Abstract
Spontaneous intracerebral hemorrhage (ICH) defines a potentially life-threatening neurological malady that accounts for 10-15% of all stroke-related hospitalizations and for which no effective treatments are available to date1,2. Because of the heterogeneity of ICH in humans, various preclinical models are needed to thoroughly explore prospective therapeutic strategies3. Experimental ICH is commonly induced in rodents by intraparenchymal injection of either autologous blood or bacterial collagenase4. The appropriate model is selected based on the pathophysiology of hemorrhage induction and injury progression. The blood injection model mimics a rapidly progressing hemorrhage. Alternatively, bacterial collagenase enzymatically disrupts the basal lamina of brain capillaries, causing an active bleed that generally evolves over several hours5. Resultant perihematomal edema and neurofunctional deficits can be quantified from both models. In this study, we described and evaluated a modified double injection model of autologous whole blood6 as well as an ICH injection model of bacterial collagenase7, both of which target the basal ganglia (corpus striatum) of male CD-1 mice. We assessed neurofunctional deficits and brain edema at 24 and 72 hr after ICH induction. Intrastriatal injection of autologous blood (30 μl) or bacterial collagenase (0.075U) caused reproducible neurofunctional deficits in mice and significantly increased brain edema at 24 and 72 hr after surgery (p<0.05). In conclusion, both models yield consistent hemorrhagic infarcts and represent basic methods for preclinical ICH research.
Keywords: Medicine, Issue 67, Physiology, Neuroscience, Immunology, experimental stroke, animal model, autologous blood, collagenase, intracerebral hemorrhage, basal ganglia, brain injury, edema, behavior, mouse
Protocol
All procedures were conducted in compliance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee at Loma Linda University.
1. Presurgical Preparations
Aseptic techniques are recommended for all surgical procedures. Disinfect the stereotactic apparatus and prepare sterile surgical tools prior to surgery. Wear personal protective equipment (PPE) during all animal handling. Use a heating pad during surgery to maintain the animal's physiologic body temperature.
Weigh the 8-12 week old mouse using a triple beam animal scale.
Co-inject ketamine (100 mg/kg) and xylazine (10 mg/kg) intraperitoneally then allow 7-10 min for the anesthesia to take effect (monitor for an adequate sedation).
Place the mouse onto a thermal blanket and shave the scalp.
Apply ophthalmic ointment to both eyes.
Secure the airway, by gently moving the tongue laterally, and carefully secure the mouse's head onto the stereotactic apparatus. Note: The head must be secured horizontally to the basis of the stereotactic frame.
Disinfect the surgical area with Betadine, and rinse with 70% ethanol. Repeat alternating applications of Betadine and 70% ethanol for a total of three times. Cotton-tipped applicators can be used for this purpose.
2. Blood Injection Model
Make a 1 cm long midline incision of the scalp with a #10 scalpel blade.
Use cotton-tipped applicators to clear away the soft tissue covering the skull, in order to expose the perpendicular intersection point of the coronal and sagittal suture (bregma).
Mount the Hamilton syringe (250 μl) onto the injection pump, and stereotaxically direct the needle (26 Gauge) over bregma.
Next, adjust the stereotactic manipulator arms to position the needle 0.2 mm anterior and 2 mm laterally to the right. At these coordinates make a small cranial burr hole, using a variable speed drill with a 1 mm drill bit.
Suspend the animal's tail and disinfect its lower surface with 70% ethanol.
Puncture the central tail artery with a sterile needle (e.g. 26 Gauge) and collect the arterial blood into an unheparinized capillary tube.
Transfer the blood quickly from the capillary tube into the glass barrel of the Hamilton syringe, then insert the plunger.
Reattach the now 30 μl or more of arterial blood containing Hamilton syringe onto the injection pump and insert the needle (with its beveled edge facing the sagittal suture) through the burr hole just until its bevel is no longer visible.
From this point advance the needle 3 mm ventrally and inject 5 μl of autologous blood at a rate of 2 μl/min.
After completion of the first injection advance the needle 0.7 mm further in depth.
Wait for 5 min then inject 25 μl of blood into the right striatum.
Upon completion of the second injection, leave the needle in position for additional 10 min, before withdrawing it at a rate of 1 mm/min.
Seal the burr hole with bone wax and suture the skin.
For postoperative analgesia inject 0.05 mg/kg of buprenorphine subcutaneously in pre-warmed fluids (normal saline).
3. Collagenase Injection Model
Following the presurgical preparations, repeat steps 1-4 as described for the blood injection model.
Fill the Hamilton syringe (10 μl) with 0.075U of bacterial (clostridial) collagenase VII-S dissolved in 0.5 μl of saline. Avoid the formation of air bubbles.
Reattach the Hamilton syringe onto the injection pump and insert the needle (26 Gauge), through the burr hole just until its bevel is no longer visible.
Advance the needle 3.7 mm ventrally and inject the 0.075U of collagenase into the right striatum at a rate of 2 μl/min.
Upon completion of the injection, leave the needle in position for additional 10 min, before withdrawing it at a rate of 1 mm/min.
Seal the burr hole with bone wax and suture the skin.
Inject 0.05 mg/kg of buprenorphine subcutaneously in pre-warmed post-operative fluids.
4. Sham Operation
Following the presurgical preparations, repeat steps 1-4 as described for the blood injection model.
Insert the needle (26 Gauge) 3.7 mm ventrally through the burr hole. The needle should remain in position for 10 min before being withdrawn at a rate of 1 mm/min.
Seal the burr hole with bone wax and suture the skin.
Inject 0.05 mg/kg of buprenorphine subcutaneously in pre-warmed post-operative fluids.
5. Representative Results
Experimental intrastriatal hemorrhage evokes morphological as well as behavioral changes in rodents. These changes can be evaluated to ensure an adequate execution of the procedure, or to investigate the effects of potential treatments. Generating the bleed in a targeted brain area (e.g. basal ganglia) is most essential for a reproducible approach, and can be verified on gross or histologically stained brain sections (Figure 1-2). Injury to the basal ganglia results in sensorimotor deficits, which can be quantified via various behavioral assessments. Results of the corner turn test showed that, after experimental right-sided ICH, mice turned significantly more often ipsilaterally and away from the impaired contralateral (left) side, than sham operated animals at 24 and 72 hr after surgery (Figure 3 A). Furthermore, the ability to adequately place the impaired (left) forelimb on a surface, following vibrissae stimulation, was evaluated via the forelimb placing test. At 24 and 72 hr after surgery, mice subjected to right-sided ICH showed significantly fewer placements than sham operated animals. Measurement of brain edema is frequently employed to quantify the extent of brain injury after experimental ICH. Intracerebral injections of autologous blood (30 μl) or bacterial collagenase (0.075 U) led to a significant increase of brain water content in the ipsilateral cortex and basal ganglia at 24 (Figure 4 A) and 72 hr (Figure 4 B) after surgery (compared to sham). The outcome of the behavior tests (Figure 3) and the extent of brain edema (Figure 4) showed no difference between the blood and collagenase injection models at given volumes.
 Figure 1. Modeling ICH in mice. (A) The simplified schematic of a coronal brain section 0.2 mm anterior of bregma illustrates the proposed location of autologous blood or collagenase injection. The lateral ventricle is marked LV. CPu stands for caudate-putamen, a part of the striatum, and GP identifies the globus pallidus. Both, the striatum as well as the globus pallidus belong to a group of sub-cortical nuclei, also known as basal ganglia. (B) Representative photomicrograph of a coronal brain section 0.2mm anterior of bregma, obtained at 24 hr after intrastriatal injection of autologous whole blood.
Figure 1. Modeling ICH in mice. (A) The simplified schematic of a coronal brain section 0.2 mm anterior of bregma illustrates the proposed location of autologous blood or collagenase injection. The lateral ventricle is marked LV. CPu stands for caudate-putamen, a part of the striatum, and GP identifies the globus pallidus. Both, the striatum as well as the globus pallidus belong to a group of sub-cortical nuclei, also known as basal ganglia. (B) Representative photomicrograph of a coronal brain section 0.2mm anterior of bregma, obtained at 24 hr after intrastriatal injection of autologous whole blood.
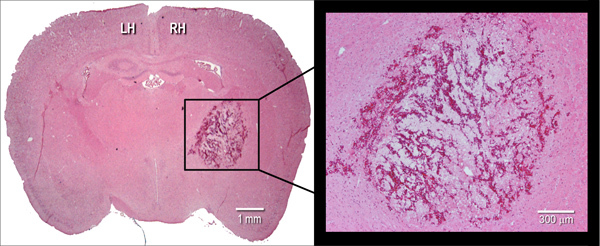 Figure 2. Histological manifestation of the hematoma. Representative hematoxylin and eosin (H&E) stained coronal cryosection (10 μm) of a mouse brain, illustrating hematoma size at 24 hr after intrastriatal injection of bacterial collagenase (0.075 U). LH=left hemisphere, RH=right hemisphere.
Figure 2. Histological manifestation of the hematoma. Representative hematoxylin and eosin (H&E) stained coronal cryosection (10 μm) of a mouse brain, illustrating hematoma size at 24 hr after intrastriatal injection of bacterial collagenase (0.075 U). LH=left hemisphere, RH=right hemisphere.
 Figure 3. Neurofunctional assessments following experimental ICH in mice. Intrastriatal injection of autologous blood (30 μl) or bacterial collagenase (0.075 U) caused reproducible neurofunctional deficits. (A) Mice after experimental ICH showed significantly more right turns than sham operated animals at 24 and 72 hr after surgery. (B) Forepaw placing capacity of the left limb was impaired after ICH at 24 and 72 hr after surgery. Values were expressed as mean±S.E.M. and analyzed with Kruskal-Wallis One Way Analysis of Variance on Ranks, followed by the Student-Newman-Keuls Method. A P value of <0.05 was considered statistically significant; n=6-12 per group, *P<0.05 compared to sham. Click here to view larger figure.
Figure 3. Neurofunctional assessments following experimental ICH in mice. Intrastriatal injection of autologous blood (30 μl) or bacterial collagenase (0.075 U) caused reproducible neurofunctional deficits. (A) Mice after experimental ICH showed significantly more right turns than sham operated animals at 24 and 72 hr after surgery. (B) Forepaw placing capacity of the left limb was impaired after ICH at 24 and 72 hr after surgery. Values were expressed as mean±S.E.M. and analyzed with Kruskal-Wallis One Way Analysis of Variance on Ranks, followed by the Student-Newman-Keuls Method. A P value of <0.05 was considered statistically significant; n=6-12 per group, *P<0.05 compared to sham. Click here to view larger figure.
 Figure 4. Evaluation of brain water content following experimental ICH in mice. Intracerebral injection of autologous blood (30 μl) or bacterial collagenase (0.075 U) led to significant increase of brain water content in the ipsilateral cortex and basal ganglia at 24 (A) and 72 hr (B) after ICH-induction. Values were expressed as mean±S.E.M. and analyzed with One Way Analysis of Variance, followed by Tukey post hoc test. A P value of <0.05 was considered statistically significant; n=6-10 per group, *P<0.05 compared to sham. Click here to view larger figure.
Figure 4. Evaluation of brain water content following experimental ICH in mice. Intracerebral injection of autologous blood (30 μl) or bacterial collagenase (0.075 U) led to significant increase of brain water content in the ipsilateral cortex and basal ganglia at 24 (A) and 72 hr (B) after ICH-induction. Values were expressed as mean±S.E.M. and analyzed with One Way Analysis of Variance, followed by Tukey post hoc test. A P value of <0.05 was considered statistically significant; n=6-10 per group, *P<0.05 compared to sham. Click here to view larger figure.
Discussion
Animal models of intracerebral hemorrhage (ICH) contribute greatly to an advanced understanding of the disease's pathophysiology, and are commonly used to develop and evaluate novel therapeutic strategies in a preclinical setting. Intraparenchymal injections of autologous blood or bacterial collagenase are well-established methods to generate ICH in rodents. Both methods were initially developed in the rat; however, due to the rapidly increasing availability of transgenic and knockout strains, mice became indispensable to further elucidate mechanisms of hemorrhagic brain injury8.
In humans, basal ganglia hemorrhage accounts for approximately 50% of all hemorrhagic strokes, and patients surviving the initial event frequently develop deleterious neurofunctional deficits1. Accordingly, experimental ICH in rodents, involving the basal ganglia, evokes sensorimotor deficits in the animal's contralateral extremities. To date, several behavior assessments have been developed to characterize those impairments in mice and rats9,10.
In the present study, we described and evaluated a modified double injection model of autologous whole blood6 as well as an ICH injection model of bacterial collagenase7, both targeting the basal ganglia (corpus striatum) in mice. We evaluated behavioral deficits via the corner turn and forelimb placing test9,11 and observed increased sensorimotor impairments in both models at 24 and 72 hr after surgery (Figure 3). At these time points no significant differences were found between the ICH groups; however, previous studies suggested a prolonged injury progression after collagenase injection, making it a more suitable model for long-term outcome studies5. Brain edema (brain water content) was measured using the wet-weight/dry-weight method as previously reported12,13. Our results showed a significant increase in perihematomal brain edema at 24 and 72 hr after ICH induction (Figure 4). All mice subjected to experimental ICH or sham surgery survived until day of sacrifice (mortality=0%).
The two described ICH models employ a stereotactically-assisted surgery to ensure precise and reproducible injections of either blood or collagenase into the targeted brain area. A small craniotomy (1 mm burr hole) is needed for this purpose. It is essential to avoid perforation of the dura by the drill bit, since this inaccuracy would aggravate the injury and result in backflow of blood or collagenase during the injection.
Initially, the blood injection model was developed as a single intracerebral injection14, but it often produced inconsistent outcomes due to backflow of blood along the needle tract15. To minimize this complication, a double injection method was developed, in which a small amount of blood is injected right above the targeted brain area, followed by a second injection of blood into the basal ganglia6. Coagulated blood of the first injection, prevents the backflow along the needle tract. This model imitates a rapidly developing hematoma, but does not induce the actual rupture of cerebral blood vessels. A major benefit of the autologous blood injection model is that no confounding factors, such as exogenous proteins, are used to induce ICH. Alternatively, the bacterial collagenase model mimics a spontaneous intracerebral bleed that develops over several hours, as exhibited in approximately 30% of all ICH patients5. Bacterial collagenase is a protease that lyses the extracellular matrix around the cerebral capillaries and weakens them, causing vessel rupture and consequent blood extravasation16. This model is generally utilized to investigate mechanisms of hematoma enlargement as well as to develop prospective treatments that affect homeostasis. However, bacterial collagenase, may amplify the inflammatory response and present neurotoxic effects at high doses3. Furthermore, extensive bleeds following the intracerebral collagenase injection may produce - in contrast to human ICH pathology- an ischemic cerebral injury.
Interestingly, female mice subjected to experimental ICH showed a significantly faster recovery of neurofunctional deficits than male animals8. Similar findings have been made in ischemic stroke models, thus male rodents are more commonly implemented for studies of stroke pathology and treatment evaluation17.
In these experiments we utilized intraperitoneal co-injections of the anesthetics ketamine (100 mg/kg) and xylazine (10 mg/kg) for both ICH models; however, previous studies have reported the incidence of acute hyperglycemia in anesthetized rodents, starting within 20 minutes after ketamine and xylazine injection18. Furthermore, ketamine, an N-methyl d-aspartate (NMDA) receptor antagonist, may possibly reduce NMDA receptor-dependant excitotoxicity, and therefore ameliorate outcomes in brain injury models. Volatile anesthetics, such as isoflurane, are alternatively used in preclinical ICH research and hold unique advantages over injectable agents, including the quick alteration of anesthesia depth and short recovery times19. The main disadvantage of gas anesthesia is the need of elaborate equipment (vaporizer, flow-meter, mask breathing circuit) as well as the possibility of human gas exposure. Moreover, isoflurane has been reported to reduce apoptotic cell death following hemorrhagic stroke in mice20. The optimal anesthesia needs to be adapted depending on length of surgery, animal species or strain, and on the outcome measurements of interest.
We described and demonstrated two ICH models that have unique strengths and weaknesses, while representing specific ICH properties. Each models' representative characteristics must be taken into consideration, when used in preclinical ICH investigations.
Disclosures
No conflicts of interest declared.
Acknowledgments
This study was partially supported by NIH grant RO1NS053407 to J.H. Zhang. We would like to thank Mr. Damon Klebe for his valuable contributions.
References
- Broderick JP. Guidelines for the management of spontaneous intracerebral hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999;30:905–915. doi: 10.1161/01.str.30.4.905. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLellan CL, Silasi G, Auriat AM, Colbourne F. Rodent models of intracerebral hemorrhage. Stroke. 2010;41:95–98. doi: 10.1161/STROKEAHA.110.594457. [DOI] [PubMed] [Google Scholar]
- James ML, Warner DS, Laskowitz DT. Preclinical models of intracerebral hemorrhage: a translational perspective. Neurocrit Care. 2008;9:139–152. doi: 10.1007/s12028-007-9030-2. [DOI] [PubMed] [Google Scholar]
- MacLellan CL. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J. Cereb. Blood Flow Metab. 2008;28:516–525. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- Belayev L. Experimental intracerebral hemorrhage in the mouse: histological, behavioral, and hemodynamic characterization of a double-injection model. Stroke. 2003;34:2221–2227. doi: 10.1161/01.STR.0000088061.06656.1E. [DOI] [PubMed] [Google Scholar]
- Clark W, Gunion-Rinker L, Lessov N, Hazel K. Citicoline treatment for experimental intracerebral hemorrhage in mice. Stroke. 1998;29:2136–2140. doi: 10.1161/01.str.29.10.2136. [DOI] [PubMed] [Google Scholar]
- Nakamura T. Intracerebral hemorrhage in mice: model characterization and application for genetically modified mice. J. Cereb. Blood Flow Metab. 2004;24:487–494. doi: 10.1097/00004647-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman R, Lekic T, Rojas H, Tang J, Zhang JH. Assessing functional outcomes following intracerebral hemorrhage in rats. Brain Res. 2009;1280:148–157. doi: 10.1016/j.brainres.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- Tang J. Mmp-9 deficiency enhances collagenase-induced intracerebral hemorrhage and brain injury in mutant mice. J. Cereb. Blood Flow Metab. 2004;24:1133–1145. doi: 10.1097/01.WCB.0000135593.05952.DE. [DOI] [PubMed] [Google Scholar]
- Ma Q. Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. J. Cereb. Blood Flow Metab. 2010. [DOI] [PMC free article] [PubMed]
- Bullock R, Mendelow AD, Teasdale GM, Graham DI. Intracranial haemorrhage induced at arterial pressure in the rat. Part 1: Description of technique, ICP changes and neuropathological findings. Neurol Res. 1984;6:184–188. doi: 10.1080/01616412.1984.11739687. [DOI] [PubMed] [Google Scholar]
- Yang GY, Betz AL, Chenevert TL, Brunberg JA, Hoff JT. Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood-brain barrier permeability in rats. J. Neurosurg. 1994;81:93–102. doi: 10.3171/jns.1994.81.1.0093. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–807. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- Saha JK, Xia J, Grondin JM, Engle SK, Jakubowski JA. Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: mechanisms and implications for preclinical models. Exp Biol Med. (Maywood) 2005;230:777–784. doi: 10.1177/153537020523001012. [DOI] [PubMed] [Google Scholar]
- Fujiwara N. Effect of normobaric oxygen therapy in a rat model of intracerebral hemorrhage. Stroke. 2011;42:1469–1472. doi: 10.1161/STROKEAHA.110.593350. [DOI] [PubMed] [Google Scholar]
- Khatibi NH. Isoflurane posttreatment reduces brain injury after an intracerebral hemorrhagic stroke in mice. Anesth Analg. 2011;113:343–348. doi: 10.1213/ANE.0b013e31821f9524. [DOI] [PMC free article] [PubMed] [Google Scholar]


