Abstract
In response to injury, blood coagulation is activated and results in generation of the clotting protease, thrombin. Thrombin cleaves fibrinogen to fibrin which forms an insoluble clot that stops hemorrhage. Factor V (FV) in its activated form, FVa, is a critical cofactor for the protease FXa and accelerator of thrombin generation during fibrin clot formation as part of prothrombinase 1, 2. Manual FV assays have been described 3, 4, but they are time consuming and subjective. Automated FV assays have been reported 5-7, but the analyzer and reagents are expensive and generally provide only the clot time, not the rate and extent of fibrin formation. The microplate platform is preferred for measuring enzyme-catalyzed events because of convenience, time, cost, small volume, continuous monitoring, and high-throughput 8, 9. Microplate assays have been reported for clot lysis 10, platelet aggregation 11, and coagulation Factors 12, but not for FV activity in human plasma. The goal of the method was to develop a microplate assay that measures FV activity during fibrin formation in human plasma.
This novel microplate method outlines a simple, inexpensive, and rapid assay of FV activity in human plasma. The assay utilizes a kinetic microplate reader to monitor the absorbance change at 405nm during fibrin formation in human plasma (Figure 1) 13. The assay accurately measures the time, initial rate, and extent of fibrin clot formation. It requires only μl quantities of plasma, is complete in 6 min, has high-throughput, is sensitive to 24-80pM FV, and measures the amount of unintentionally activated (1-stage activity) and thrombin-activated FV (2-stage activity) to obtain a complete assessment of its total functional activity (2-stage activity - 1-stage activity).
Disseminated intravascular coagulation (DIC) is an acquired coagulopathy that most often develops from pre-existing infections 14. DIC is associated with a poor prognosis and increases mortality above the pre-existing pathology 15. The assay was used to show that in 9 patients with DIC, the FV 1-stage, 2-stage, and total activities were decreased, on average, by 54%, 44%, and 42%, respectively, compared with normal pooled human reference plasma (NHP).
The FV microplate assay is easily adaptable to measure the activity of any coagulation factor. This assay will increase our understanding of FV biochemistry through a more accurate and complete measurement of its activity in research and clinical settings. This information will positively impact healthcare environments through earlier diagnosis and development of more effective treatments for coagulation disorders, such as DIC.
Keywords: Immunology, Issue 67, Factor V, Microplate, Coagulation assay, Human plasma, Disseminated intravascular coagulation (DIC), blood clotting
Protocol
1. Preparation of FV-deficient Human Plasma
This method is a modification of the procedure originally described by Bloom et al 4.
Obtain whole human blood from a consenting healthy adult volunteer (Male or Female, age 18 - 50 yrs) that is not taking any medication. Wear latex gloves and a laboratory coat throughout the procedure.
Prepare 100 ml of 3.2% (w/v) trisodium citrate in distilled water. Load 6.0 ml of this solution into separate 60 ml syringes. Remove air by depressing plunger carefully to the 6.0 ml mark on the syringe.
Use an alcohol swab to clean area of cubital vein between bicep and forearm. Connect 20 gauge butterfly apparatus to a 3 ml syringe. Insert butterfly needle into cubital vein and draw gently on plunger until blood fills to the 3 ml mark. Discard syringe with blood. This step is performed to remove any tissue factor released from the damaged endothelium at the needle injury site that may activate the coagulation process and interfere with preparation of FV-deficient plasma.
Connect butterfly needle to 60 ml syringe 'loaded' with 6.0 ml trisodium citrate and draw gently on the plunger until the blood reaches the 60 ml mark on the syringe. (Final citrate concentration 10.9mM).
Remove syringe from butterfly needle and gently mix syringe while keeping a gloved finger or thumb on the syringe outlet.
Continue drawing blood into 60 ml syringes each filled with 6.0 ml of 3.2% trisodium citrate as described above. One should anticipate recovery of approx 50% of the citrated blood volume as plasma after centrifugation and that 50 μl of FV-deficient plasma is required per sample in the microplate assay. Our laboratory routinely processes 300-420 ml of citrated blood at a time using 5-7 x 60 ml syringes each with 6.0 ml of 3.2% trisodium citrate/syringe. This is sufficient FV-deficient plasma for approximately 3,000 microplate assays (See below).
Remove butterfly needle from volunteer's arm and press a sterile gauze pad over site of vein puncture for 1.0 min. Cover site of vein puncture with a sterile bandage.
Centrifuge citrated blood at 3,300 x g for 20 min at room temperature. Recover upper yellow plasma layer to a plastic beaker with a stir bar using a plastic transfer pipette taking care not to disturb the lower red and white blood cell layer.
Measure volume of plasma in ml and retain 100 μl of plasma before EDTA addition on ice for future prothrombin clot time (PT) testing (See below).
Slowly add solid EDTA to citrated plasma with gentle stirring at room temperature to achieve 5mM (Final concentration). Once the EDTA has dissolved, adjust pH to 7.0 by dropwise addition of 1.0M NaOH with gentle stirring.
Cover beaker with Saran Wrap and incubate for 8-10 hr without stirring at 37 °C.
Every 2 hr, recover 100 μl of the EDTA-treated plasma on ice and determine the PT at the time of incubation with EDTA and compare to the PT of plasma prior to EDTA addition (See above).
For the PT determinations, place removable 8 well immunomodule strips into plastic template holder and insert into microplate reader.
Orient immunomodule strips in microplate reader as: empty, empty, sample, empty, empty, sample, empty, and empty from left to right in the template holder to minimize light scatter interference from neighbouring sample-containing strips. Also, use only wells 2-7 from top to bottom in each 8 well immunomodule strip to minimize light scatter interference from edges of plastic template holder.
The FV microplate assay is capable of measuring fibrin clot formation in at least 12 different samples simultaneously (6 samples per immunomodule strip over 2 immunomodule strips).
Using a multichannel pipette, add 50 μl of HBS (20mM HEPES, 150mM NaCl, pH 7.4) to each microplate well for each of sample to be assayed. Using a single pipette, add 50 μl of normal human pooled reference plasma (NHP) or plasma before addition of EDTA or at various incubation times after adding EDTA to separate microplate wells.
Using a multichannel pipette, add 50 μl thromboplastin (See below for preparation method) to all microplate wells with plasma to be assayed. Incubate in microplate reader at 25 °C for 1 min and program the microplate reader to shake the plate during the 15-25 sec of the 1 min incubation.
Using the computer interfaced with the microplate reader, access SoftMax Pro software microplate application. Set the reader to Absorbance, Wavelength to 405nm, mode to Kinetic, temperature to 25 °C, and program the microplate reader to shake for 5 sec, and read the absorbance at 405nm every 5 sec for 6 min.
Using a multichannel pipette, add calcium chloride (50 μl of 25mM in distilled water; 2.1mM final concentration) to all of the microplate wells, wait 15 sec, and press the 'Start' icon on the microplate SoftMax Pro menu to begin the assay.
Determine the PTs from the Absorbance vs. Time profiles in SoftMax Pro as the time to reach the half maximal increase in absorbance at 405nm (The mid-point of the sigmoidal Absorbance vs. Time curve produced after thromboplastin and calcium chloride addition. See below, Figure 1).
Calculate the FV 1-stage activity from the Log Clot Time (sec) vs. Log FV Activity (Units/ml) as illustrated below in Figure 2A. For quantitation purposes, 1Unit of FV activity is defined as the activity present in 1.0 ml NHP prior to intentional activation with added thrombin (See below, Assaying human plasma samples with the FV 1-stage and 2-stage microplate coagulation assay).
Prepare 4.0l of 20mM HEPES containing 0.15M NaCl in distilled water with gentle stirring. Adjust pH to 7.4 with slow dropwise addition of 5.0M NaOH (HBS, pH 7.4).
Obtain a sufficient length of dialysis tubing and rinse thoroughly with distilled water. Tie a knot in one end of the tubing. Transfer EDTA treated plasma to dialysis tubing with a plastic transfer pipette, remove air, and tie a knot in the other end of the tubing.
Carefully hang the EDTA-treated plasma in the tubing into the HBS with gentle stirring. Cover with Saran Wrap and dialyze for 14-16h with gentle stirring at 4 °C.
Carefully remove the EDTA-treated/dialyzed plasma in 3.0 ml aliquots to 15.0 ml screw capped tubes and retain 100 μl for PT assay of 'final' FV-deficient plasma on ice. Store the FV-deficient plasma in at -80 °C until use. Under the conditions described above, the PTs increase from 10-15 sec before EDTA addition to 90-100 sec 8-10 hr after EDTA addition. The FV-deficient plasma prepared by this method routinely contains less than 0.1% of the active FV present in the starting fresh human plasma.
2. Preparation of Thromboplastin
Dialysis is required to remove any calcium from this reagent in order to permit precisely timing fibrin clot formation from the time of calcium chloride addition to initiate coagulation process.
Prepare 100 ml and 4.0l of 20mM HEPES and 0.15M NaCl in distilled water in a plastic beaker with gentle stirring. Adjust pH to 7.4 with slow dropwise addition of 5.0M NaOH (HBS, pH 7.4).
Add 6.0 ml of HBS, pH 7.4 to each vial of thromboplastin reagent, replace lid, and shake gently to dissolve. Incubate reagent in capped vials for 15 min at room temperature to dissolve dried material. Shake gently to completely dissolve.
Cut a sufficient length of dialysis tubing, rinse well with distilled water, and tie off one end of the tubing with a knot. Transfer thromboplastin to dialysis tubing, remove air, and tie off other end of tubing with a knot.
Carefully hang dialysis tubing in 4.0l of HBS, pH 7.4 and gently stir covered with Saran Wrap at 4 °C for 14-16 hr.
Transfer 3.0 ml aliquots of the dialyzed thromboplastin to 15 ml screw capped tubes and store at -80 °C until used.
3. Preparation of FV 1-stage Microplate Coagulation Assay Activity Standard Curves
Thaw FV-deficient plasma, NHP, and thromboplastin at 37 °C for 10 min. Gently mix FV-deficient plasma and thromboplastin independently, transfer to separate plastic washing trays, and incubate on ice. Store the NHP on ice after gently mixing. Store calcium chloride (25mM in distilled water) in a separate washing tray at room temperature.
Prepare 200 μl each of 2-fold serial dilutions of NHP from 0-, 2-, 4-, 8-, 16-, 32-, 64-, 128-, 256-, 512-fold in HBS, pH 7.4 using 1.5 ml microcentrifuge tubes. Vortex well and incubate on ice.
Place removable 8 well immunomodule strips into plastic template holder and insert into microplate reader.
Orient immunomodule strips in microplate reader as: empty, empty, sample, empty, empty, sample, empty, and empty from left to right in the template holder to minimize light scatter interference from neighbouring sample-containing strips. Also, use only wells 2-7 from top to bottom in each 8 well immunomodule strip to minimize light scatter interference from edges of plastic template holder.
The FV microplate assay is capable of measuring fibrin clot formation in at least 12 different samples simultaneously (6 samples per immunomodule strip over 2 immunomodule strips).
Turn on microplate reader and access SoftMax Pro microplate application software.
Using a multichannel pipette, make simultaneous additions of FV-deficient plasma (50 μl) to all sample microplate wells. Use a single pipette, add 50 μl of the 10 serial dilutions of NHP prepared above to separate microplate wells.
Using a multichannel pipette, add thromboplastin (50 μl) to all sample wells. Incubate in the microplate reader at 25 °C for 1 min and program the microplate reader to shake the plate during the 15-25 sec of the 1 min incubation.
Using the computer interfaced with the microplate reader, access SoftMax Pro software microplate application. Set the reader to Absorbance, Wavelength to 405nm, mode to Kinetic, temperature to 25 °C, and program the microplate reader to shake for the first 5 sec after calcium chloride addition, and measure absorbance at 405nm every 5 sec for 6 min thereafter. The 5 sec shaking interval is not included in the calculated clot times (See below).
Using a multichannel pipette, add calcium chloride (50 μl of 25mM in distilled water; final concentration 3.5mM) to all sample microplate wells, wait 15 sec, press the 'Start' icon in the microplate application in SoftMax Pro from computer to begin the assay.
The clot times are calculated as the time in seconds to reach the midpoint between the minimum and maximum absorbance at 405nm after thromboplastin and calcium chloride addition.
The initial rates of clot formation are calculated as the rate of increase in absorbance at 405nm (mUnits/min) that encompass the first 5 time points of clot formation in the linear portion of the Absorbance vs. Time curve.
The extent of clot formation was calculated as the difference between the maximal and minimal absorbance at 405nm (Units) achieved during the clot formation event.
4. Assaying Human Plasma Samples with the FV 1-stage and 2-stage Microplate Coagulation Assay
Thaw FV-deficient plasma, NHP, sample plasmas, and thromboplastin at 37 °C for 10 min. Gently mix FV-deficient plasma and thromboplastin independently, transfer to separate plastic ELISA washing trays, and incubate on ice. Store the NHP and sample plasmas on ice after gently mixing. Store calcium chloride (25mM in distilled water) in a separate ELISA washing tray at room temperature.
Prepare 200 μl each of 40-fold dilutions of NHP and sample plasmas in HBS, pH 7.4 using 1.5 ml microcentrifuge tubes. Vortex well and incubate on ice.
Insert removable 8 well immunomodule strips into plastic template holder and insert into microplate reader.
Alternate empty, empty, sample, empty, empty, sample, empty, and empty immunomodule strips from left to right in the template holder to minimize light scatter interference from neighbouring sample-containing strips. Use only wells 2-7 from top to bottom in each immunomodule strip to minimize light scatter interference from edges of plastic template holder.
Turn on microplate reader and access SoftMax Pro microplate application software.
Using a multichannel pipette, add FV-deficient plasma (50 μl) to all sample microplate wells. Using a single pipette, add 50 μl of 40-fold diluted NHP or sample plasmas prepared above to separate microplate wells.
Using a multichannel pipette, add thromboplastin (50 μl) to all sample wells. Incubate in the microplate reader at 25 °C for 1 min and program the microplate reader to shake the plate during the 15-25 sec of the 1 min incubation.
Using the computer interfaced with the microplate reader, access SoftMax Pro software microplate application. Set the reader to Absorbance, Wavelength to 405nm, mode to Kinetic, temperature to 25 °C, and program the microplate reader to shake for the first 5 sec after calcium chloride addition, and measure absorbance at 405nm every 5 sec for 6 min thereafter. The 5 sec shaking interval is not included in the calculated clot times (See below).
Using a multichannel pipette, add calcium chloride (50 μl of 25mM in distilled water; 6.25mM final concentration) to all sample microplate wells and immediately press the 'Start' icon in the microplate application in SoftMax Pro from computer to begin the assay.
At the end of the run, determine the time, initial rate, and extent of clot formation for 40-fold diluted NHP and sample plasmas from the absorbance vs. time profiles in SoftMax Pro.
Taking into account the 40-fold dilution, use the clot time for each sample to calculate the FV activity in Units/ml from the FV 1-stage activity standard curve of Log Clot Time vs. Log FV Activity (Figure 2A). This represents the FV-1 stage activity or that without 'intentional' activation by thrombin.
Since FV is activated with thrombin to the active cofactor, FVa 1, a second assay after addition and incubation with thrombin is critical to accurately measure the FV 2-stage activity of a plasma sample (With 'intentional' activation by added thrombin).
For the FV 2-stage assay, prepare 200-300 μl of purified thrombin 18 at 100nM in HBS, pH 7.4 and incubate on ice. Plan to use 10 μl of diluted thrombin for each plasma sample to be assayed with the FV 2-stage assay.
Dilute 120 μl of the above NHP and sample plasmas from 40-fold to 100-fold with 170 μl of HBS, pH 7.4 containing 2.8mM calcium chloride. Add 10 μl thrombin (10nM final concentration) to each sample. Vortex well to mix and incubate at 37 °C for 1 min.
Dilute NHP and sample plasmas to 500-fold by adding 400 μl of HBS, pH 7.4, vortex well, and assay 50 μl of each plasma sample in the FV 1-stage microplate assay as described above.
Determine the clot time for NHP and each plasma sample assayed from the Absorbance vs. Time profiles in SoftMax Pro. Taking into account the 500-fold dilution, use the clot time for each sample to calculate the FV 2-stage activity from the FV 1-stage standard curve of Log Clot Time vs. Log FV Activity (Figure 2A).
The total FV activity may then be calculated as FV 2-stage activity - FV 1-stage activity.
Representative Results
Representative Results - Preparation of FV 1-stage microplate coagulation assay activity standard curves
A representative example of fibrin clot formation in the FV assay over time generated by the microplate reader is illustrated in Figure 1. The FV assay accurately measures the time, initial rate, and extent of fibrin clot formation. Inspection of the wells upon assay completion confirmed that clot formation occurred. All reactions reached approximately the same extent of clot formation with a general change in absorbance at 405nm of 0.35 - 0.45 Units between the starting absorbance before and the maximal absorbance after thromboplastin and calcium chloride addition. Representative examples of FV 1-stage activity standard curves of Log Clot Time (in seconds) vs. Log FV Activity (Units/ml) and Log Initial Rate of Clot Formation (in mUnits/min) vs. Log FV Activity (Units/ml) for serial dilutions of NHP is shown in Figure 2A and Figure 2B, respectively. Fitting the Log-Log plot of Clot Time vs. FV 1-Activity indicated a strong relationship between these variables after linear regression analysis (Figure 2A; R2 = 0.980). Fitting the Log-Log plot of the Initial Rate of Clot Formation vs. FV Activity also indicated a strong relationship between these variables after linear regression analysis (Figure 2B; R2 = 0.983). The relationship of both Log Clot Time and Log Initial Rate of Clot Formation vs. Log FV Activity remained linear for NHP diluted up to 512-fold. Since FV circulates in NHP at approx 12-40nM 16, the microplate assay is sensitive to approx 24-80pM FV in NHP. Given that the dissociation constant of the interaction of FVa-FXa-lipid in prothrombinase is approximately 1nM 17, the FV microplate assay is entirely suitable for measurement of FV levels in the physiologically relevant nM range for FVa function in prothrombinase.
Using the FV microplate assay, it was also determined that the normal range of FV activity in the FV 1-stage activity assay of 15 healthy control plasmas (Male and Female, Age 18-20yrs) was (Mean ± Standard deviation; Range): 0.96 ± 0.14U/ml; 0.68-1.11U/ml. This agrees well with the normal healthy FV activity and range (0.66-1.14U/ml) reported by Cutler et al. for the FV 1-stage activity determined with an automated analyzer 7. The intra-assay variability of the time, extent, and initial rate of clot formation in the FV 1-stage assay in 6 wells on 8 different days was 3.4%, 4.4%, and 3.1%, respectively. The inter-assay variability of the time, extent, and initial rate of clot formation in the FV 1-stage assay in 6 wells of 8 different experiments on 8 different days was 7.1%, 7.8%, and 9.2%, respectively. Thus, the intra- and inter-assay variability of these three measured variables was at a low and acceptable level for robust assay performance within and between microplate assay of multiple samples simultaneously (Up to 12).
Representative Results - Assaying human plasma samples with the FV 1-stage and 2-stage microplate coagulation assay
The standard curve of Log Clot Time vs. Log FV Activity (Figure 2A) was used to measure the FV activity in NHP and 9 DIC patient plasmas which were not intentionally activated with added thrombin (FV 1-stage activity) or were intentionally activated with added thrombin (FV 2-stage activity) and the results are shown in Table 1. All 9 DIC patient plasmas exhibited FV 1-stage activities and initial rates of clot formation that were decreased on average, by 54% and 18%, respectively, from NHP. The extents of clot formation in the FV 1-stage assay in the DIC patients were not largely different from NHP, and increased on average, by 13% from NHP.
Activation of NHP with thrombin generated an approximate 8-fold increase in FV 2-stage activity above the FV 1-stage activity (Table 1). This indicates that the FV in NHP was mainly present in its unactivated form and agrees with the previously reported results using manual tilt-tube FV assays 3, 4 and automated FV assays 5-7. The FV 2-stage and total activity were also decreased in the DIC patients on average, by 44% and 42%, respectively, from NHP. The initial rates and extents of clot formation in the FV 2-stage assay in the DIC patients were not significantly different from NHP, and varied on average, by approx 9% and 4%, respectively, from that observed with NHP. These results indicated that compared with the FV in NHP, the FV in the DIC patient plasmas resulted in a prolonged time and decreased rate of fibrin clot formation and that the patient FV was on average, only 56% as activatable with thrombin as well.
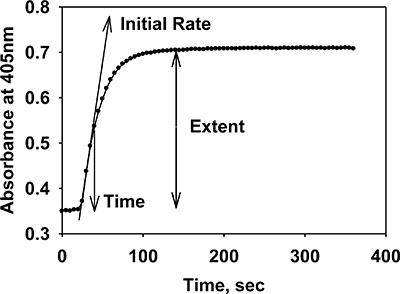 Figure 1. Clot formation in normal pooled human reference plasma measured with the kinetic microplate FV 1-stage coagulation assay. Fibrin clot formation in NHP was continuously monitored at 405nm using a kinetic microplate reader. The plot is the microplate reader output of a 6 min reaction of 32-fold diluted NHP, FV-deficient plasma, thromboplastin, and calcium chloride in a microplate well. The vertical axis represents the change in absorbance at 405nm that occurred as a result of fibrin formation in plasma. The time of fibrin formation was defined as the time to reach the half maximal increase in absorbance or the midpoint of curve (36.40 sec). The initial rate of clot formation was defined as the rate of change of absorbance at 405nm over the first 5 time points of the linear increase of the absorbance portion of the curve (611.88 mUnits/min). The extent of clot formation was defined as the difference between the maximum and minimum absorbance at 405 nm (0.35 Units).
Figure 1. Clot formation in normal pooled human reference plasma measured with the kinetic microplate FV 1-stage coagulation assay. Fibrin clot formation in NHP was continuously monitored at 405nm using a kinetic microplate reader. The plot is the microplate reader output of a 6 min reaction of 32-fold diluted NHP, FV-deficient plasma, thromboplastin, and calcium chloride in a microplate well. The vertical axis represents the change in absorbance at 405nm that occurred as a result of fibrin formation in plasma. The time of fibrin formation was defined as the time to reach the half maximal increase in absorbance or the midpoint of curve (36.40 sec). The initial rate of clot formation was defined as the rate of change of absorbance at 405nm over the first 5 time points of the linear increase of the absorbance portion of the curve (611.88 mUnits/min). The extent of clot formation was defined as the difference between the maximum and minimum absorbance at 405 nm (0.35 Units).
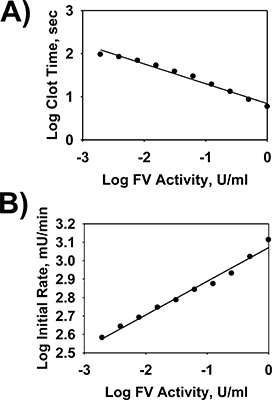 Figure 2. Standard curves of time and initial rate of clot formation vs. Factor V activity in normal pooled human reference plasma using the FV 1-stage microplate assay. NHP was serially diluted (0- to 512-fold in HBS) and assayed with the FV 1-stage microplate assay as described in the protocol. Log-Log plots of the time and initial rate of clot formation vs. FV activity in the FV 1-stage microplate assay are shown after linear regression modeling of the data in panels A and B, respectively.
Figure 2. Standard curves of time and initial rate of clot formation vs. Factor V activity in normal pooled human reference plasma using the FV 1-stage microplate assay. NHP was serially diluted (0- to 512-fold in HBS) and assayed with the FV 1-stage microplate assay as described in the protocol. Log-Log plots of the time and initial rate of clot formation vs. FV activity in the FV 1-stage microplate assay are shown after linear regression modeling of the data in panels A and B, respectively.
| Sample | 1-stage assay Activity (Units/ml) | 1-stage assay Extent (Units) | 1-stage assay Initial Rate (mUnits/min) | 2-stage assay Activity (Units/ml) | 2-stage assay Extent (Units) | 2-stage assay Initial Rate (mUnits/min) | Total activity (Units/ml) |
| NHP | 1.02 | 0.363 | 744.96 | 7.93 | 0.433 | 375.84 | 6.91 |
| Patient 1 | 0.36 | 0.404 | 583.80 | 3.84 | 0.432 | 249.96 | 3.48 |
| Patient 2 | 0.67 | 0.416 | 704.04 | 5.28 | 0.469 | 323.16 | 4.61 |
| Patient 3 | 0.31 | 0.435 | 562.08 | 3.92 | 0.453 | 294.96 | 3.61 |
| Patient 4 | 0.39 | 0.435 | 617.04 | 4.14 | 0.462 | 372.60 | 3.75 |
| Patient 5 | 0.73 | 0.401 | 641.40 | 5.91 | 0.433 | 354.24 | 5.18 |
| Patient 6 | 0.45 | 0.403 | 600.72 | 4.16 | 0.445 | 393.96 | 3.71 |
| Patient 7 | 0.49 | 0.395 | 575.40 | 4.89 | 0.449 | 357.48 | 4.40 |
| Patient 8 | 0.19 | 0.448 | 489.00 | 2.89 | 0.450 | 330.48 | 2.70 |
| Patient 9 | 0.64 | 0.423 | 699.48 | 5.11 | 0.455 | 417.24 | 4.47 |
Table 1 FV Activity in NHP and in 9 Patients That Developed Disseminated Intravascular Coagulation (DIC). The FV 1-stage, 2-stage, and total activity in NHP and 9 DIC patient plasmas were determined from the FV 1-stage microplate assay standard curve of time of clot formation vs. FV activity (Figure 2A) and are given in Units/ml. The initial rates (Initial rate of increase in A405nm over first five time points in mUnits/min) and extents (Maximum A405nm - Minimum A405nm) of clot formation in the FV 1- and 2-stage microplate assay are also shown.
Discussion
The kinetic microplate assay method outlines the development of a novel, rapid, inexpensive, and convenient technique for measurement of FV coagulation activity in samples of human plasma which have not (FV 1-stage assay) or have been intentionally activated with added thrombin (FV 2-stage assay). The assay utilizes a kinetic microplate reader and all the materials and reagents required are commercially available or may be made in-house. The assay continuously monitors the change in the light scatter of plasma during fibrin clot formation at 405nm. The microplate assay has the advantage of using small plasma sample volumes (μl) and is amenable for analysis of multiple samples simultaneously (up to 12). These assay attributes are advantageous when expensive equipment and reagents are used (Automated analyzers and Factor-deficient plasmas); only small sample volumes are available (Patient plasmas) or analyzing a large number of samples (During FV purification from plasma).
The FV microplate assay has the added advantages that it is convenient, fast, and does not require the isolation and purification of the required constituent components. Compared with manual tilt-tube 3, 4 and automated 5-7 FV assays that have been reported, the FV microplate assay has comparable useful range of clot times (25-75 sec) and corresponding FV levels in the sample (0.5-0.005 Units/ml), and sensitivity/detection limits (20-80pM). Compared with manual tilt-tube FV assays which suffer from a subjective visual assessment of clot formation 3, 4, the FV microplate assay permits objective and quantitative measurement of the clot time, initial rate, and extent of fibrin clot formation. Compared with automated FV assays which suffer from the requirement of expensive analyzers and reagents 5-7, the FV microplate assay reagents and materials are inexpensive and all the required materials may be purchased commercially or made in-house. Manual tilt-tube 3, 4 and automated 5-7 FV assays generally only provide the time for clot formation; not the time, initial rate, and extent of fibrin clot formation which are all accurately measured spectrophotometrically with the FV microplate assay. Finally, manual tilt-tube 3,4 and automated 5-7 FV assays suffer from only being able to measure the FV activity in plasma samples one at a time; whereas the FV microplate assay is amenable to high-throughput and 12 samples may be assayed simultaneously.
The microplate assay was used to demonstrate that the FV in 9 DIC patient plasmas was less active than in NHP because of a combination of delayed clot times and lower initial rates of clot formation in the FV 1-stage assay. The initial rates of clot formation in the FV 2-stage assay in the DIC patient plasmas were not changed from NHP. The extents of clot formation in the DIC patient plasmas were also not changed from NHP in the FV 1-stage and 2-stage assays. Decreased FV 1-stage, 2-stage, and total activities may have been due to increased FV consumption 19 and/or inactivation 6 in accordance with other studies during the pathogenesis of this acquired blood disorder. Given the extent of clot formation in the DIC plasmas was the same as observed with NHP, this variable was most likely a result of the fibrinogen concentration in the FV-deficient plasma and not related to the characteristics of the patient plasmas.
The results from the microplate assay indicated that measurement of FV activity in samples of human plasma may be obtained from the time and initial rate of clot formation from the FV 1-stage assay and the time of clot formation and total activity from the FV 2-stage assay. It was not possible to obtain quantitative measurement of FV activity in a plasma sample based on the extent of clot formation in the FV 1- and 2-stage assays or the initial rate of clot formation in the FV 2-stage assay. Given all reactions reached approximately the same extent of clot formation of 0.35 - 0.45 Units between the initial absorbance before and the maximal absorbance after thromboplastin and calcium chloride addition, measurement of the extent of clot formation does not provide a quantitative assessment of FV activity in a given plasma sample.
The novel microplate assay will find use in research and clinical settings for measurement of FV activity in samples of human plasma and fractions during its purification from human and animal plasma. The microplate assay may be used to measure the time, initial rate, and extent of clot formation; parameters not generally monitored simultaneously in manual tilt-tube assays and automated coagulation analyzers. This information provides more of a complete quantitative assessment of the involvement of FV during the clot formation event in human plasma than has been previously reported 3-7. This information is especially important in both research and clinical laboratories when measuring significant changes in FV activity in samples of plasma or with purified FV or FVa. The FV microplate assay may also be used to characterize and measure compounds that may activate or inactivate FV and FVa, measure the FV activity in patients at risk for venous thrombosis as a result of the FV Leiden mutation 20, 21 and to monitor the activity of FV during its purification from plasma.
The FV microplate assay is easily adaptable to measure the activity of any coagulation factor in the extrinsic, intrinsic, or common pathway using the appropriate factor-deficient plasma and initiating reagents for fibrin formation. The assay will increase our understanding of FV biochemistry through a more accurate and complete measurement of its activity in research and clinical laboratories. Ultimately, this information will positively impact healthcare environments through earlier diagnosis and development of more effective treatments for individuals afflicted with coagulation disorders, such as DIC.
Disclosures
No conflicts of interest declared.
Acknowledgments
The research was supported with Professional Development and Research Startup Funds from The University of Ontario Institute of Technology (UOIT) to Dr. John A. Samis and a Canadian Institutes of Health Research Health Professional Student Research Award to Irina Levit. The authors acknowledge Shannon Everett (Teaching and Learning Centre, UOIT) for her assistance preparing the video. The authors acknowledge Dr. Michael E. Nesheim (Department of Biochemistry, Queen's University, Kingston, ON) for his mentorship, insight, and helpful discussions. The authors also acknowledge Dr. Cheng Hock Toh (Roald Dahl Haemostasis and Thrombosis Centre, Royal Liverpool University Hospital, Liverpool, UK) for providing the DIC patient plasmas used in the study.
References
- Orfeo T, Brufatto N, Nesheim ME, Xu H, Butenas S, Mann KG. The factor V activation paradox. J. Biol. Chem. 2004;279:19580–19591. doi: 10.1074/jbc.M400727200. [DOI] [PubMed] [Google Scholar]
- Bukys MA, Blum MA, Kim PY, Brufatto N, Nesheim ME, Kalafatis M. Incorporation of factor Va into prothrombinase is required for coordinated cleavage of prothrombin by factor Xa. J. Biol. Chem. 2005;280:27393–273401. doi: 10.1074/jbc.M503435200. [DOI] [PubMed] [Google Scholar]
- Nesheim ME, Katzmann JA, Tracy PB, Mann KG. Factor V. Methods. Enzymol. 1981;80:249–274. doi: 10.1016/s0076-6879(81)80023-7. [DOI] [PubMed] [Google Scholar]
- Bloom JW, Nesheim ME, Mann KG. A rapid technique for the preparation of factor V deficient plasma. Thromb. Res. 1979;15:595–599. doi: 10.1016/0049-3848(79)90169-5. [DOI] [PubMed] [Google Scholar]
- Samis JA, Stewart KA, Nesheim ME, Taylor FB., Jr Factor V cleavage and inactivation are temporally associated with elevated elastase during experimental sepsis. J. Thromb. Haemost. 2007;5:2559–2561. doi: 10.1111/j.1538-7836.2007.02778.x. [DOI] [PubMed] [Google Scholar]
- Samis JA, Stewart KA, Toh CH, Day A, Downey C, Nesheim ME. Temporal changes in factors associated with neutrophil elastase and coagulation in intensive care patients with a biphasic waveform and disseminated intravascular coagulation. J. Thromb. Haemost. 2004;2:1535–1544. doi: 10.1111/j.1538-7836.2004.00826.x. [DOI] [PubMed] [Google Scholar]
- Cutler JA, Patel R, Rangarajan S, Tait RC, Mitchell MJ. Molecular characterization of 11 novel mutations in patients with heterozygous and homozygous FV deficiency. Haemophilia. 2010;16:937–942. doi: 10.1111/j.1365-2516.2010.02330.x. [DOI] [PubMed] [Google Scholar]
- Ihara M, Suzuki T, Kobayashi N, Goto J, Ueda H. Open-sandwich enzyme immunoassay for one-step noncompetitive detection of corticosteroid 11-deoxycortisol. Anal. Chem. 2009;81:8298–8304. doi: 10.1021/ac900700a. [DOI] [PubMed] [Google Scholar]
- Janke R, Genzel Y, Wahl A, Reichl U. Measurement of key metabolic enzyme activities in mammalian cells using rapid and sensitive microplate-based assays. Biotechnol. Bioeng. 2010;107:566–581. doi: 10.1002/bit.22817. [DOI] [PubMed] [Google Scholar]
- Beebe DP, Aronson DL. An automated fibrinolytic assay performed in microtiter plates. Thromb. Res. 1987;47:123–128. doi: 10.1016/0049-3848(87)90249-0. [DOI] [PubMed] [Google Scholar]
- Frantantoni JC, Poindexter BJ. Measuring platelet aggregation with microplate reader. A new technical approach to platelet aggregation studies. Am. J. Clin. Pathol. 1990;94:613–617. doi: 10.1093/ajcp/94.5.613. [DOI] [PubMed] [Google Scholar]
- Pratt CW, Monroe DM. Microplate coagulation assays. Biotechniques. 1992;13:430–433. [PubMed] [Google Scholar]
- Tilley D, Levit I, Samis JA. Development of a microplate coagulation assay for Factor V in human plasma. Thromb. J. 2011;9:11–16. doi: 10.1186/1477-9560-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh CH, Downey C. Back to the future: Testing in disseminated intravascular coagulation. Blood Coagul. Fibrinolysis. 2005;16:535–542. doi: 10.1097/01.mbc.0000187905.54087.91. [DOI] [PubMed] [Google Scholar]
- Spero JA, Lewis JH, Hasiba U. Disseminated intravascular coagulation: Findings in 346 patients. Thromb. Haemost. 1980;43:28–33. [PubMed] [Google Scholar]
- Tracy PB, Eide LL, Bowie EJ, Mann KG. Radioimmunoassay of factor V in human plasma and platelets. Blood. 1982;60:59–63. [PubMed] [Google Scholar]
- Krishnaswamy S, Nesheim ME, Pryzdial ELG, Mann KG. Assembly of prothrombinase complex. Meths. Enzymol. 1993;222 (Part A):261–280. doi: 10.1016/0076-6879(93)22018-b. [DOI] [PubMed] [Google Scholar]
- Bajzar L, Manuel R, Nesheim ME. Purification and characterization of TAFI, a thrombin-activable fibrinolysis inhibitor. J. Biol. Chem. 1995;270:14477–14784. doi: 10.1074/jbc.270.24.14477. [DOI] [PubMed] [Google Scholar]
- Mammen EF. Disseminated intravascular coagulation (DIC) Clin. Lab. Sci. 2000;13:239–2345. [PubMed] [Google Scholar]
- Dahlbäck B, Carlsson M, Svensson PJ. Familial thrombophilia due to a previously unrecognized mechanism characterized by poor anticoagulant response to activated protein C: prediction of a cofactor to activated protein C. PNAS. 1993;90:1004–108. doi: 10.1073/pnas.90.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B. Advances in understanding pathogenic mechanisms of thrombophilic disorders. Blood. 2008;112:19–27. doi: 10.1182/blood-2008-01-077909. [DOI] [PubMed] [Google Scholar]


