Abstract
Mesenchymal stem cells (MSCs) represent a heterogeneous population of progenitor cells with self-renewal and multipotent differentiation potential. Aside from their regenerative role, extensive in vitro and in vivo studies have demonstrated that MSCs are capable of potent immunomodulatory effects on a variety of innate and adaptive immune cells. In this article, we will review recent experimental studies on the characterization of a unique population of MSCs derived from human oral mucosa and gingiva, especially their immunomodulatory and anti-inflammatory functions and their application in the treatment of several in vivo models of inflammatory diseases. The ease of isolation, accessible tissue source, and rapid ex vivo expansion, with maintenance of stable stem-cell-like phenotypes, render oral mucosa- and gingiva-derived MSCs a promising alternative cell source for MSC-based therapies.
Keywords: gingival-derived mesenchymal stem cells, oral mucosa, multipotency, immunomodulation, inflammatory disease, regeneration
Introduction
Mesenchymal stem cells (MSCs) represent a heterogeneous population of non-hematopoietic stem cells, which were first characterized from bone marrow (Luria et al., 1971) and subsequently identified from various adult tissues, including oral tissues (Gronthos et al., 2000; Miura et al., 2003; Zhang et al., 2009). Originally, because of their multipotent capabilities, MSCs were regarded as the major source of reparative progenitor cells in tissue engineering to replace damaged tissues (Hermann et al., 2006; Kuroda et al., 2010). However, such a paradigm has been challenged by recent findings that only a very small proportion of MSCs engrafted at the injured sites could differentiate into the types of resident cells essential for the replacement of damaged tissues (Prockop, 2009; Prockop and Oh, 2012); this evidence suggests that soluble mediators or direct interaction with host cells may contribute mainly to the therapeutic effects of transplanted MSCs (Roddy et al., 2011).
The trophic property of MSCs allows them to ‘home’ to the inflammatory site, where they become activated and produce an array of bioactive mediators with various biological functions (English and Mahon, 2011; Lee et al., 2011; Prockop and Oh, 2012). Accumulating evidence suggests that the immunomodulatory and anti-inflammatory functions of MSCs are flexible or plastic depending on their distinct tissue origins, the types of targeted immune cells, and specific pathophysiological settings (English and Mahon, 2011; Lee et al., 2011). Additionally, the lack of expression of MHC class II molecules and most of the classical co-stimulatory molecules may contribute to the low immunogenicity or immune privilege of MSCs (Salem and Thiemermann, 2010). In comparison with the well-studied MSCs derived from bone marrow (BMSC) and adipose tissues (ADSC), the immunomodulatory properties of MSCs derived from oral tissues remain largely unexplored. Herein, we focus on the characteristics of a unique population of MSCs derived from human gingiva and oral mucosa and their promising role as an easily accessible and feasible alternative source of MSCs in the treatment of several inflammation-related diseases.
Gingiva and Oral Mucosa: Expression of Stem-Cell-Related Genes
The gingiva and oral mucosa share similarities to skin in histological structures and biological functions, specifically, oral defense and resistance to shear stress or friction (Stephens and Genever, 2007). However, the gingiva, in addition to its unique microenvironmental niche fueled by food residues, microbial flora, and saliva, has also been recognized for its sensitivity to inflammation, fibrosis response, and proneness to drug-induced overgrowth (Nakasone et al., 2009; Garlet, 2010). These biological properties suggest that MSCs derived from gingiva might possess some intrinsic properties distinct from those of oral mucosa-derived MSCs (Tang et al., 2011). Recent studies have shown that a population of clustered cells in the lamina propria layer of human gingiva displays positive signals for pluripotency-related markers, Oct-4, SSEA-4, and Stro-1 (Zhang et al., 2009; Tang et al., 2011), with some co-expressing Oct-4/SSEA-4 or Oct4/Stro-1 (Zhang et al., 2009). Additionally, the human oral mucosal/gingival lamina propria (OMLP) has been shown to harbor a population of cells positive for low-affinity neurotrophin (p75), a marker of neural stem cells, organized in cord-like structures that are also positively stained for Oct-4 and Sox2 (Marynka-Kalmani et al., 2010). These findings suggest that human oral mucosa and gingival tissues harbor progenitors or adult stem cells; however, the potential biological differences between these 2 related populations of oral MSCs remain to be determined.
Characterization of MSCs From Human Oral Mucosa and Gingiva
While progenitor cells isolated from the subepithelial layers of oral mucosa and gingival have been designated under different terms—i.e., gingiva-derived mesenchymal stem/stromal cells (GMSCs) (Zhang et al., 2009; Tang et al., 2011; Wang et al., 2011), gingival-tissue-derived stem cells (GT-MSCs) (Tomar et al., 2010), gingival multipotent progenitor cells (GMPCs) (Fournier et al., 2010), human oral mucosa stem cells (hOMSCs) (Marynka-Kalmani et al., 2010), and oral mucosa lamina propria progenitor cells (OMLP-PCs) (Davies et al., 2010)—they share similarities in MSC-associated properties.
Self-renewal
The self-renewal capabilities of human oral mucosa- and gingival propria-derived MSCs have been demonstrated by CFU-F assay (Zhang et al., 2009; Davies et al., 2010; Fournier et al., 2010; Marynka-Kalmani et al., 2010; Mitrano et al., 2010; Tomar et al., 2010; Tang et al., 2011; Wang et al., 2011). Importantly, human oral mucosa- and gingiva-derived MSCs invariably display a higher proliferation rate than do BMSCs (Zhang et al., 2009; Davies et al., 2010; Marynka-Kalmani et al., 2010; Tomar et al., 2010; Tang et al., 2011), which was likely attributed to the constitutive expression of human reverse telomerase transcriptase (hTERT) (Zhang et al., 2009; Davies et al., 2010). Moreover, the in vivo self-renewal capacity of gingiva-derived MSCs has been demonstrated by serial subcutaneous (s.c.) transplantation in immunocompromised mice (Zhang et al., 2009; Tang et al., 2011). These findings support that a population of MSCs with potent self-renewal and proliferative potentials can be readily isolated from human oral mucosa and gingival tissues and reliably expanded ex vivo for large-scale culture.
Multipotent Differentiation
Like BMSCs and ADSCs, human oral mucosa-/gingiva-derived MSCs can also differentiate into osteoblasts, adipocytes, and chondrocytes under specific in vitro differentiating conditions (Zhang et al., 2009; Davies et al., 2010; Fournier et al., 2010; Marynka-Kalmani et al., 2010; Mitrano et al., 2010; Tomar et al., 2010; Tang et al., 2011; Wang et al., 2011). In addition to these tri-lineage potentials, oral mucosa-/gingiva-derived MSCs are capable of differentiating into endodermal and ectodermal lineages, including various types of neural cells (Zhang et al., 2009; Davies et al., 2010; Marynka-Kalmani et al., 2010). As found in vivo, oral mucosa-/gingiva-derived MSCs embedded with carriers and subcutaneously transplanted into immunocompromised mice can generate connective tissue-like structures (Zhang et al., 2009; Tang et al., 2011), bone matrix (Fournier et al., 2010; Wang et al., 2011) and even 2 germ-layer-derived (teratoma-like) tissues (Marynka-Kalmani et al., 2010).
Expression of a Panel of MSC-associated Cell-surface Markers
Despite the lack of a specific cell-surface marker for adult MSCs of distinct tissue origins (Nombela-Arrieta et al., 2011), they invariably express a panel of mesenchymal cell markers such as CD73, CD90, CD105, and CD44 but are negative for endothelial and hematopoietic markers such as CD31, CD34, and CD45 (Dominici et al., 2006). Similarly, human oral mucosa- and gingiva-derived MSCs consistently express CD29, CD44, CD73, and CD90 (> 80%) and are negative for CD34 and CD45, but are positive for CD105, CD146, and Stro-1 in variable population subsets (Table).
Table.
Cell Surface Marker Profiles of Human Gingiva/Mucosa-derived MSCs
| CD29 (%) | CD44 (%) | CD73 (%) | CD90 (%) | CD105 (%) | CD106 (%) | CD13 (%) | CD146 (%) | CD166 (%) | SSEA-4 (%) | Stro-1 (%) | HLA-DR(%) | CD34 (%) | CD45 (%) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 99.8 | 99.9 | 100 | 29.9 | 7.1 | 36.9 | 18.3 | 0.1 | Zhang et al., 2009 | ||||||
| 78.74 | 95.25 | 98.03 | 98.32 | 97.16 | 3.37 | 3.21 | Tomar et al., 2010 | |||||||
| 100 | 100 | 100 | 100 | 100 | 3-17 | 35 | 0 | 0 | 0 | Fournier et al., 2010 | ||||
| 99.4 | 98.98 | 99.52 | 96.1 | 99.48 | 0.95 | 0.85 | Mitrano et al., 2010 | |||||||
| 99.98 | 92.87 | 34.75 | 17.89 | 0.01 | 0.41 | Wang et al., 2011 | ||||||||
| 82.4 | 90 | 76.4 | 92.5 | 93.3 | 75.6 | 0.3 | 0.5 | Tang et al., 2011 | ||||||
| 95.04 | 96.98 | 97.87 | 96.64 | 35.37 | 14.2 | 98.94 | 35.87 | 0 | 0 | 0 | Marynka-Kalmani et al., 2010 |
Collectively, these fundamental biological properties conferred by human oral mucosa-/gingiva-derived progenitor cells fit the minimal criteria for human MSCs as proposed by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (Dominici et al., 2006). Lately, several studies have suggested the potential neural crest origin of this unique population of MSCs (Zhang et al., 2009; Davies et al., 2010; Marynka-Kalmani et al., 2010); however, like other heterogeneous populations of tissue-resident MSCs, the in vivo identity and physiological functions of oral mucosa- and gingiva-derived MSCs remain largely unclear.
Immunomodulatory and Anti-inflammatory Properties of Human Oral Mucosa-/Gingiva-derived MSCs
While the self-renewal and multipotent differentiation capabilities of human oral mucosa-/gingiva propria-derived MSCs have been well-characterized, their immunomodulatory and anti-inflammatory functions remain unexplored relative to BMSCs and ADSCs. Most recently, our group has performed serial in vitro and in vivo studies to investigate the immunomodulatory effects of human gingiva-derived MSCs (GMSCs) and their interplay with various types of innate and adaptive immune cells, as well as their potential clinical application in the treatment of several inflammation-related disease models in mice.
Effects of GMSCs on T-cells
GMSCs exhibit potent suppressive effects on the proliferation and activation of human peripheral blood mononuclear cells (PBMC) stimulated either by phytohemagglutinin (PHA) (Zhang et al., 2009) or allogenic lymphocytes in mixed lymphocyte reactions (MLRs) (Mitrano et al., 2010; Tang et al., 2011). GMSCs suppress PHA-stimulated T-lymphocyte proliferation and activation in a cell-cell contact-independent manner, apparently mediated via IDO (Zhang et al., 2009); whereas the inflammatory cytokine IFN-γ secreted by activated T-lymphocytes in the co-culture system serves as a feedback signal in the cross-talk between GMSCs and T-cells (Zhang et al., 2009) (Fig. 1). Davies et al. have recently reported that oral mucosa lamina-propria-derived progenitor cells induced inhibitory effects on activated T-lymphocytes independent of cell-cell contact, cell dose, or apoptosis, while IFN-γ or co- culture with T-lymphocytes also led to the up-regulation of IDO expression (Davies et al., 2012). Similar immunomodulatory mechanisms mediated by elevated IDO have also been reported for other types of oral MSCs, particularly human periodontal ligament stem cells (Wada et al., 2009). Additionally, findings from both in vitro and in vivo studies have indicated that GMSCs could significantly inhibit Th17 cells and simultaneously promote the expansion of CD4+CD25+FoxP3+ regulatory T-cells (Tregs) (Zhang et al., 2009, 2010; Su et al., 2011; Tang et al., 2011). However, further studies are needed to elucidate the underlying mechanisms of interplay between gingiva-derived MSCs and specific types of T-helper cells.
Figure 1.
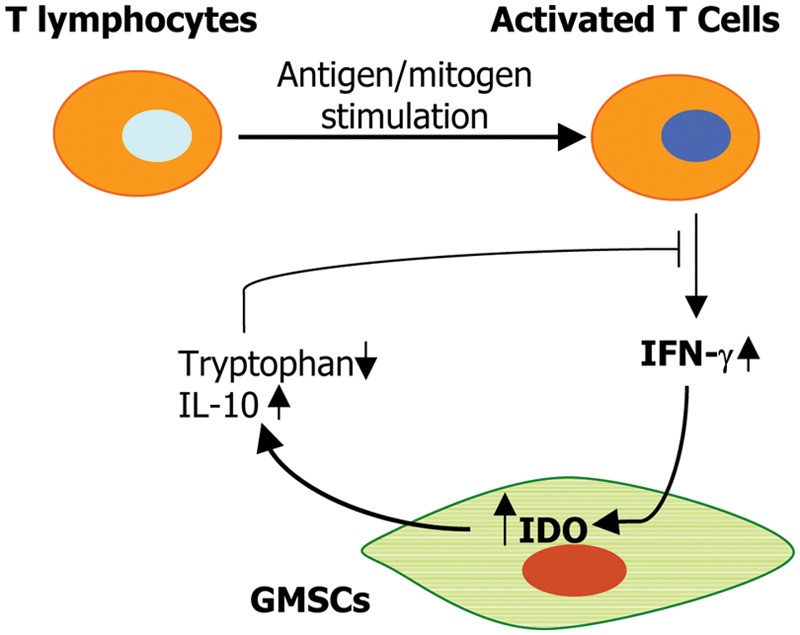
Potential interactions between activated T-lymphocytes and gingiva-derived MSCs. In response to antigen or mitogen stimulation, T-lymphocytes are activated and secrete the pro-inflammatory cytokine, interferon (IFN)-γ. Upon stimulation by IFN-γ, GMSCs express increased levels of IDO and IL-10, which subsequently dampen the pro-inflammatory function of activated T-cells. IDO, indoleamine 2, 3-dioxygenase.
Effects of GMSCs on Innate Immune Cells
The innate immune system is the first line of host defense, which consists of several types of innate immune cells (Galli et al., 2011). Similar to BMSCs (English and Mahon, 2011; Lee et al., 2011), GMSCs exhibit potent immunomodulatory effects on several types of innate immune cells, particularly dendritic cells (DCs), macrophages, and mast cells (Zhang et al., 2010; Su et al., 2011).
Dendritic Cells
Dendritic cells (DCs) can initiate and regulate effector T-cell activation and subsequently serve as major antigen-presenting cells that link the innate and adaptive immune responses (Galli et al., 2011). Previous studies have shown that MSCs possess profound capabilities to inhibit the maturation and activation of DCs under different settings (Spaggiari et al., 2009; Chiesa et al., 2011; Choi et al., 2012; Kapoor et al., 2012). Similarly, human GMSCs can significantly blunt the maturation and activation of DCs through the production of prostaglandin E2 (PGE2) (Su et al., 2011). This is in agreement with previous findings that MSC-derived PGE2 plays a central role in BMSC-mediated inhibition of monocyte-derived DC maturation and functions (Spaggiari et al., 2009).
Macrophages
Macrophages constitute another essential cellular component of innate immune responses (Galli et al., 2011), which are generally categorized into M1 and M2 macrophages. Usually, M1 macrophages display pro-inflammatory properties, while M2 macrophages are considered to be anti-inflammatory because of their increased production of anti-inflammatory cytokines such as IL-10 and TGF-β (Laskin et al., 2011). Recent evidence has suggested an essential role of MSCs in modulating the phenotype and function of macrophages (Kim and Hematti, 2009; Nemeth et al., 2009; Bartosh et al., 2010; Maggini et al., 2010; Nakajima et al., 2012). Mice BMSCs have been shown to re-polarize macrophages from a pro-inflammatory M1 phenotype to an anti-inflammatory M2 phenotype with enhanced interleukin-10 production (Nemeth et al., 2009), and co-culture with mouse BMSCs led to the conversion of activated macrophages to a regulatory-like profile (Maggini et al., 2010). In these studies, the secretion of PGE2 by MSCs was critical in the MSC-mediated phenotype conversion of macrophages (Nemeth et al., 2009; Maggini et al., 2010). Similarly, co-culture with human BMSCs triggers acquisition of M2 phenotype characterized by up-regulated expression of IL-10, increased phagocytic ability, and a decreased expression of pro-inflammatory cytokines (Kim and Hematti, 2009). Human BMSCs could also promote the alternative activation of infiltrated rat macrophage when they were locally transplanted at the injured spinal cord site (Nakajima et al., 2012). Additionally, MSC-mediated polarization of M2 macrophages displays increased phagocytic and antimicrobial activities (Kim and Hematti, 2009; Nemeth et al., 2009; Maggini et al., 2010; Zhang et al., 2010), which may contribute to the emerging role of MSCs in host defense against infectious challenges (Auletta et al., 2012), as evidenced in a mouse model for sepsis (Nemeth et al., 2009; Krasnodembskaya et al., 2012) and zymozan-induced peritonitis (Bartosh et al., 2010; Choi et al., 2011). Likewise, GMSCs were shown to be capable of polarizing macrophages into the M2 phenotype via enhanced secretion of IL-6 and GM-CSF (Zhang et al., 2010) (Fig. 2). Given the unique anatomic location of oral mucosa and gingival MSCs in the oral cavity, a complex ecosystem that contains a diverse assemblage of micro-organisms with different pathogenic potentials, it would be conceivable to further investigate whether GMSCs are capable of antimicrobial activity as compared with BMSCs.
Figure 2.
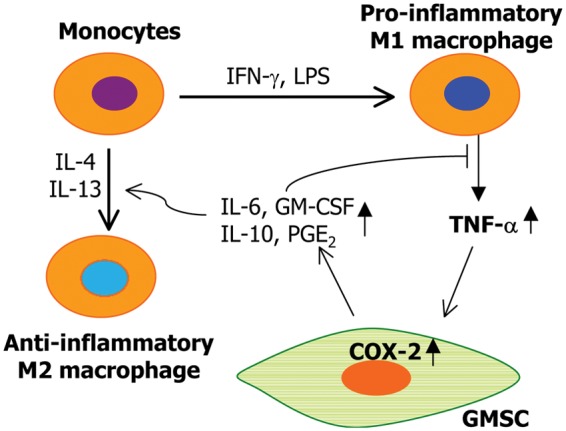
Potential interactions between macrophages and gingiva-derived MSCs. Activated by IFN-γ, TNF-α, or LPS, M1 macrophages produce TNF-α; which positively feeds back on MSCs to increase a variety of immunosuppressive or anti-inflammatory factors, some of which negatively regulate the M1 inflammatory responses. Other immunosuppressive factors produced by GMSCs promote the polarization of the M2 phenotype or the conversion of M1 to M2 macrophages. LPS, lipopolysaccharides; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2.
Mast Cells
Mast cells (MCs) are critical innate immune effector cells in allergic and inflammatory disorders (Sayed et al., 2008). To date, the immunomodulatory effect of MSCs on MCs is largely unknown. Most recently, it has been shown that mouse BMSCs and human GMSCs exhibit striking suppressive effects on specific functions of MCs in vitro and in vivo (Brown JM et al., 2011; Su et al., 2011). We found that human BMSCs and GMSCs suppressed de novo synthesis of the major pro-inflammtory cytokine, TNF-α, from activated human HMC-1 mast cells in a cell-cell contact-independent manner; however, it had no obvious inhibitory effects on their degranulation in vitro (Su et al., 2011). However, mouse BMSCs suppressed not only the production of pro-inflammatory cytokines by MCs, but also their degranulation, chemokinesis, and chemotaxis (Brown JM et al., 2011). Such discrepancies in MSC-mediated inhibitory effects on MCs may be due to the distinct cell contexts of both MSCs and MCs. However, in both studies, in vivo administration of BMSCs or GMSCs led to the suppression of MC degranulation in mouse skin and the peritoneal cavity (Brown JM et al., 2011; Su et al., 2011). The inhibitory effects of both human GMSCs and mouse BMSCs on MC functions were dependent on the COX2/PGE2 pathway (Brown JM et al., 2011; Su et al., 2011), and were facilitated through the activation of EP4 receptors in mouse MCs (Brown JM et al., 2011). These findings suggest that the TNF-α/COX2/PGE2 axis constitutes a negative feedback loop in the cross-talk between GMSCs and MCs (Su et al., 2011) (Fig. 3) and highlight the immunomodulatory functions of BMSCs and GMSCs on MCs and their potential application in cell-based therapy for MC-driven inflammatory diseases.
Figure 3.
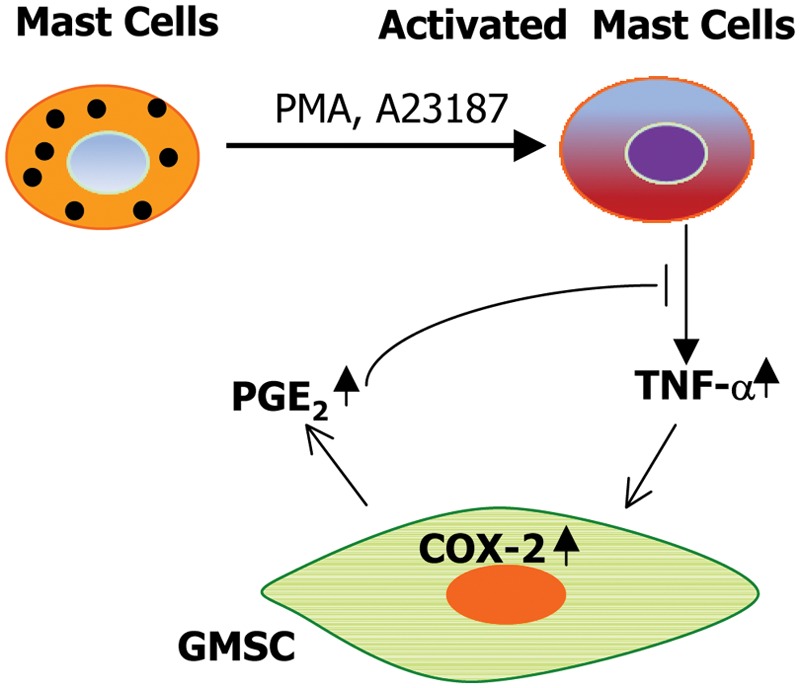
Potential interactions between activated mast cells and gingiva-derived MSCs. In response to PMA stimulation, activated mast cells synthesize and secrete the pro-inflammatory cytokine, TNF-α, which acts on GMSCs to induce increased levels of COX-2 and PGE-2. These factors negatively feed back and dampen the pro-inflammatory activity of activated mast cells. PMA, phorbol 12-myristate 13-acetate; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2.
Treatment of Animal Models of Wound Healing and Inflammatory Diseases With Human GMSCs
The compelling findings that human oral mucosa-/gingiva-derived MSCs possess potent immunomodulatory effects on several types of innate and adaptive immune cells prompted us to further explore their in vivo immunomodulatory functions and therapeutic effects in several inflammation-related disease models in mice.
Wound Healing
Wound healing is a complex process involving the participation of many types of immune and resident cells. Using a chemotherapy-induced oral mucositis (OM) mouse model, a compromised wound model in oral mucosa, we showed that systemic infusion of human GMSCs could mitigate the pathology of OM, as evidenced by reversal of body weight loss and restoration of the disrupted epithelial lining and proliferative basal cells (Zhang et al., 2011). In addition, Wang et al. found that local application of human GMSCs could significantly promote the repair of mandibular wounds and calvarial defects in rats (Wang et al., 2011). In a murine excisional full-thickness skin wound model, systemic infusion of human GMSCs significantly accelerated the repair process, as evidenced by rapid re-epithelialization and increased angiogenesis (Zhang et al., 2010). Compared with normal skin, increased numbers of infused MSCs were detected at the wound bed, where they were close to and interacted with resident macrophages, potentially contributing to their conversion to an anti-inflammatory M2 phenotype (Zhang et al., 2010). Meanwhile, systemic infusion of GMSCs significantly suppressed the local infiltration of inflammatory cells and pro-inflammatory cytokines such as TNF-α and IL-6, but simultaneously increased IL-10 (Zhang et al., 2010). These findings suggest that GMSCs enhance skin wound healing by promoting polarization of infiltrated monocytes or reprogramming resident macrophages into the M2 phenotype, thus preparing a special microenvironment for tissue repair and remodeling.
Dextran Sulfate Sodium (DSS)-induced Murine Colitis
The immunomodulatory and anti-inflammatory effects of GMSCs were also tested in a dextran sulfate sodium (DSS)-induced murine colitis model, in which Th1 and Th17 cells play an essential role (Brown JB et al., 2012). Systemic administration of GMSCs could reverse body weight loss, improve the overall colitis score, and restore normal intestinal architecture (Zhang et al., 2009). At the cellular level, GMSC treatment strikingly reduced the infiltration of CD4+IFNγ+ (Th1) and CD4+IL-17+ (Th17) cells at the colitic sites, and increased the recruitment of Tregs. At the molecular level, GMSCs remarkably suppressed pro-inflammatory cytokines such as IL-6, IL-17, and IFN-γ and increased IL-10 (Zhang et al., 2009). These findings suggest that GMSCs ameliorate inflammation-related tissue destruction caused by experimental acute colitis by suppressing the pro-inflammatory function of Th1 and Th17 cells and promoting the infiltration of Tregs.
Allergy-related Inflammatory Diseases
The pathological process of allergic contact dermatitis (ACD) or contact hypersensitivity (CHS) is comprised of multiple overlapping stages characterized by a dynamic and complex cellular network, including dendritic cells, CD8+ T-cells, CD4+IFNγ+ (Th1), CD4+IL-17+ (Th17), mast cells, and Tregs, as well as their cytokines (Vocanson et al., 2009; Fonacier et al., 2010). Using a hapten (oxazolone)-induced murine CHS model, we showed that both prophylactic and therapeutic administration of GMSCs could mitigate clinical signs of CHS (Su et al., 2011). Following GMSC treatment, we observed a reduced infiltration of dendritic cells (DCs), CD8+ T-cells, Th17, total and degranulated mast cells (MCs), a decreased level of a variety of inflammatory cytokines, and a reciprocal increased infiltration of Tregs and expression of IL-10 at regional lymph nodes and inflammatory areas. The underlying mechanism of GMSC-mediated attenuation of CHS involves the COX2/PGE2 axis (Su et al., 2011). These findings suggest that GMSCs suppress CHS through targeting multiple types of innate and adaptive immune cells (Su et al., 2011), and the use of MSCs in cell-based therapy potentially contributes a novel modality for the treatment of allergic diseases.
Mouse Skin Allograft Model
Aside from our in vivo studies on cutaneous wound healing and inflammatory diseases, Tang et al. have recently reported that systemic infusion of GMSCs exhibited remarkable immune tolerance and promoted the survival of skin allografts, whereby the increased infiltration of Tregs may play a major role (Tang et al., 2011). These immunosuppressant capabilities in the graft vs. host disease model further extend the clinical spectrum based on the unique immunomodulatory functions conferred by GMSCs.
Role of Human Oral Mucosa-/Gingiva-derived MSCs in Tissue Regeneration
Recently, accumulating evidence has challenged the previous paradigm that MSCs mediate tissue regeneration by virtue of their multipotent capabilities that enable them to replace damaged cells (Hermann et al., 2006; Kuroda et al., 2010). More studies have supported the new paradigm that MSCs promote tissue regeneration specifically through interaction with host/resident cells and production of a large array of trophic factors, capable of immunomodulatory and anti-inflammatory functions (Prockop, 2009; Roddy et al., 2011; Prockop and Oh, 2012). Despite the reported multipotent capabilities of oral mucosa- and gingiva-derived MSCs, both in vitro and in vivo (Zhang et al., 2009; Davies et al., 2010; Fournier et al., 2010; Marynka-Kalmani et al., 2010; Mitrano et al., 2010; Tomar et al., 2010; Tang et al., 2011; Wang et al., 2011), evidence supporting their direct role in tissue regeneration or replacement remains scanty. Using a chemotherapy-induced oral mucositis model, we demonstrated that only a very few GMSCs were found to ‘home’ to the injured sites and transdifferentiate into epithelial-like cells (Zhang et al., 2011). Mechanistically, the regenerative effects mediated by cultured GMSCs might be due to an increased expression of various chemokines and growth factors, as well as an increased resistance to oxidant stress-induced apoptosis (Zhang et al., 2011). In mouse models of skin wound and colitis, we showed that the mechanisms underlying GMSC-mediated acceleration of cutaneous and intestinal healing and regeneration may involve both pro-angiogenic and anti-inflammatory functions (Zhang et al., 2009, 2010). These findings further support that GMSCs, like other MSCs, may have promoted tissue regeneration via their trophic factors, not just their multipotent capabilities. Previous studies have implied that basal fibroblast growth factor (bFGF) can stimulate BMSCs to regenerate both bone and soft tissues, thus serving as an important growth factor for tissue regeneration (Sahoo et al., 2010; Tasso et al., 2012). However, its effect on GMSCs remains to be determined.
GMSCs vs. Gingival Fibroblasts
Fibroblasts are the most abundant stromal cells in the connective tissue proper. It appears that fibroblasts share several common features with MSCs, including a spindle-like cell morphology, plastic adherence, and overlapping cell-surface-marker profile (Haniffa et al., 2009). Some studies have reported that fibroblasts derived from different tissue origins can exhibit multilineage differentiation potentials (Lysy et al., 2007; Sudo et al., 2007; Lorenz et al., 2008; Bouffi et al., 2011) and immunomodulatory functions (Haniffa et al., 2007; Cappellesso-Fleury et al., 2010; Bouffi et al., 2011; Pinchuk et al., 2011; Wada et al., 2011). Recent studies indicated that a population of MSC-like cells enriched from gingiva-derived fibroblasts grown on chitosan membranes expressed increased Stro-1, Oct4, Nanog, and Sox-10 and enhanced chondrogenic differentiation (Hsu et al., 2012a,b). Mostafa et al. have reported that human gingival fibroblasts (HGFs) can be induced to differentiate into osteocytes in vitro (Mostafa et al., 2011). In addition, heterotopic gingival fibroblasts have been used as transplanted cells to facilitate tracheal epithelial regeneration (Kobayashi et al., 2007, 2010), periodontal tissue regeneration (Nakajima et al., 2008), and skin wound healing (Nishi et al., 2010). Moreover, HGFs display immunosuppressive effects on T-lymphocytes similar to those of periodontal ligament stem cells (Wada et al., 2009). These findings suggest that HGFs share similar properties with GMSCs. However, because of the lack of a specific marker and the unknown in vivo identity of MSCs, the exact relationship between MSCs and fibroblasts remains elusive. There has been some evidence that fibroblasts may represent a more differentiated subpopulation of MSCs, or, under certain conditions, may in fact be derived from MSCs (Haniffa et al., 2009; Aghajanova et al., 2010; Lee et al., 2010). Since the frequency of MSCs is very low in vivo as compared with the relative abundance of fibroblasts, further elucidation of the exact identity or relationship between these 2 populations of stromal cells would lead to the identification of an alternative source of stromal cells for cell-based tissue regeneration and therapy of immune- and inflammation-related diseases.
Concluding Remarks
The potent immunomodulatory and anti-inflammatory properties of human oral mucosa-/gingiva-derived MSCs position them as a promising cell source for MSC-based therapies for wound repair and a wide range of inflammation-related diseases. Further research on this unique population of MSCs will undoubtedly contribute to a deeper understanding of the mechanisms underlying their immunomodulatory and tissue-regenerative functions under different pathophysiological settings. Some topics to be addressed include: (1) What is the real identity or developmental origin of this population of cells? Are they identical to or different from MSCs isolated from other post-natal tissues? (2) Do GMSCs and gingival fibroblasts belong to the same hierarchical lineage of stromal cell? (3) Do these oral mucosa-/ gingiva-derived MSCs with unique trophic properties exhibit distinct secretomes in response to specific stimuli? (4) Because of their specific anatomic location in the oral cavity, do these MSCs differ from BMSCs in terms of host defense immune response? Do these MSC-induced immunomodulatory effects contribute to the complexity of the oral mucosal immune network in mucosal wounds? Answers to these questions will substantially enhance our understanding of the biological properties of oral mucosa-/gingiva-derived MSCs and their important roles in tissue regeneration and cell-based therapy of immune- and/or inflammation-related diseases.
Footnotes
This work was supported by a National Institutes of Health Research Grant (R01DE 019932) and an Oral and Maxillofacial Surgery Foundation (OMSF) Research Grant.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aghajanova L, Horcajadas JA, Esteban FJ, Giudice LC. (2010). The bone marrow-derived human mesenchymal stem cell: potential progenitor of the endometrial stromal fibroblast. Biol Reprod 82:1076-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auletta JJ, Deans RJ, Bartholomew AM. (2012). Emerging roles for multipotent, bone marrow-derived stromal cells in host defense. Blood 119:1801-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosh TJ, Ylöstalo JH, Mohammadipoor A, et al. (2010). Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci USA 107:13724-13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouffi C, Bony C, Jorgensen C, Noël D. (2011). Skin fibroblasts are potent suppressors of inflammation in experimental arthritis. Ann Rheum Dis 70:1671-1676. [DOI] [PubMed] [Google Scholar]
- Brown JB, Cheresh P, Zhang Z, Ryu H, Managlia E, Barrett TA. (2012). P-selectin glycoprotein ligand-1 is needed for sequential recruitment of T-helper 1 (Th1) and local generation of Th17 T cells in dextran sodium sulfate (DSS) colitis. Inflamm Bowel Dis 18:323-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Nemeth K, Kushnir-Sukhov NM, Metcalfe DD, Mezey E. (2011). Bone marrow stromal cells inhibit mast cell function via a COX2-dependent mechanism. Clin Exp Allergy 41:526-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellesso-Fleury S, Puissant-Lubrano B, Apoil PA, Titeux M, Winterton P, Casteilla L, et al. (2010). Human fibroblasts share immunosuppressive properties with bone marrow mesenchymal stem cells. J Clin Immunol 30:607-619. [DOI] [PubMed] [Google Scholar]
- Chiesa S, Morbelli S, Morando S, Massollo M, Marini C, Bertoni A, et al. (2011). Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc Natl Acad Sci USA 108:17384-17389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. (2011). Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood 118:330-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Jeong JA, Lim DS. (2012). Mesenchymal stem cell-mediated immature dendritic cells induce regulatory T cell-based immunosuppressive effect. Immunol Invest 41:214-229. [DOI] [PubMed] [Google Scholar]
- Davies LC, Locke M, Webb RD, Roberts JT, Langley M, Thomas DW, et al. (2010). A multipotent neural crest-derived progenitor cell population is resident within the oral mucosa lamina propria. Stem Cells Dev 19:819-830. [DOI] [PubMed] [Google Scholar]
- Davies LC, Lonnies H, Locke M, Sundberg B, Rosendahl K, Gotherstrom C, et al. (2012). Oral mucosal progenitor cells are potently immunosuppressive in a dose-independent manner. Stem Cells Dev 21:1478-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315-317. [DOI] [PubMed] [Google Scholar]
- English K, Mahon BP. (2011). Allogeneic mesenchymal stem cells: agents of immune modulation. J Cell Biochem 112:1963-1968. [DOI] [PubMed] [Google Scholar]
- Fonacier LS, Dreskin SC, Leung DY. (2010). Allergic skin diseases. J Allergy Clin Immunol 125(2 Suppl 2):S138-S149. [DOI] [PubMed] [Google Scholar]
- Fournier BP, Ferre FC, Couty L, Lataillade JJ, Gourven M, Naveau A, et al. (2010). Multipotent progenitor cells in gingival connective tissue. Tissue Eng Part A 16:2891-2899. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Borregaard N, Wynn TA. (2011). Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 12:1035-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlet GP. (2010). Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res 89:1349-1363. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 97:13625-13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, et al. (2007). Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol 179:1595-1604. [DOI] [PubMed] [Google Scholar]
- Haniffa MA, Collin MP, Buckley CD, Dazzi F. (2009). Mesenchymal stem cells: the fibroblasts' new clothes? Haematologica 94:258-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A, Liebau S, Gastl R, Fickert S, Habisch HJ, Fiedler J, et al. (2006). Comparative analysis of neuroectodermal differentiation capacity of human bone marrow stromal cells using various conversion protocols. J Neurosci Res 83:1502-1514. [DOI] [PubMed] [Google Scholar]
- Hsu SH, Huang GS, Feng F. (2012a). Isolation of the multipotent MSC subpopulation from human gingival fibroblasts by culturing on chitosan membranes. Biomaterials 33:2642-2655 [DOI] [PubMed] [Google Scholar]
- Hsu SH, Huang GS, Lin SY, Feng F, Ho TT, Liao YC. (2012b). Enhanced chondrogenic differentiation potential of human gingival fibroblasts by spheroid formation on chitosan membranes. Tissue Eng Part A 18:67-79. [DOI] [PubMed] [Google Scholar]
- Kapoor S, Patel SA, Kartan S, Axelrod D, Capitle E, Rameshwar P. (2012). Tolerance-like mediated suppression by mesenchymal stem cells in patients with dust mite allergy-induced asthma. J Allergy Clin Immunol 129:1094-1101. [DOI] [PubMed] [Google Scholar]
- Kim J, Hematti P. (2009). Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol 37:1445-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Suzuki T, Nomoto Y, Tada Y, Miyake M, Hazama A, et al. (2007). Potential of heterotopic fibroblasts as autologous transplanted cells for tracheal epithelial regeneration. Tissue Eng 13:2175-2184. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Suzuki T, Nomoto Y, Tada Y, Miyake M, Hazama A, et al. (2010). A tissue-engineered trachea derived from a framed collagen scaffold, gingival fibroblasts and adipose-derived stem cells. Biomaterials 31:4855-4863. [DOI] [PubMed] [Google Scholar]
- Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, et al. (2012). Human mesenchymal stem cells reduce mortality and bacteremia in Gram negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol 302:L1003-L1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, et al. (2010). Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci USA 107:8639-8643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin DL, Sunil VR, Gardner CR, Laskin JD. (2011). Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol 51:267-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Shah B, Moioli EK, Mao JJ. (2010). CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest 120:3340-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Oh JY, Choi H, Bazhanov N. (2011). Therapeutic factors secreted by mesenchymal stromal cells and tissue repair. J Cell Biochem 112:3073-3078. [DOI] [PubMed] [Google Scholar]
- Lorenz K, Sicker M, Schmelzer E, et al. (2008). Multilineage differentiation potential of human dermal skin-derived fibroblasts. Exp Dermatol 17:925-932. [DOI] [PubMed] [Google Scholar]
- Luria EA, Panasyuk AF, Friedenstein AY. (1971). Fibroblast colony formation from monolayer cultures of blood cells. Transfusion 11:345-349. [DOI] [PubMed] [Google Scholar]
- Lysy PA, Smets F, Sibille C, Najimi M, Sokal EM. (2007). Human skin fibroblasts: from mesodermal to hepatocyte-like differentiation. Hepatology 46:1574-1585. [DOI] [PubMed] [Google Scholar]
- Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzon IM, Nepomnaschy I, et al. (2010). Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One 5:e9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marynka-Kalmani K, Treves S, Yafee M, Rachima H, Gafni Y, Cohen MA, et al. (2010). The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cells 28:984-995. [DOI] [PubMed] [Google Scholar]
- Mitrano TI, Grob MS, Carrion F, Nova-Lamperti E, Luz PA, Fierro FS, et al. (2010). Culture and characterization of mesenchymal stem cells from human gingival tissue. J Periodontol 81:917-925. [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. (2003). SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA 100:5807-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa NZ, Uludağ H, Varkey M, Dederich DN, Doschak MR, El-Bialy TH. (2011). In vitro osteogenic induction of human gingival fibroblasts for bone regeneration. Open Dent J 5:139-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, et al. (2012). Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma 29:1614-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Abe T, Tanaka M, Hara Y. (2008). Periodontal tissue engineering by transplantation of multilayered sheets of phenotypically modified gingival fibroblasts. J Periodontal Res 43:681-688. [DOI] [PubMed] [Google Scholar]
- Nakasone N, Kubota T, Hoshino C, Nohno K, Itagaki M, Shimizu T, et al. (2009). Differential gene and protein expression of tissue inhibitors of metalloproteinases (TIMP)-3 and TIMP-4 in gingival tissues from drug induced gingival overgrowth. Arch Oral Biol 54:634-641. [DOI] [PubMed] [Google Scholar]
- Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. (2009). Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15:42-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi H, Ohta K, Takechi M, Yoneda S, Hiraoka M, Kamata N. (2010). Wound healing effects of gingival fibroblasts cultured in animal-free medium. Oral Dis 16:438-444. [DOI] [PubMed] [Google Scholar]
- Nombela-Arrieta C, Ritz J, Silberstein LE. (2011). The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol 12:126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchuk IV, Beswick EJ, Saada JI, Boya G, Schmitt D, Raju GS, et al. (2011). Human colonic myofibroblasts promote expansion of CD4(+) CD25(high) Foxp3(+) regulatory T cells. Gastroenterology 140:2019-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ. (2009). Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther 17:939-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ, Oh JY. (2012). Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther 20:14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, et al. (2011). Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells 29:1572-1579. [DOI] [PubMed] [Google Scholar]
- Sahoo S, Toh SL, Goh JC. (2010). A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials 31:2990-2998. [DOI] [PubMed] [Google Scholar]
- Salem HK, Thiemermann C. (2010). Mesenchymal stromal cells: current understanding and clinical status. Stem Cells 28:585-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed BA, Christy A, Quirion MR, Brown MA. (2008). The master switch: the role of mast cells in autoimmunity and tolerance. Annu Rev Immunol 26:705-739. [DOI] [PubMed] [Google Scholar]
- Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. (2009). MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood 113:6576-6583. [DOI] [PubMed] [Google Scholar]
- Stephens P, Genever P. (2007). Non-epithelial oral mucosal progenitor cell populations. Oral Dis 13:1-10. [DOI] [PubMed] [Google Scholar]
- Su WR, Zhang QZ, Shi SH, Nguyen AL, Le AD. (2011). Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E(2)-dependent mechanisms. Stem Cells 29:1849-1860. [DOI] [PubMed] [Google Scholar]
- Sudo K, Kanno M, Miharada K, Ogawa S, Hiroyama T, Saijo K, et al. (2007). Mesenchymal progenitors able to differentiate into osteogenic, chondrogenic, and/or adipogenic cells in vitro are present in most primary fibroblast-like cell populations. Stem Cells 25:1610-1617. [DOI] [PubMed] [Google Scholar]
- Tang L, Li N, Xie H, Jin Y. (2011). Characterization of mesenchymal stem cells from human normal and hyperplastic gingiva. J Cell Physiol 226:832-842. [DOI] [PubMed] [Google Scholar]
- Tasso R, Gaetani M, Molino E, et al. (2012). The role of bFGF on the ability of MSC to activate endogenous regenerative mechanisms in an ectopic bone formation model. Biomaterials 33:2086-2096. [DOI] [PubMed] [Google Scholar]
- Tomar GB, Srivastava RK, Gupta N, Barhanpurkar AP, Pote ST, Jhaveri HM, et al. (2010). Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem Biophys Res Commun 393:377-383. [DOI] [PubMed] [Google Scholar]
- Vocanson M, Hennino A, Rozieres A, Poyet G, Nicolas JF. (2009). Effector and regulatory mechanisms in allergic contact dermatitis. Allergy 64:1699-1714. [DOI] [PubMed] [Google Scholar]
- Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S. (2009). Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol 219:667-676. [DOI] [PubMed] [Google Scholar]
- Wada N, Bartold PM, Gronthos S. (2011). Human foreskin fibroblasts exert immunomodulatory properties by a different mechanism to bone marrow stromal/stem cells. Stem Cells Dev 20:647-659. [DOI] [PubMed] [Google Scholar]
- Wang F, Yu M, Yan X, Wen Y, Zeng Q, Yue W, et al. (2011). Gingiva-derived mesenchymal stem cell-mediated therapeutic approach for bone tissue regeneration. Stem Cells Dev 20:2093-2102. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, et al. (2009). Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol 183:7787-7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, et al. (2010). Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells 28:1856-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Nguyen AL, Shi S, Hill C, Wilder-Smith P, Krasieva TB, et al. (2011). Three-dimensional spheroid culture of human gingiva-derived mesenchymal stem cells enhances mitigation of chemotherapy-induced oral mucositis. Stem Cells Dev 21:937-947. [DOI] [PMC free article] [PubMed] [Google Scholar]


