Abstract
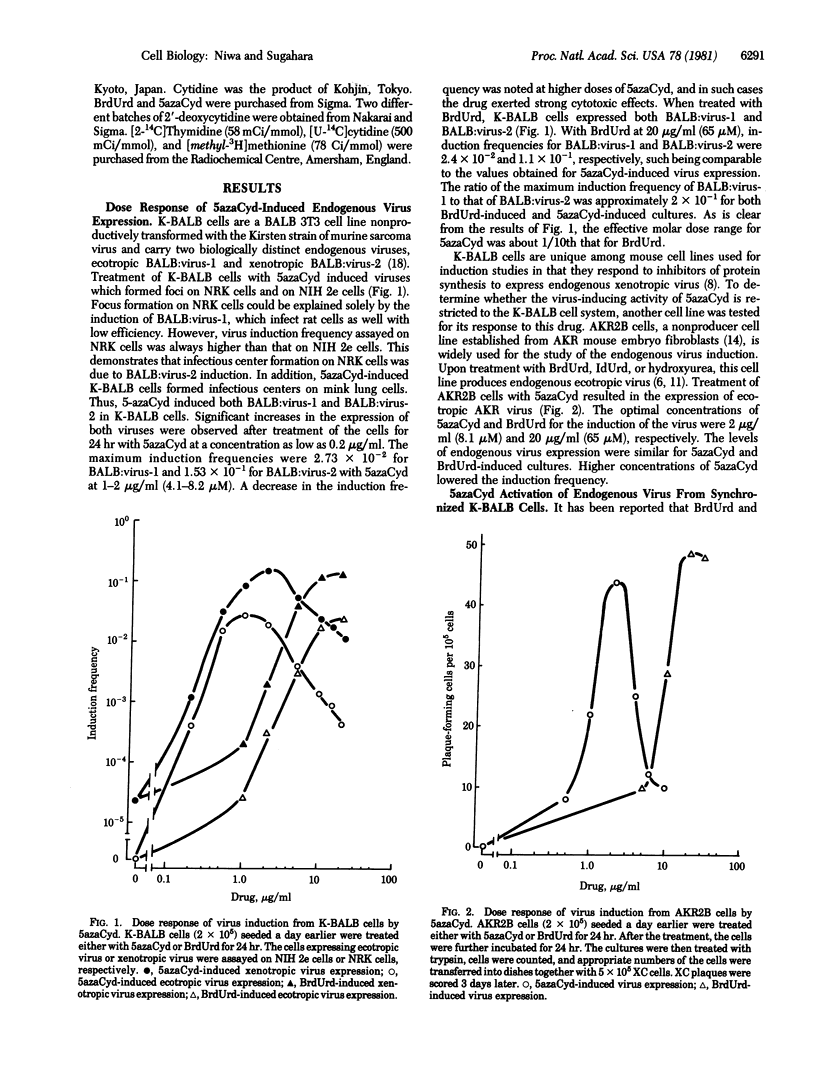
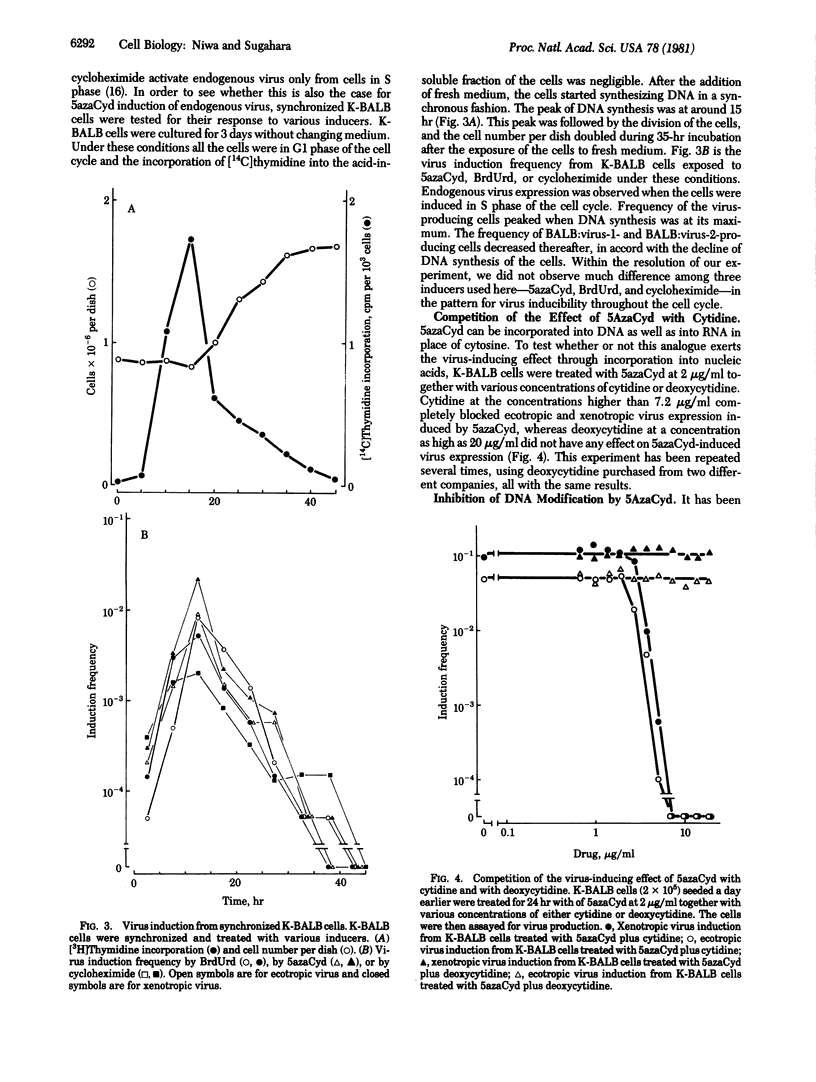
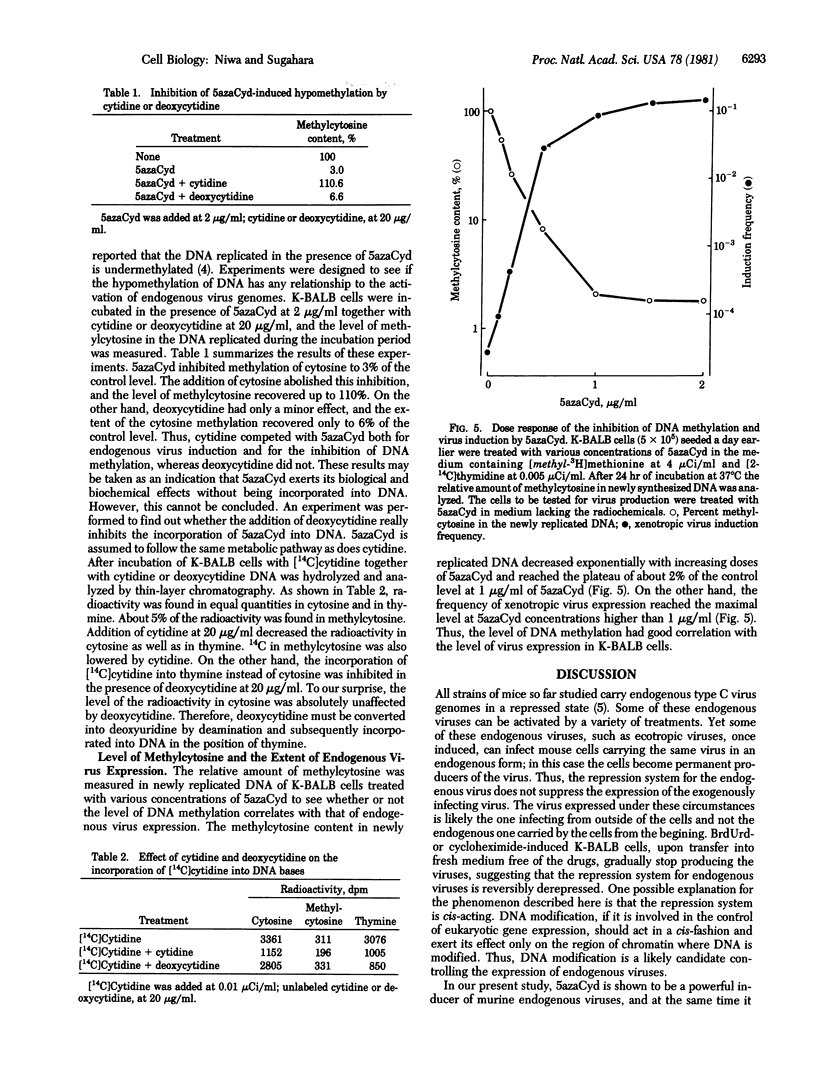
5-Azacytidine was found to induce the expression of BALB:virus-1 and BALB:virus-2 from K-BALB cells and ecotropic endogenous virus from AKR2B cells. Efficiency of the induction was high and comparable to that by 5-bromodeoxyuridine. The level of methylcytosine in newly synthesized DNA was drastically decreased when K-BALB cells were treated with 5-azacytidine. There was an inverse relationship between the level of DNA modification and the frequency of virus expression.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Chemical induction of focus-forming virus from nonproducer cells transformed by murine sarcoma virus. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3069–3072. doi: 10.1073/pnas.68.12.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Dunn C. Y. High-frequency C-type virus induction by inhibitors of protein synthesis. Science. 1974 Feb 1;183(4123):422–424. doi: 10.1126/science.183.4123.422. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Weaver C. A. Characterization of murine sarcoma virus (Kirsten) transformation of mouse and human cells. J Gen Virol. 1971 Nov;13(2):245–252. doi: 10.1099/0022-1317-13-2-245. [DOI] [PubMed] [Google Scholar]
- Aksamit R. R., Long C. W. Short communications induction of endogenous murine type C virus by an arginine analog: L-canavanine. Virology. 1977 May 15;78(2):567–570. doi: 10.1016/0042-6822(77)90131-3. [DOI] [PubMed] [Google Scholar]
- Bird A. P. Use of restriction enzymes to study eukaryotic DNA methylation: II. The symmetry of methylated sites supports semi-conservative copying of the methylation pattern. J Mol Biol. 1978 Jan 5;118(1):49–60. doi: 10.1016/0022-2836(78)90243-7. [DOI] [PubMed] [Google Scholar]
- Cabradilla C. D., Robbins K. C., Aaronson S. A. Induction of mouse type-C virus by translational inhibitors: evidence for transcriptional derepression of a specific class of endogenous virus. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4541–4545. doi: 10.1073/pnas.73.12.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Jay G., Lander M. R., Levine A. S. Correlation of the induction of transcription of the AKR mouse genome 5-lododeoxyuridine with the activation of an endogenous murine leukemia virus. Cancer Res. 1979 May;39(5):1539–1546. [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lowy D. R., Teich N. M., Levine A. S., Rowe W. P. Qualitative and quantitative studies of AKR-type murine leukemia virus sequences in mouse DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1085–1101. doi: 10.1101/sqb.1974.039.01.124. [DOI] [PubMed] [Google Scholar]
- Cohen J. C. Methylation of milk-borne and genetically transmitted mouse mammary tumor virus proviral DNA. Cell. 1980 Mar;19(3):653–662. doi: 10.1016/s0092-8674(80)80042-0. [DOI] [PubMed] [Google Scholar]
- Constantinides P. G., Jones P. A., Gevers W. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature. 1977 May 26;267(5609):364–366. doi: 10.1038/267364a0. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Mulder C., Fleckenstein B. Methylation of Herpesvirus saimiri DNA in lymphoid tumor cell lines. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3839–3843. doi: 10.1073/pnas.76.8.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger J. S., Aaronson S. A. Cycloheximide induction of xenotropic type C virus from synchronized mouse cells: metabolic requirements for virus activation. J Virol. 1975 Jan;15(1):64–70. doi: 10.1128/jvi.15.1.64-70.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthert U., Schweiger M., Stupp M., Doerfler W. DNA methylation in adenovirus, adenovirus-transformed cells, and host cells. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3923–3927. doi: 10.1073/pnas.73.11.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Li L. H., Olin E. J., Buskirk H. H., Reineke L. M. Cytotoxicity and mode of action of 5-azacytidine on L1210 leukemia. Cancer Res. 1970 Nov;30(11):2760–2769. [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Mandel J. L., Chambon P. DNA methylation: organ specific variations in the methylation pattern within and around ovalbumin and other chicken genes. Nucleic Acids Res. 1979 Dec 20;7(8):2081–2103. doi: 10.1093/nar/7.8.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Ginder G. D. Specific DNA methylation sites in the vicinity of the chicken beta-globin genes. Nature. 1979 Aug 2;280(5721):419–420. doi: 10.1038/280419a0. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Niwa O., Decléve A., Liberman M., Kaplan H. S. Adaptation of plaque assay methods to the in vitro quantitation of the radiation leukemia virus. J Virol. 1973 Jul;12(1):68–73. doi: 10.1128/jvi.12.1.68-73.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa O., Sugahara T. Radiation induction of endogenous type C virus from mouse cells transformed in vitro by murine sarcoma virus. J Natl Cancer Inst. 1979 Feb;62(2):329–335. [PubMed] [Google Scholar]
- Otten J. A., Quarles J. M., Tennant R. W. Cell division requirement for activation of murine leukemia virus in cell culture by irradiation. Virology. 1976 Mar;70(1):80–87. doi: 10.1016/0042-6822(76)90237-3. [DOI] [PubMed] [Google Scholar]
- Rascati R. J., Tennant R. W. Involvement of DNA damage in hydroxyurea-mediated induction of endogenous murine retrovirus. Virology. 1979 Apr 30;94(2):273–281. doi: 10.1016/0042-6822(79)90461-6. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W., Lander M. R., Pugh W. E., Teich N. Noninfectious AKR mouse embryo cell lines in which each cell has the capacity to be activated to produce infectious murine leukemia virus. Virology. 1971 Dec;46(3):866–876. doi: 10.1016/0042-6822(71)90087-0. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Maniatis T. Tissue-specific DNA methylation in a cluster of rabbit beta-like globin genes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6634–6638. doi: 10.1073/pnas.77.11.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanyushin B. F., Tkacheva S. G., Belozersky A. N. Rare bases in animal DNA. Nature. 1970 Mar 7;225(5236):948–949. doi: 10.1038/225948a0. [DOI] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]


