Abstract
Contraction or relaxation of smooth muscle cells within the walls of resistance arteries determines the artery diameter and thereby controls flow of blood through the vessel and contributes to systemic blood pressure. The contraction process is regulated primarily by cytosolic calcium concentration ([Ca2+]cyt), which is in turn controlled by a variety of ion transporters and channels. Ion channels are common intermediates in signal transduction pathways activated by vasoactive hormones to effect vasoconstriction or vasodilation. And ion channels are often targeted by therapeutic agents either intentionally (e.g. calcium channel blockers used to induce vasodilation and lower blood pressure) or unintentionally (e.g. to induce unwanted cardiovascular side effects).
Kv7 (KCNQ) voltage-activated potassium channels have recently been implicated as important physiological and therapeutic targets for regulation of smooth muscle contraction. To elucidate the specific roles of Kv7 channels in both physiological signal transduction and in the actions of therapeutic agents, we need to study how their activity is modulated at the cellular level as well as evaluate their contribution in the context of the intact artery.
The rat mesenteric arteries provide a useful model system. The arteries can be easily dissected, cleaned of connective tissue, and used to prepare isolated arterial myocytes for patch clamp electrophysiology, or cannulated and pressurized for measurements of vasoconstrictor/vasodilator responses under relatively physiological conditions. Here we describe the methods used for both types of measurements and provide some examples of how the experimental design can be integrated to provide a clearer understanding of the roles of these ion channels in the regulation of vascular tone.
Keywords: Physiology, Issue 67, Molecular Biology, Medicine, Anatomy, Vascular smooth muscle, mesenteric artery, patch clamp, Kv channel, vasoconstriction, electrophysiology
Protocol
1. Surgical Excision of the Small Intestinal Mesenteric Vascular Arcade
Anesthetize a 300-400 g Sprague-Dawley rat with isoflurane (4%) administered by inhalation.
Perform a midline laparotomy to expose the small intestinal mesentery. Exteriorize the small and large intestine through the abdominal incision with great care to avoid trauma to the exposed bowel and mesentery. Gently fan the mesentery out over sterile gauze.
Surgically excise the small intestine and a portion of the large intestine, including the caecum (blood vessels carefully cut along the margins and at the origins from the primary branches of the superior mesenteric artery/vein).
Transfer the excised tissue to a beaker containing ice cold dissecting solution (Table 1).
Following excision of the intestine, the rat should be humanely euthanized while under anesthesia.
2. Dissection and Cleaning of Arteries
Transfer the excised intestine and mesenteric arcade to a cooled dissecting chamber (4-5 °C) containing dissecting solution. The chamber consists of a 100-mm glass Petri dish with a SYLGARD 184 elastomer base to accommodate dissecting pins; the Petri dish is placed in a cooled base that is maintained at 4-5 °C by a circulating water bath.
Using surgical scissors cut an approximately 10-12 cm segment out of the whole intestine. Cut at each end of the intestinal segment keeping its vasculature attached, and then remove the unused intestine from the chamber leaving the one segment with its attached vasculature (mesenteric arcade) in isolation.
Pin down the segment of small intestine, spreading the mesentery over the near side of the chamber such that the primary branch of the superior mesenteric artery/vein is in the center and the subsequent orders of branches radiate from the center (Figure 1). Place the pins only on the intestinal segments (not on the blood vessels).
Distinguish arteries from veins by their relatively bright red color, thick walls and characteristic V-shaped branches, rather than the U-shaped branches seen in veins.
Visualizing through an illuminated dissecting microscope, carefully clear the adipose tissue and the connective tissue surrounding the 2nd- 4th order arteries close to the intestines (dashed circle in Figure 1) using micro forceps and scissors. Holding the adjacent veins facilitates clearing the adipose tissue and connective tissue. Avoid unnecessary stretching of the arteries.
Mesenteric artery segments cleared of connective tissue can then be dissected out and used for cell isolation (for single-cell patch clamp studies, Sections 3-4) or cannulated for pressure myography (Section 5). Cut segments between branch points, usually up to 1 cm in length.
3. Cell Isolation for Patch Clamp Studies
Prepare 0.5 L of Isolation Solution (Table 1) and divide it into 2 portions before adjusting the pH. The first portion (usually 100 ml) should be warmed up to 37 °C and pH should be adjusted to 7.2 at 37 °C (37 °C Isolation Solution). The second portion of the Isolation Solution should be cooled down to 4 °C and pH should be adjusted to 7.2 at 4 °C (Ice Cold Isolation Solution). After pH adjustment, the osmolarity of both solutions must to be adjusted to 298 mOsm by adding an appropriate amount of D-glucose or H2O.
Transfer 2 ml of 37 °C Isolation Solution to a glass vial and incubate at 37 °C for use in step 3.4. Add 20 mg of bovine serum albumin (BSA) to 10 ml of 37 °C Isolation Solution, dissolve, re-warm to 37 °C and readjust the pH to 7.2. To 2 ml of this solution add 4 mg collagenase type VIII (Sigma, Lot 089K8612: 633 units/mg Collagenase activity, 285 units/mg Caseinase activity, 1.6 units/mg Clostripain activity), 0.64 mg elastase type IV (Sigma, Lot 110M7025V 5.9 units/mg; add 40 μl of a 16 mg/ml stock prepared in 37 °C Isolation Solution) and 2 μl of 1 M DL-dithiothreitol (DTT Sigma); pre-warm this Enzyme Solution to 37 °C in preparation for step 3.5.
Transfer 2-3 segments of the mesenteric artery into a 35-mm tissue culture dish containing 2 ml of Ice Cold Isolation Solution and place the dish on ice. Cut mesenteric artery segments into 3-4 mm-long pieces.
Using a Pasteur pipette with fire polished tip (to prevent damaging of arteries) transfer the arterial segments into a glass vial (15 x 45 mm, 1 dram) containing 2 ml pre-warmed 37 °C Isolation Solution and incubate for 30 min at 37 °C.
After 30 min, carefully aspirate the 37 °C Isolation Solution using a Pasteur pipette, add 2 ml warm Enzyme Solution and incubate for 35 min at 37 °C.
Immediately after the 35 min incubation in the enzyme solution, place the vial on ice, aspirate the enzyme solution and add 2 ml of Ice Cold Isolation Solution (repeat aspiration and addition of fresh Ice Cold Isolation Solution 4 times). Then, in a volume of 1 ml of Ice Cold Isolation Solution, gently triturate arterial segments with the polished Pasteur pipette 10-20 times to release individual mesenteric artery smooth muscle cells (MASMCs).
Periodically verify myocytes appearance by placing a drop of the cell suspension on a 35-mm dish and viewing under a microscope. Healthy MASMCs should have a smooth elongated appearance. Not all of the cells in the arterial wall will be released. The cell suspension should be kept on ice.
Another useful indicator of MASMC health is their tolerance of physiological Ca2+ concentrations. Fill recording chamber (Bioscience Tools, #CSC-25L) with bath solution containing 2 mM CaCl2 (pH 7.3 at room temperature (RT), for composition see Table 1) and position agar bridge (bent glass capillary filled with 2% agar in 2 M KCl, inserted into the reference electrode (Warner Instrument, REF-3L)) inside the chamber. Using the polished Pasteur pipette, transfer approximately 100-200 μl cell suspension to a recording chamber and allow the cells to adhere for 15 min. Undamaged cells should not contract significantly in the 2 mM CaCl2-containing solution (cells should appear to be elongated or rod-shaped, not rounded up into balls).
After adding the first aliquot of the cell suspension to the recording chamber, 0.25 ml of bath solution containing 2 mM CaCl2 should be added to the remaining volume of cell suspension. This remaining cell suspension can be maintained in this solution on ice and used for patch clamp recording for up to 6 hr.
4. Use of Whole Cell Patch Clamp to Measure Kv7 Potassium Currents or Membrane Voltage
After myocytes adhere to the glass base of the recording chamber (25-mm No. 1 round cover slip, Warner Instruments), initiate continuous perfusion of the bath solution (Table 1). For recording of Kv7 currents in isolation from delayed rectifier potassium currents of Kv1 and Kv2 families, the bath solution should be supplemented with 100 μM GdCl3.
Fabricate a patch pipette from borosilicate glass (Kimax-51, Kimble Chase) using a pipette puller and microforge; its resistance should be 4-5 MΩ when filled with internal solution (Table 1). Coat the lower 1/3 of the pipette (~1-2 mm above the tip) with SYLGARD 184.
For recording of Kv7 currents in MASMCs, the perforated patch whole cell voltage clamp technique should be used. Use of a perforated patch configuration helps to prevent depletion of membrane phosphatidylinositol 4,5-bisphosphate (PIP2) and subsequent fast (within 5 min) rundown of the Kv7 currents, which is commonly observed using the conventional ruptured patch configuration. Supplement the internal solution (Table 1) with 120 μg/ml Amphotericin B: add 3 μl of 2 mg/ml stock of Amphotericin B (prepared in DMSO and kept frozen in small aliquots and protected from light) to 0.5 ml of internal solution. Fill the tip of the patch pipette with Amphotericin B-free internal solution by dipping the pipette tip in the Amphotericin B-free internal solution and then applying suction on the other end using a 10 ml syringe attached via a piece of tubing (Tygon R-3603). Add Amphotericin B-containing pipette solution from the top using an Eppendorf Microloader Pipette Tip until the pipette is about half filled with solution. Remove any air bubbles by gently tapping the pipette. Use the pipette immediately.
Insert the pipette into the electrode holder and use a micromanipulator to lower the pipette to the surface of an elongated myocyte (close to, but not on top of the nucleus, Figure 2). When resistance in the pipette increases to 6-10 MΩ, apply suction to achieve a giga-seal (>10 GΩ).
Set holding voltage to 0 mV while waiting for membrane perforation. Apply 100 ms voltage steps to +10 mV and then to -10 mV, and use the amplifier to compensate fast pipette capacitance transients. Concurrent with the appearance of whole cell capacitance transients, small sustained positive currents (usually 2-10 pA) will indicate the presence of functional Kv7 channels.
Successful perforation with Amphotericin B will reduce access resistance to 35 MΩ or less. Current recording can then be initiated using a 5 sec voltage step protocol from -4 mV holding voltage. We use the -4 mV holding voltage to achieve near maximal inactivation of other types of delayed rectifier potassium currents (mediated predominantly by Kv1 and Kv2 channels) that would otherwise contaminate the recording of the relatively small non-inactivating Kv7 currents. The long (5 sec) voltage step protocol is necessary to achieve steady-state activation of Kv7 channels, which exhibit slow voltage-dependent activation and deactivation. The Kv7 currents do not inactivate during the sustained voltage steps.
To record the Kv7 current-voltage (I-V) relationship, apply 5 sec voltage steps to voltages ranging from -84 mV, at which the open probability of Kv7 channels is minimal, to +16 mV at which the open probability of Kv7 channels reaches a plateau ; after 5 sec at each test voltage, step back to the holding voltage (-4 mV) for 10 sec. It will take approximately 3.5 min for the full series of test pulses (Figure 3A). Perform 2-3 control I-V recordings to ensure that the recorded currents are stable over time. Plot average end pulse currents (average currents recorded for last 1 sec) versus voltage to create an I-V curve (Figure 3D). The control I-V curves should be approximately superimposed if the currents are stable.
Before applying any pharmacological agents initiate a time course protocol designed to continuously record current at -20 mV. Ensure stable recording of the current for at least 5 min prior to the drug application. Apply the pharmacological agents for 5-15 min or until a steady-state is achieved (Figure 3C); then terminate the time course protocol and record two I-V curves using the voltage-step protocol. Washout the drug and record the time course of drug washout, followed by recording of two additional I-V curves.
At the end of the experiment apply 10 μM linopirdine or 10 μM XE991, irreversible inhibitors of Kv7 channels; these drugs should fully inhibit the currents recorded at voltages negative to -20 mV (Figure 3D).
After each experiment wash the chamber and perfusion system with detergent, ethanol and hot water to remove any traces of the drugs used.
5. Use of Intact Artery Segments for Pressure Myography
A pressure myograph system purchased from Danish Myo Technology (DMT A/S, Denmark, Model 110P) is used for the pressure myography experiment. Mount the artery segment in the stainless steel chamber by inserting the horizontally fixed left glass cannula into one end and the movable right glass cannula into the other end. Two pressure transducers (P1 on the right and P2 on the left side) are built in to monitor the pressure within the artery lumen. Fluid from the right glass cannula will be used to adjust the pressure inside the artery to its approximate physiological level. To begin, pre-fill an open 10 ml syringe (serving as a gravity feed pressure column), with lumen solution (Table 1). Raise the syringe to push solution via polyethylene tubing into the right glass cannula, taking care to avoid air bubbles in the tubing or cannula. The left cannula should be pre-filled with lumen solution via a 1 ml syringe. Fill the pressure myograph chamber with 10 ml of the vessel bath solution (Table 1). Loosely suspend two pre-made fine nylon sutures on each side adjacent to the glass cannulae.
Carefully transfer the dissected mesenteric artery segment (usually a 3rd or 4th order segment, around 200 - 350 μm in diameter; from step 2.6) from the dissecting chamber to the pressure myograph chamber by holding on to one end of the segment using micro forceps.
Visualizing through an illuminated dissecting microscope, hold one end of the artery segment and gently slide it over the left glass cannula and secure the end using the nylon sutures. Gently flush the lumen fluid through the mounted vessel to remove blood and debris within the vessel. The valve to the left syringe should then be closed so that this side presents a static column of fluid against which the pressure is adjusted from the right side.
Using the micromanipulator, position the movable right glass cannula closer to the artery segment and pull the right end of the segment over it, finally securing it with the nylon sutures. Clip off the excess suture threads.
Mount the pressure myograph unit on the stage over the pressure myograph microscope. Place the cover over the pressure myograph chamber. The cover for the chamber contains the superfusion inlet and the suction pipe attached to it. Connect the superfusion inlet to a manifold to allow superfusion of room temperature bath or 60 mM KCl solution into the pressure myograph chamber by gravity. Test substances, such as drugs or hormones that are found in the patch clamp measurements to affect Kv7 channel function, are generally applied directly to the bath solution, not via the bath superfusion or in the lumen solution. The superfusion of bath solution will be used to wash out the KCl solution. Attach the suction pipe to a vacuum system to remove the excess superfusate. Insert the temperature sensor into the bath solution through the opening provided in the myograph chamber cover.
Connect the pressure myograph unit to the myo-interface system, the hardware that enables communication of the myograph unit to the myoview software (DMT).
Open the myoview software in the computer. In the parameter window, set the target temperature as 35 °C with a temperature tolerance of 1 °C, target inflow pressure as 80 mmHg and target outflow pressure as 80 mmHg. Load the calibration file and calibrate the vessel outer diameter so that the changes in artery diameter are recorded accurately in microns. Adjust the contrast as necessary to enable a good view of the mounted segment against the background. A detailed description for how to use the software is provided in the user manual from DMT.
Gradually raise the 10 ml syringe (serving as a gravity feed pressure column) over a period of 15 min until the P1 pressure transducer reads 80 mmHg. While raising the pressure column, check for any leaks in the vessel. If there are any leaks, discard the segment and mount another artery segment (repeat steps from 5.2). Adjust the distance between the cannulae when necessary. The distance should be adjusted in such a way that the pressurized artery segment is approximately straight (but not stretched) and forms a shoulder like-pattern where the ties are made (Figure 4A).
Turn on the heating of the pressure myograph chamber through the controls in the myo-interface system. Allow the bath solution to warm gradually until it reaches 36-37 °C.
Use the XYZ adjustments of the objective in the DMT microscope when necessary to keep the vessel in focus and facilitate accurate tracking of the changes in outer vessel diameter.
Check the viability of the mounted vessel by transient superfusion (~30 sec) with 60 mM KCl. A viable vessel constricts quickly on addition of KCl and dilates immediately upon washout of the 60 mM KCl (superfuse approximately 60 ml of bath solution to wash out the KCl). After this test, readjust the volume of the bath solution to exactly 10 ml. The temperature of the bath is reduced during the KCl test and washout because these solutions are at room temperature, but the chamber heater will restore the temperature to 36-37 °C within a few minutes.
Allow the artery to equilibrate for at least 30 min (the diameter should be stable for at least the last 10 min of this period). Add the concentrated test substance to the bath solution directly and pipette back and forth gently near the edges of the chamber to mix the test substance in the bath solution, yielding the appropriate final concentration in the 10 ml chamber volume. Changes in the vessel diameter following addition of test substance(s) can be monitored in the live video image and also charted in the image analysis window while the experiment is in progress.
After completion of the experiment, the stored data (*.myo) file can be opened as a spreadsheet file for further analysis.
6. Representative results
The results shown in Figure 3 illustrate typical recordings of Kv7 currents from mesenteric artery myocytes using a voltage step protocol before (Figure 3A,B black traces) and during treatment with a Kv7 channel activator Zn pyrithione (ZnPyr, 100 nM; Figure 3A,B red traces). Similar voltage step data from 3 cells were averaged to prepare the current-voltage (I-V) plots shown in Figure 3D. A representative time course of enhancement of current amplitude (measured by continuous voltage clamp at -20 mV) during treatment with 100 nM Zn pyrithione is shown in Figure 3C. Figure 4B illustrates the time course of the effect of ZnPyr on mesenteric artery outer diameter measured using pressure myography; the vessel was pre-constricted with 100 pM arginine vasopressin (AVP) prior to addition of 100 nM ZnPyr. Averaged dose-response data for ZnPyr-induced mesenteric artery dilation are shown in Figure 4C.
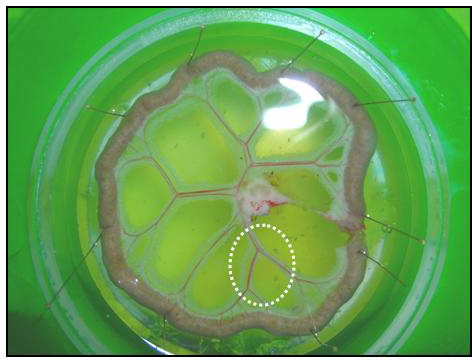 Figure 1. Picture of a mesenteric arcade in the dissecting chamber.
Figure 1. Picture of a mesenteric arcade in the dissecting chamber.
 Figure 2. Picture of a myocyte with a patch pipette on its surface.
Figure 2. Picture of a myocyte with a patch pipette on its surface.
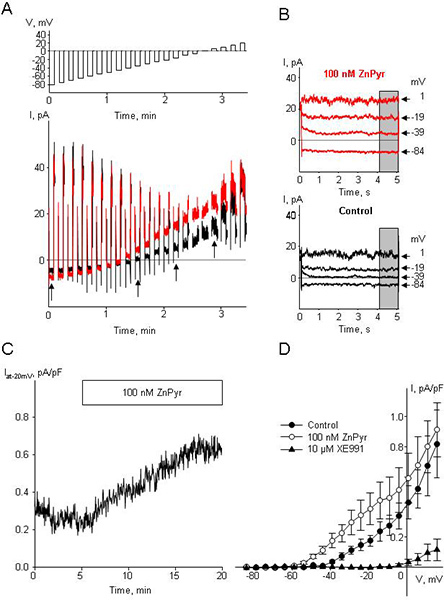 Figure 3. Original traces of Kv7 currents, time course of drugs application, I-V curves. A. Family of sequential current traces (lower panel) recorded in response to voltage steps (top panel) before treatment (control, black) and following stabilization in the presence of 100 nM ZnPyr (red) in a single MASMC. B. Representative current traces from A (as indicated by arrowheads) for control (black) and for 100 nM ZnPyr (red). Gray bars indicate time interval where averaged current amplitudes were measured for current-voltage (I-V) plots. Step voltages are indicated on the right by arrowheads. C. Time course of Kv7 current enhancement by 100 nM ZnPyr measured with continuous voltage clamp at -20 mV (bar). D. Average I-V curves (n=3) derived from Kv7 current densities recorded before (control, filled circles), during treatment with 100 nM ZnPyr (open circles), and during treatment with 10 μM XE991 (filled triangles). Non-specific leak currents were subtracted as described by Passmore et al. 1.
Figure 3. Original traces of Kv7 currents, time course of drugs application, I-V curves. A. Family of sequential current traces (lower panel) recorded in response to voltage steps (top panel) before treatment (control, black) and following stabilization in the presence of 100 nM ZnPyr (red) in a single MASMC. B. Representative current traces from A (as indicated by arrowheads) for control (black) and for 100 nM ZnPyr (red). Gray bars indicate time interval where averaged current amplitudes were measured for current-voltage (I-V) plots. Step voltages are indicated on the right by arrowheads. C. Time course of Kv7 current enhancement by 100 nM ZnPyr measured with continuous voltage clamp at -20 mV (bar). D. Average I-V curves (n=3) derived from Kv7 current densities recorded before (control, filled circles), during treatment with 100 nM ZnPyr (open circles), and during treatment with 10 μM XE991 (filled triangles). Non-specific leak currents were subtracted as described by Passmore et al. 1.
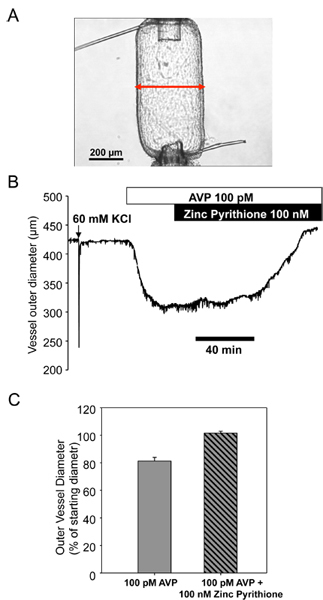 Figure 4. ZnPyr induced relaxation of a mesenteric artery pre-constricted with 100 pM AVP. A. Photograph of a mesenteric artery mounted in the pressure myograph set-up. B. Time course of changes in outer diameter of the vessel in response to application of 100 pM AVP (open bar) followed by application of the 100 nM ZnPyr (filled bar). C. Bar graph summarizing results of 100 nM ZnPyr-induced vasodilaion.
Figure 4. ZnPyr induced relaxation of a mesenteric artery pre-constricted with 100 pM AVP. A. Photograph of a mesenteric artery mounted in the pressure myograph set-up. B. Time course of changes in outer diameter of the vessel in response to application of 100 pM AVP (open bar) followed by application of the 100 nM ZnPyr (filled bar). C. Bar graph summarizing results of 100 nM ZnPyr-induced vasodilaion.
Discussion
The methods and experimental approaches described here are quite robust and can produce clear and reproducible results when applied with meticulous attention to detail. Good electrophysiological recordings and constriction/dilation of arterial segments are dependent on the health of the cells and artery segments, respectively. Cell preparations can vary from day to day, even using the same protocol. Isolation Solutions can be used for up to 2 weeks, but if the quality of the cell preparation is low on two consequent days new Isolation Solutions should be prepared. We have found that enzyme activities vary from batch to batch and therefore isolation conditions (the times of incubation and concentrations of enzymes) require optimization with each new batch of enzymes. We have also found that the cell isolation protocol described here for rat mesenteric artery is not optimal for other vascular beds or other species, which may have a different mix or density of extracellular matrix components. Each vascular preparation requires its own optimization to identify conditions that produce the healthiest cells. The criteria that we find most helpful in identifying healthy cells are: 1) cell morphology: smooth, elongated, but slightly constricted appearance after triturating MA segments in the Isolation Solution containing 50 μM Ca2+, and retaining the same appearance when transferred to 2 mM Ca2+-containing external solution. Not all cells will retain their nearly fully relaxed elongated shape, but only nearly fully relaxed myocytes should be used for electrophysiological recording; 2) Cells should be optically dense, which indicates an intact undamaged membrane; 3) Resting membrane voltages of healthy myocytes used to record potassium currents or for current-clamp recordings of membrane voltage should be more negative than -40 mV (usually in the range of -45 mV to -56 mV under the ionic conditions described in our protocol here).
To ensure successful electrophysiological recordings, the bath solution and the pipette solution (for composition see Table 1) should be prepared on the day of the experiment. It is crucial to adjust osmolality of the both internal and bath solutions to be identical (298-299 mOsM).
Isobaric arterial function measurements made in small arteries (<500 μm) using pressure myography more closely approximate physiological conditions than do isometric force measurements made using a wire myograph. The vessels in a pressure myograph are typically more sensitive to vasoconstrictor agonists2-4, more closely approximating the sensitivities measured in vivo5, 6. The increased sensitivity to low concentrations of agonists has enabled the identification of signal transduction mechanisms induced by physiologically relevant picomolar concentrations of AVP7, 8.
Pressure myography preserves the geometry of the vessel and maintains the integrity of the endothelium. Endothelium-mediated effects of drugs can be distinguished from smooth muscle-mediated effects by conducting parallel measurements in intact and endothelium-denuded vessels9. The intentional disruption of the endothelium can be accomplished by physically damaging the endothelium (e.g. by passing a human hair back and forth through the lumen) or by perfusing air through the lumen of the artery. The loss of endothelial function (but not vascular smooth muscle function) should be confirmed by measuring a reduced endothelium-mediated vasodilator response to acetylcholine in vessels pre-constricted with an agonist9.
Parallel measurements of ionic currents/membrane voltage in mesenteric artery myocytes using patch clamp techniques and arterial constriction/dilation of mesenteric arteries using pressure myography can enable elucidation of the roles of specific ion channels in both physiological signal transduction cascades and in the actions of therapeutic agents. Treatments targeting specific classes of ion channels or specific signal transduction intermediates can be applied additively or in sequence to provide mechanistic information about the roles of these channels in the regulation of myocyte function at the cellular level and in constriction/dilation of intact arteries. Convergent results of treatments that enhance or inhibit particular types of ionic currents with corresponding effects on artery diameter provide more reliable interpretations than can be obtained using either method by itself. Ideally, the concentration-dependence of the agents under study should be determined in both systems. To assess physiological or therapeutic relevance, the concentrations tested should be related to concentrations measured in the physiological environment or achieved with clinical therapies. Examples of these types of experimental approaches can be found in our previous publications8-10.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was funded by a grant from National Heart, Lung, and Blood Institute (NIH R01-HL089564) to KLB and pre-doctoral fellowships from the American Heart Association (09PRE2260209) and Arthur J. Schmitt Foundation to BKM.
References
- Passmore GM. KCNQ/M Currents in Sensory Neurons: Significance for Pain Therapy. J. Neurosci. 2003;23:7227–7236. doi: 10.1523/JNEUROSCI.23-18-07227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falloon BJ. Comparison of small artery sensitivity and morphology in pressurized and wire-mounted preparations. Am. J. Physiol. Heart Circ. Physiol. 1995;268:H670–H678. doi: 10.1152/ajpheart.1995.268.2.H670. [DOI] [PubMed] [Google Scholar]
- Dunn WR. Enhanced resistance artery sensitivity to agonists under isobaric compared with isometric conditions. Am. J. Physiol. Heart Circ. Physiol. 1994;266:H147–H155. doi: 10.1152/ajpheart.1994.266.1.H147. [DOI] [PubMed] [Google Scholar]
- Buus NH. Differences in sensitivity of rat mesenteric small arteries to agonists when studied as ring preparations or as cannulated preparations. Br. J. Pharmacol. 1994;112:579–587. doi: 10.1111/j.1476-5381.1994.tb13114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhalim MA. Effects of big endothelin-1 in comparison with endothelin-1 on the microvascular blood flow velocity and diameter of rat mesentery in vivo. Microvasc. Res. 2006;72:108–112. doi: 10.1016/j.mvr.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Altura BM. Dose-response relationships for arginine vasopressin and synthetic analogs on three types of rat blood vessels: possible evidence for regional differences in vasopressin receptor sites within a mammal. J. Pharmacol. Exp. Ther. 1975;193:413–423. [PubMed] [Google Scholar]
- Henderson KK. Vasopressin-induced vasoconstriction: two concentration-dependent signaling pathways. J. Appl. Physiol. 2007;102:1402–1409. doi: 10.1152/japplphysiol.00825.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie AR. Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance. J. Pharmacol. Exp. Ther. 2008;325:475–483. doi: 10.1124/jpet.107.135764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI. Differential effects of selective cyclooxygenase-2 inhibitors on vascular smooth muscle ion channels may account for differences in cardiovascular risk profiles. Mol. Pharmacol. 2009;76:1053–1061. doi: 10.1124/mol.109.057844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, editor; Kaneez FS, editor. Patch Clamp Technique. Intech; 2012. [Google Scholar]
- Berra-Romani R. TTX-sensitive voltage-gated Na+ channels are expressed in mesenteric artery smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H137–H145. doi: 10.1152/ajpheart.01156.2004. [DOI] [PubMed] [Google Scholar]


