institute on Aging grants P30AG10161, R01AG15819, R01AG17917, and R01AG34374.
Footnotes
Author contributions
All authors contributed to data collection. Dr. Bennett had full access to all the data in the study and had final responsibility for the decision to submit for publication and takes full responsibility for the manuscript. Drs. Wilson, Boyle, Buchman and Schneider made critical revisions to the text.
Conflicts of Interest
The authors have no conflicts of interest to report.
References
- 1.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galvin JE, Powlishta KK, Wilkins K, et al. Predictors of preclinical Alzheimer disease and dementia: a clinicopathologic study. Arch Neurol. 2005;62:758–765. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–72. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 5.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 6.Mormino EC, Kluth JT, Madison CM, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009 May;132:1310–23. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 8.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–76. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- 9.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 10.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67:441–5. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- 11.Bennett DA, Wilson RS, Schneider JA, et al. Apolipoprotein E4 allele, Alzheimer's disease pathology, and the clinical expression of Alzheimer's disease. Neurology. 2003;60:246–252. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- 12.Bennett DA, Schneider JA, Arnold SE, Tang Y, Wilson RS. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. The Lancet Neurology. 2006;5:406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 13.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load and with clinical Alzheimer's disease and level of cognitive function. Archives of Neurology. 2004;61:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 14.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–8. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42:722–7. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SAS Institute Inc. SAS OnlineDocR 9.1.3. SAS Institute Inc.; Cary, NC: 2004. [Google Scholar]
- 17.Crystal HA, Dickson DW, Sliwinski MJ, et al. Pathological markers associated with normal aging and dementia in the elderly. Ann Neurol. 1993;34:566–573. doi: 10.1002/ana.410340410. [DOI] [PubMed] [Google Scholar]
- 18.Riley KP, Snowdon DA, Markesbery WR. Alzheimer's neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 19.Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 20.Troncoso JC, Cataldo AM, Nixon RA, et al. Neuropathology of preclinical and clinical late-onset Alzheimer's disease. Ann Neurol. 1998;43:673–676. doi: 10.1002/ana.410430519. [DOI] [PubMed] [Google Scholar]
- 21.Abner EL, Kryscio RJ, Schmitt FA, et al. “End-stage” neurofibrillary tangle pathology in preclinical Alzheimer's disease: fact or fiction? J Alzheimers Dis. 2011;25:445–53. doi: 10.3233/JAD-2011-101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.SantaCruz KS, Sonnen JA, Pezhouh MK, Desrosiers MF, Nelson PT, Tyas SL. Alzheimer disease pathology in subjects without dementia in 2 studies of aging: the Nun Study and the Adult Changes in Thought Study. J Neuropathol Exp Neurol. 2011;70:832–40. doi: 10.1097/NEN.0b013e31822e8ae9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol. 2003;60:729–736. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- 24.Haroutunian V, Purohit DP, Perl DP, et al. Neurofibrillary tangles in nondemented elderly subjects and mild Alzheimer disease. Arch Neurol. 1999;56:713–718. doi: 10.1001/archneur.56.6.713. [DOI] [PubMed] [Google Scholar]
- 25.Sonnen JA, Santa Cruz K, Hemmy LS, et al. Ecology of the aging human brain. Arch Neurol. 2011;68:1049–56. doi: 10.1001/archneurol.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–95. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 27.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55:370–6. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 28.Price JL, McKeel DW, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–36. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Driscoll I, Resnick SM, Troncoso JC, An Y, O'Brien R, Zonderman AB. Impact of Alzheimer's pathology on cognitive trajectories in nondemented elderly. Ann Neurol. 2006;60:688–695. doi: 10.1002/ana.21031. [DOI] [PubMed] [Google Scholar]
- 30.Carey CL, Kramer JH, Josephson SA, et al. Subcortical lacunes are associated with executive dysfunction in cognitively normal elderly. Stroke. 2008;39:397–402. doi: 10.1161/STROKEAHA.107.491795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim C, Alexander MP. Stroke and episodic memory disorders. Neuropsychologia. 2009;47:3045–58. doi: 10.1016/j.neuropsychologia.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Kantarci K, Lowe V, Przybelski SA, et al. APOE modifies the association between Aβ load and cognition in cognitively normal older adults. Neurology. doi: 10.1212/WNL.0b013e31824365ab. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson PT, Abner EL, Schmitt FA, et al. Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J Neuropathol Exp Neurol. 2009;68:774–84. doi: 10.1097/NEN.0b013e3181aacbe9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J Neurochem. 2009;110:1129–34. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- 35.Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci. 2011;45:384–9. doi: 10.1007/s12031-011-9589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 37.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 38.Braak H, Braak E. Neuropathological stageing of Alzheimerrelated changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 39.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghoshal N, García-Sierra F, Wuu J, et al. Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer's disease. Exp Neurol. 2002;177:475–93. doi: 10.1006/exnr.2002.8014. [DOI] [PubMed] [Google Scholar]
- 41.Raji CA, Lopez OL, Kuller LH, Carmichael OT. White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.08.010. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]; Ghoshal N, García-Sierra F, Wuu J, et al. Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer's disease. Exp Neurol. 2002;177:475–93. doi: 10.1006/exnr.2002.8014. [DOI] [PubMed] [Google Scholar]
- 42.Buchman AS, Shulman JM, Nag S, Leurgans SE, Arnold SE, Morris MC, Schneider JA, Bennett DA. Nigral pathology and parkinsonian signs in elders without Parkinson's disease. Annals of Neurology. 2012;71:258–66. doi: 10.1002/ana.22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson RS, Yu L, Schneider JA, Arnold SE, Buchman AS, Bennett DA. Lewy bodies and olfactory dysfunction in old age. Chem Senses. 2011;36:367–73. doi: 10.1093/chemse/bjq139. [DOI] [PMC free article] [PubMed] [Google Scholar]