Abstract
Embryogenesis is a dynamic process that is best studied by using techniques that allow the documentation of developmental changes in vivo. The use of genetically-encoded fluorescent proteins has proven a valuable strategy for elucidating dynamic morphogenetic processes as they occur in the intact organism. During the past decade, the development of photoactivatable and photoconvertible fluorescent proteins has opened the possibility to investigate the fate of discrete subpopulations of tagged proteins1. Unlike photoactivatable proteins, photoconvertible fluorescent proteins (PCFPs) are readily tracked and imaged in their native emission state prior to photoconversion, making it easier to identify and select regions by optical inspection. PCFPs, such as Kaede2, KikGR3, Dendra4 and EosFP5, can be shifted from green to red upon exposure to UV or blue light due to a His-Tyr-Gly tripeptide sequence which forms a green chromophore that can be photoconverted to a red one by a light-catalyzed β-elimination and subsequent extension of a π-conjugated system3. PCFPs and their monomeric variants are useful tools for tracking cells6-10 and studying protein dynamics11-14, respectively. During recent years, PCFPs have been expressed in different animal model, such as zebrafish6, chicken7,8 and mouse9,10 for cell fate tracking. Here we report a protocol for cell-specific photoconversion of PCFPs in the living zebrafish embryo and further tracking of photoconverted proteins at later developmental stages. This methodology allows studying, in a tissue-specific manner, cell biological events underlying morphogenesis in the zebrafish animal model.
Keywords: Developmental Biology, Issue 67, Cellular Biology, Molecular Biology, Cell tracking, live cell imaging, photoconvertible fluorescent proteins, tissue morphogenesis, Danio rerio, zebrafish, embryo
Protocol
1. Obtaining Embryos for the Photoconversion Assay
Upon in-crossing of transgenic zebrafish expressing a PCFP the brightest which are homozygote embryos are chosen, and grown at 28.5 °C until the desired embryonic stage15.
Note 1: This preselection will allow scanning embryos with low laser power thereby preventing phototoxicity and photobleaching.
Note 2: We suggest to use low-intensity light when visualizing embryos and to maintain them in the dark conditions due to high sensitivity of PCFPs, such as Kaede and KikGR, to light exposure including daylight.
2. Embedding
In a glass container with egg-water, dechorionate embryos under stereomicroscope using forceps.
Anesthetize embryos by transferring a dechorionated embryo into 0.16 mg/ml Tricaine (3-amino benzoic acidethylester, Sigma-Aldrich) solution (40μl of 4 mg/ml stock solution in 1 ml of E3 medium 1X).
Note 1: Transfer embryos with a glass pipette or a cut-tip (P1000) to minimize the risk of damaging the embryos.
Note 2: Young dechorionated embryos should not be exposed to air or lifted up to the water/air interface, since the eggs easily rupture when subjected to mechanical stress.
Transfer the anesthetized embryo onto the cover of a culture dish and maintain the embryo within the smallest possible volume of medium.
Transfer the embryo into agarose by pipetting the embryo with 1% low melting temperature agarose (LMA, Lonza) at 30 °C containing 0.16 mg/ml Tricaine, and place it onto the cover-glass of a cover-glass-bottomed culture dish (MatTek Corp. Ashland MA USA). Subsequently, orient the embryo with the help of a smooth plastic tip (e.g. a cut Microloader Pipette Tip, Eppendorf) and, when the LMA has polymerized, cover agarose with 0.16 mg/ml Tricaine solution.
Note 1: Melt 1% LMA at 60 °C and maintain melted agarose at 30 °C.
3. Photoconversion
On an inverted confocal microscope equipped with an 405 nm, 488nm and 561nm laser source, such as the Zeiss LSM 710, visualize the sample by a z-Stack scan covering the entire structure of the specimen to be photoconverted and tracked later on using the 488 nm laser (i.e., 30 mW Ar laser,at 5-7 % intensity, detector set at 493-540 nm) and the 561 nm laser (i.e., 10 mW DPSS 561 laser, at 7-9 % intensity, detector set at 587-651) to scan the tissue/organ for the green and red fluorescence of the photoconvertible proteins, respectively.
Note 1: For the scan before photoconversion, we used a Plan Apochromat 20x/NA 0.8 objective.
Note 2: Always scan for the red fluorescence prior to the experiment to exclude that any accidentally generated red converted PCFP is present.
Select the Time series tool and set for 2 cycles with no interval (one for pre- and one for post photoconvertion).
Select the Regions tool and define one or more regions of interest (ROI) for photoconversion.
Note 1: We recommend to first determine the precision with which the region actually photo-converted by the scanning laser in the ROI matches the ROI selected in the software by measuring the area of photoconverted tissue/cells in all three (xy and z direction) in a fixed specimen where no cell or protein movement is expected. For the settings described within this application, the ROI matched the photoconverted region with great precision but this depends on the objective used and the amount of laser applied for photoconversion. The size of the ROI can be reduced to the size of a single cell depending on the numerical aperture of the objective, the zoom factor and the amount of laser light used for photoconversion.
Select the Bleaching tool and set for start bleaching after scan 1 of 2. We used a 30 mW 405 nm diode laser for photoconversion and carefully optimized for the minimal amount of laser light necessary for complete photoconversion which is mostly achieved by varying the intensity of the laser light, the scan iterations of the ROI and the pixel time (i.e., scan speed).
Note 1: For photoconversion we used a Plan Apochromat 20x/NA 0.8 objective.
Note 2: The power of the laser and the number of iterations must be determined empirically for each experimental setting and should ensure the complete photoconversion from green to red PCFP within the ROI with the minimal necessary amount of laser light to reduce phototoxicity. We recommend to initiate tests using low laser power (e.g. 3 %, corresponding to 0.03 mW in the objective plan) combined with low numbers of iterations (e.g. 20), and to subsequently increase the laser power until complete photoconversion is achieved which in our hands was at 10% laser power (corresponding to 0.1 mW in the objective plan). Under these conditions, all embryos tested survived the treatment plan. Nevertheless, we observed that the yolk membrane is sensitive to laser pulses and can easily be damaged causing death of the embryo. For this reason, embryo lethality after photoconversion may be caused if the position of the target tissue is close to the yolk body.
Scan the sample by a z-Stack using lasers for 488 nm and 561 nm to visualize any remaining green and the photoconverted red fluorescence of the PCFP.
Note 1: For the scan after photoconversion, we used a Plan Apochromat 20x/NA 0.8.
4. Remove Embryo from Agarose
Discard the solution and carefully remove the embryo from the 1% LMA with the help of a needle or a smooth plastic tip.
Maintain the embryo in egg water in the dark at 28.5 °C until the desired developmental stage.
Note 1: It is important that embryos continue their development in egg water, particularly during the first days of development during extension of the embryonic body. Indeed, some embryonic tissues/organs change positions during development and hence require specific orientations during embedding which depends on the particular tissue/organ of interest and the desired developmental stage of analysis.
5. Re-embedding
Proceed like under point 2.) of this protocol.
6. Tracking Photoconverted Tissue at Later Embryonic Stages
Scan of the region of interest (z-Stack) using lasers for 488 nm and 561 nm for green and red fluorescent signals, respectively.
Note 1: For the scan x hours post-photoconversion, we used a Plan Apochromat 20x/NA 0.8.
Note 2: As this protocol does not affect the normal development of the embryo, it is possible to observe the photoconverted embryo at later stages of development (for this, proceed as outlined under points 4 - 6).
7. Representative Results
An example of a photoconversion assay is shown in Figure 1. We tracked endothelial cells during zebrafish development (for a review of the vascular anatomy of the developing zebrafish see16). Accordingly, we used a zebrafish Tg(kdrl:nlsKikGR)hsc7 reporter line expressing KikGR within endothelial nuclei17. Homozygous and brightly expressing embryos were raised at 28.5 °C until 48 hr post fertilization (hpf). Upon dechorionation, embryos were anesthetized and embedded in LMA positioning the anterior-dorsal side of the embryo towards the cover glass for better visualization of head vessels. We use an inverted confocal microscope (Zeiss LSM 710 Meta) equipped with lasers operating at 405 nm, 488 nm and 561 nm, and a Plan Apochromat 20x/NA 0.8 objective for the photoconversion assay and further tracking of the photoconverted protein. As shown in Figure 1A, before photoconversion, KikGR was expressed within endothelial tissue and was only detectable as green fluorescence using a 488 nm Argon laser. Control scans with a 561 nm DPSS 561-10 laser for red fluorescence did not detect any photoconverted KikGR prior to the photoconversion (Figure 1A). Subsequently, the ROI was completely photoconverted using a 405 nm Diode laser (at 10% intensity, 20 iterations) and the head vessels were scanned again using the 488 nm and 561 nm lasers (Figure 1B). As shown in Figure 1B, green KikGR had completely switched to red within the ROI. After the photoconversion assay, the embryo was removed from the agarose and raised at 28.5 °C in egg water. Finally, the embryo was observed again 24 hr post-photoconversion, around 72 hpf, to track the photoconverted endothelial cells at later stages (Figure 1C). Thus, the embryo was embedded in LMA, and the sample was re-scanned using the 488 nm and 561 nm lasers. As shown in Figure 1C, within endothelial tissue arising from the photoconverted ROI, both non- and photoconverted KikGR proteins were observed due to the stability of the red fluorescent photoconverted KikGR and re-synthesis of new green non-photoconverted KikGR.
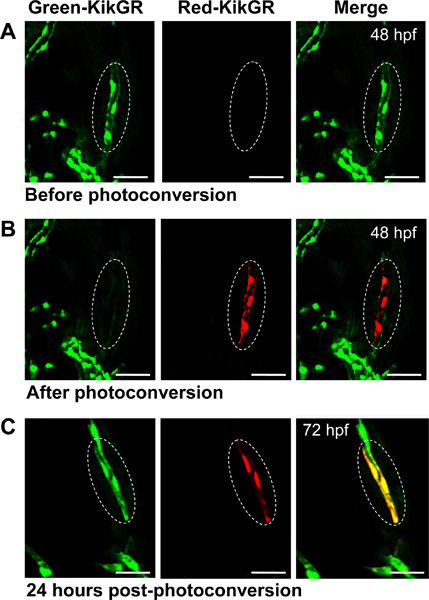 Figure 1. Photoconversion of KikGR during zebrafish development. (A,B) A specific region of the endothelial head vasculature was photoconverted at 48 hpf in Tg(kdrl:nlsKikGR)hsc7 transgenic embryos and (C) observed again 24 hr post-photoconversion (around 72 hpf). The fluorescence of KikGR (green), photoconverted KikGR (red) and merged channels are shown. Dotted circles show the ROI before, after, and 24 hr post- photoconversion. Images are dorsal views of the zebrafish embryo, anterior to the bottom. Scale bars: 50 μm.
Figure 1. Photoconversion of KikGR during zebrafish development. (A,B) A specific region of the endothelial head vasculature was photoconverted at 48 hpf in Tg(kdrl:nlsKikGR)hsc7 transgenic embryos and (C) observed again 24 hr post-photoconversion (around 72 hpf). The fluorescence of KikGR (green), photoconverted KikGR (red) and merged channels are shown. Dotted circles show the ROI before, after, and 24 hr post- photoconversion. Images are dorsal views of the zebrafish embryo, anterior to the bottom. Scale bars: 50 μm.
| Protein (Acronym) | Ex (nm) | Em (nm) | EC | QY | Quaternary Structure | Brightness | Application | Ref |
| Kaede_G | 508 | 518 | 99 | 0.9 | tetramer | 259 | Cell tracking | 2,6 |
| Kaede_R | 572 | 580 | 60 | 0.3 | tetramer | 59 | ||
| KikGR_G | 507 | 517 | 54 | 0.7 | tetramer | 112 | Cell tracking | 3,6,8-10 |
| KikGR_R | 583 | 593 | 35 | 0.6 | tetramer | 68 | ||
| mKikGR_G | 505 | 515 | 49 | 0.7 | monomer | 101 | Dynamic tracking Superresolution images | 13 |
| mKikGR_R | 580 | 591 | 28 | 0.6 | monomer | 53 | ||
| EosFP_G | 506 | 516 | 72 | 0.7 | tetramer | 150 | Cell tracking | 5,20 |
| EosFP_R | 571 | 581 | 41 | 0.5 | tetramer | 67 | ||
| tdEos_G | 506 | 516 | 34 | 0.7 | tandem dimer | 165 | Superresolution images | 12,14,21 |
| tdEos_R | 569 | 581 | 33 | 0.6 | tandem dimer | 59 | ||
| mEos2_G | 506 | 519 | 56 | 0.7 | monomer | 140 | Dynamic tracking Superresolution images | 11,18 |
| mEos2_R | 573 | 584 | 46 | 0.7 | monomer | 90 | ||
| Dendra2_G | 490 | 507 | 45 | 0.5 | monomer | 67 | Dynamic tracking Superresolution images | 4,11 |
| Dendra2_R | 553 | 573 | 35 | 0.55 | monomer | 57 |
Table I. Properties of photoconvertable fluorescent proteins. Along with the common name and/or acronym for each PCFPs, the peak excitation (Ex) and emission (Em) wavelengths, molar extinction coefficients (EC, 10-3 M-1 cm-1 ), quantum yield (QY), physiologically relevant quaternary structure, relative brightness (as % of EGFP), application, and references are shown in the table. Table adapted from Day and Davidson, 20101.
| PCFP | Zebrafish transgenic lines | Source |
| Kaede | Tg(elavl3:Kaede)rw0130a | Okamoto Lab |
| Tg(EPV.TP1-Mmu.Hbb:Kaede) | ||
| Tg(ins:Kaede) | ||
| Tg(isl1:Gal4-VP16,14xUAS:Kaede) | ||
| Tg(lhx5:Kaede)b1204/+ | ZIRC | |
| Tg(myl7:kaede) | ||
| Tg(olig2:Kaede) | ||
| Tg(UAS-E1b:Kaede)s1999t | ZIRC | |
| Tg(UAS:Kaede)rk8 | ZIRC | |
| Tg(vsx2:Kaede)nns2 | Higashijima Lab | |
| KikGR | Tg(kdrl:nlsKikGR) | |
| Tg(krt4:NTR-KikGR) | ||
| Tg(myl7:nlsKikGR) | ||
| Tg(sox10:KikGR) | ||
| Tg(UAS:KikGR) | ||
| EosFP | Tg(sox10:EosFP) | |
| Tg(sox10:nlsEosFP) | ||
| Dendra | Tg(-8mpx:Dendra2) | |
| Tg(UAS:Dendra-kras) |
Table II. Zebrafish transgenic lines expressing PCFPs. Data obtained in the Zebrafish Model Organism Database (ZFIN, http://zfin.org/). Availability of lines in the Zebrafish International Resource Center (ZIRC, http://zebrafish.org/zirc/) is indicated.
Discussion
In recent years, transgenic animal models for the expression of photoconvertible proteins such as Kaede or KikGR have been generated. These animals develop normally, indicating that these proteins have no toxic effects on embryonic development. The first report about a photoconversion assay for tracking cells in animal embryos was performed by Hatta and collaborators by injection of mRNA or DNA encoding Kaede into one-cell zebrafish embryos for ubiquitous expression, or by expression of Kaede and KikGR in neural tissue by using the Gal4-UAS system6. They were able to visualized cells movements and tracing of neural shapes, which demonstrated the versability of this tool to follow morphogenetic processes such as neurulation, placode formation and navigation of early commissural axons in the hindbrain6. In the chicken model, KikGR was introduced into the neural tube by electroporation and photoconverted in the neural crest or the neural tube and used to selectively mark subgroups of neural crest cells within migratory streams in the chick embryo8. Photoconversion of Kaede and KikGR were also compared in mouse, concluding that KikGR was the most suitable photoconvertible protein for cell labeling and lineage studies in embryonic stem cells and mice because it is developmentally neutral, bright and undergoes rapid and complete photoconversion9. Recently, the transgenic mouse line iUBC-KikGR has been generated by Griswold and collaborators, which expresses KikGR under the control of the human Ubiquitin C promoter10. Using this transgenic line, the authors tracked cells in real time by culturing the tissue of interest as explants10. In addition, monomeric variants of PCFPs, such as mEos218, Dendra211, and mKikGR13 have been reported to be useful in a wide variety of dynamic tracking experiments and can be used for precise localization in super-resolution microscopy12,14 . These reports reflect the widespread use of the photoconversion assay to study cellular processes during organ/tissue morphogenesis in different animal models. Among them, fate mapping, single cell behaviour analysis, and localization of proteins with nanometer-scale precision have been studied by this technique (see table I for an overview of the PCFPs properties and reported applications). In comparison to other models, the suitability of zebrafish for high-resolution live imaging19 and the simple generation of tissue-specific transgenic lines for the expression of fluorescent proteins, makes this vertebrate an excellent model of choice for such approaches (see table II for a list of zebrafish transgenic lines expressing PCFPs as reported in ZFIN). As depicted in this table, there is a battery of zebrafish transgenic lines expressing different PCFPs in a temporal- or tissue-specific way, as well as, in different subcellular compartments such as, cytoplasm or nuclei. This illustrates the wide variety of opportunities for using photoconversion assay in zebrafish biological processes. Here, we have exemplified a photoconversion assay to track cells during zebrafish development by using a zebrafish transgenic line expressing endothelial KikGR. Upon photoconversion, KikGR completely switched from green to red protein within the ROI. At later developmental stages, we could detect photoconverted protein within head vessels. The fact that photoconverted red KikGR is highly stable and that embryos remain viable during the assays allows for multiple, time course-dependent measurements on the same developing embryo. The stability of the converted protein could depend on the tissue within which the PCFP is expressed and on the developmental stage. We observed that 24 hours post-photoconversion the photoconverted protein expressed in endothelial tissue was stable and easily detectable. Red fluorescence was also detectable in highly proliferate tissues even though the protein would be expected to be diluted within the cytoplasm (data not shown). Here, we have laid out the methodology for tissue-specific photoconversion of cells expressing a PCFP, and further tracking of these cells in the living zebrafish embryo, which represents a powerful tool to elucidate different morphogenetic processes in intact organisms. This analysis includes to assess tissue reorganization and cell behaviors as they occur during development.
Disclosures
No conflicts of interest declared.
Acknowledgments
We thank Dr. Ian C. Scott for kindly providing the zebrafish transgenic Tg(kdrl:nlsKikGR)hsc7 line at the Hospital for Sick Children, Toronto, Canada. We thank the Confocal and 2-Photon Microscopy Core Facility (Max-Delbrueck-Center for Molecular Medicine) and Dr. Zoltan Cseresnyes, for excellent technical assistance and overall imaging support. S.A.-S. is supported by a Heisenberg fellowship of the Deutsche Forschungsgemeinschaft (DFG). This work was supported by DFG grant SE2016/7-1.
References
- Day RN, Davidson MW. The fluorescent protein palette: tools for cellular imaging. Chem. Soc. Rev. 2009;38:2887–2921. doi: 10.1039/b901966a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12651–12656. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H, Karasawa S, Shimizu H, Nukina N, Miyawaki A. Semi-rational engineering of a coral fluorescent protein into an efficient highlighter. EMBO Rep. 2005;6:233–238. doi: 10.1038/sj.embor.7400361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurskaya NG, Verkhusha VV, Shcheglov AS, Staroverov DB, Chepurnykh TV, Fradkov AF, Lukyanov S, Lukyanov KA. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat. Biotechnol. 2006;24:461–465. doi: 10.1038/nbt1191. [DOI] [PubMed] [Google Scholar]
- Wiedenmann J, Ivanchenko S, Oswald F, Schmitt F, Rocker C, Salih A, Spindler KD, Nienhaus GU. EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15905–15910. doi: 10.1073/pnas.0403668101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K, Tsujii H, Omura T. Cell tracking using a photoconvertible fluorescent protein. Nat. Protoc. 2006;1:960–967. doi: 10.1038/nprot.2006.96. [DOI] [PubMed] [Google Scholar]
- Stark DA, Kulesa PM. An in vivo comparison of photoactivatable fluorescent proteins in an avian embryo model. Dev. Dyn. 2007;236:1583–1594. doi: 10.1002/dvdy.21174. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Teddy JM, Stark DA, Smith SE, McLennan R. Neural crest invasion is a spatially-ordered progression into the head with higher cell proliferation at the migratory front as revealed by the photoactivatable protein, KikGR. Dev. Biol. 2008;316:275–287. doi: 10.1016/j.ydbio.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotschin S, Hadjantonakis AK. Use of KikGR a photoconvertible green-to-red fluorescent protein for cell labeling and lineage analysis in ES cells and mouse embryos. BMC Dev. Biol. 2009;9:49. doi: 10.1186/1471-213X-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold SL, Sajja KC, Jang CW, Behringer RR. Generation and characterization of iUBC-KikGR photoconvertible transgenic mice for live time-lapse imaging during development. Genesis. 2011;49:591–598. doi: 10.1002/dvg.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SM, Buckheit RW, 3rd, Falk MM. Green-to-red photoconvertible fluorescent proteins: tracking cell and protein dynamics on standard wide-field mercury arc-based microscopes. BMC Cell Biol. 2010;11:15. doi: 10.1186/1471-2121-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Punge A, Hollopeter G, Willig KI, Hobson RJ, Davis MW, Hell SW, Jorgensen EM. Protein localization in electron micrographs using fluorescence nanoscopy. Nat. Methods. 2011;8:80–84. doi: 10.1038/nmeth.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habuchi S, Tsutsui H, Kochaniak AB, Miyawaki A, van Oijen AM. mKikGR, a monomeric photoswitchable fluorescent protein. PLoS One. 2008;3:e3944. doi: 10.1371/journal.pone.0003944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Takizawa N, Wilson KA, Smith TC, Delprato A, Davidson MW, Lambright DG, Luna EJ. The membrane-associated protein, supervillin, accelerates F-actin-dependent rapid integrin recycling and cell motility. Traffic. 2010;11:782–799. doi: 10.1111/j.1600-0854.2010.01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev. Biol. 2001;230:278–301. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- Lazic S, Scott IC. Mef2cb regulates late myocardial cell addition from a second heart field-like population of progenitors in zebrafish. Dev. Biol. 2011;354:123–133. doi: 10.1016/j.ydbio.2011.03.028. [DOI] [PubMed] [Google Scholar]
- McKinney SA, Murphy CS, Hazelwood KL, Davidson MW, Looger LL. A bright and photostable photoconvertible fluorescent protein. Nat. Methods. 2009;6:131–133. doi: 10.1038/nmeth.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho L. Live imaging of zebrafish development. Cold Spring Harb Protoc. 2011;2011:770–777. doi: 10.1101/pdb.top119. [DOI] [PubMed] [Google Scholar]
- Curran K, Lister JA, Kunkel GR, Prendergast A, Parichy DM, Raible DW. Interplay between Foxd3 and Mitf regulates cell fate plasticity in the zebrafish neural crest. Dev. Biol. 2010;344:107–118. doi: 10.1016/j.ydbio.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhaus GU, Nienhaus K, Holzle A, Ivanchenko S, Renzi F, Oswald F, Wolff M, Schmitt F, Rocker C, Vallone B, Weidemann W, Heilker R, Nar H, Wiedenmann J. Photoconvertible fluorescent protein EosFP: biophysical properties and cell biology applications. Photochem. Photobiol. 2006;82:351–358. doi: 10.1562/2005-05-19-RA-533. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio) 4th ed. Eugene: Univ. of Oregon Press; 2000. [Google Scholar]


