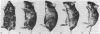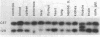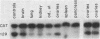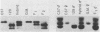Abstract
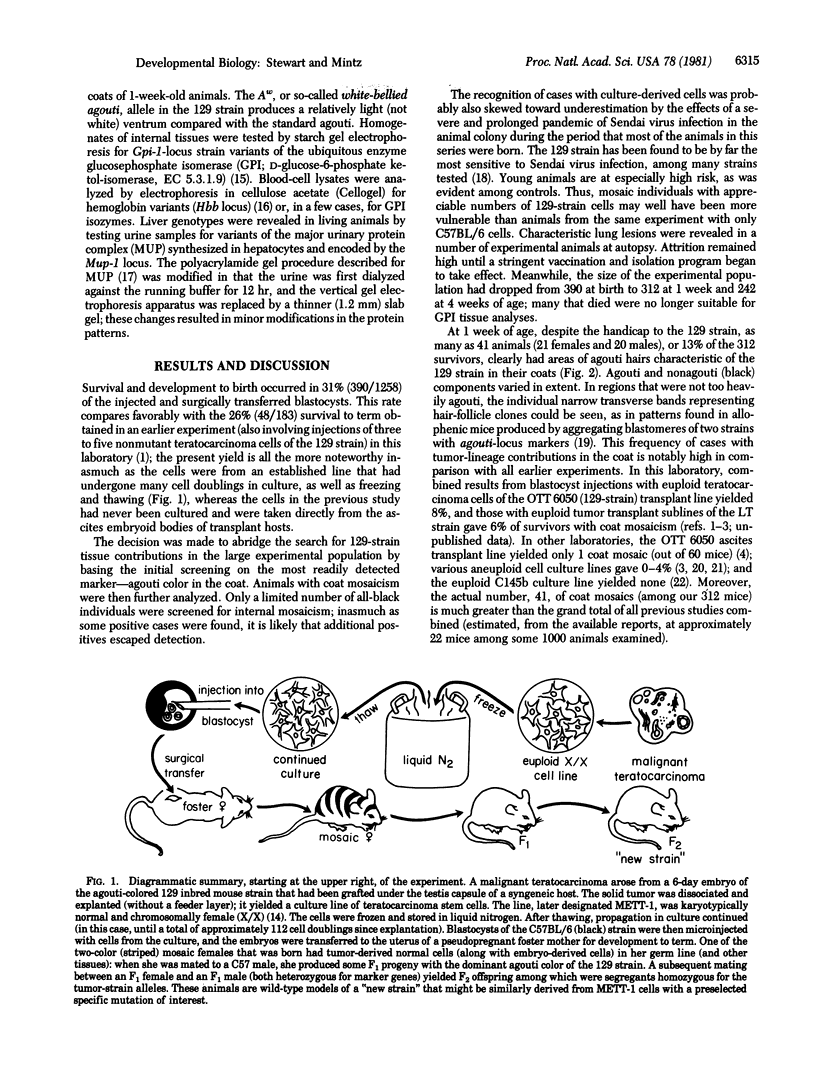
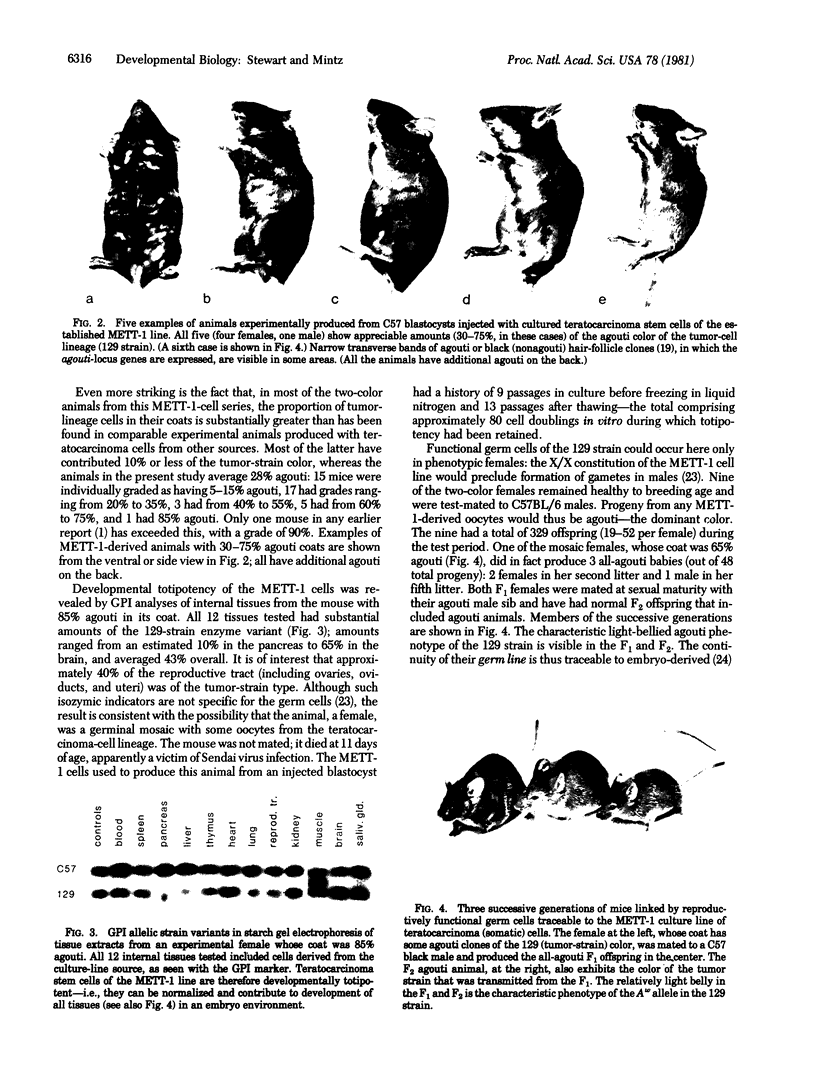
The possibility of utilizing mouse teratocarcinoma stem cells as intermediaries for production of new strains of mice with preselected mutant or foreign genes requires that, after propagation in culture (to allow for genetic manipulation and selection), the cells be capable of normalization and orderly development in carrier embryos and, ultimately, of germ-cell formation. Heretofore, no in vitro cell line has fulfilled all these requirements. A karyotypically normal teratocarcinoma culture line was recently established in this laboratory and now has been investigated as a candidate. The line, designated METT-1, is chromosomally female (X/X) and was obtained from the 129 (agouti-colored) inbred strain [Mintz, B. & Cronmiller, C. (1981) Somat Cell Genet 7, 489-505]. The developmental potential of these cells was tested, after prolonged culture and freezing and thawing, by microinjecting them into early (blastocyst stage) embryos of the C57BL/6 (black) strain. Among 312 experimental animals examined at 1 week of age, there were 41 mice (21 females and 20 males) that displayed the coat colors of both strains. This frequency (13%), as well as the extent of the coat areas derived from the cell line, greatly surpasses the contributions observed in all previous experiments, whether with other in vitro teratocarcinoma cell lines or with in vivo transplant lines. The developmental totipotency of METT-1 cells became evident from the presence of substantial amounts of 129-strain cells (bearing an isozyme marker) in all internal tissues of an individual whose coat was largely agouti. The culture-cell lineage also proved to be capable of giving rise to reproductively functional oocytes. Of nine mosaic-coat females testmated to C57BL/6 males, one produced progeny of the diagnostic agouti color in two litters; these heterozygous F1 offspring in turn transmitted their marker genes to F2 homozygous segregants. Thus, the METT-1 teratocarcinoma line bridges the gap between in vitro cell propagation and in vivo development and between the soma and the germ line. This creates the option of producing new mouse strains with predetermined genetic changes designed as probes of developmental regulation or as models of human genetic diseases.
Keywords: microinjection into blastocysts, developmental totipotency, germ-line transmission
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinster R. L. The effect of cells transferred into the mouse blastocyst on subsequent development. J Exp Med. 1974 Oct 1;140(4):1049–1056. doi: 10.1084/jem.140.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronmiller C., Mintz B. Karyotypic normalcy and quasi-normalcy of developmentally totipotent mouse teratocarcinoma cells. Dev Biol. 1978 Dec;67(2):465–477. doi: 10.1016/0012-1606(78)90212-9. [DOI] [PubMed] [Google Scholar]
- Dewey M. J., Martin D. W., Jr, Martin G. R., Mintz B. Mosaic mice with teratocarcinoma-derived mutant cells deficient in hypoxanthine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5564–5568. doi: 10.1073/pnas.74.12.5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous M., Günther E., Kemler R., Wiels J., Berger R., Guenet J. L., Jakob H., Jacob F. Association of the H-Y male antigen with beta2-microglobulin on human lymphoid and differentiated mouse teratocarcinoma cell lines. J Exp Med. 1978 Jul 1;148(1):58–70. doi: 10.1084/jem.148.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart J. D., Mintz B. Clonal origins of somites and their muscle derivatives: evidence from allophenic mice. Dev Biol. 1972 Sep;29(1):27–37. doi: 10.1016/0012-1606(72)90040-1. [DOI] [PubMed] [Google Scholar]
- Illmensee K., Mintz B. Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc Natl Acad Sci U S A. 1976 Feb;73(2):549–553. doi: 10.1073/pnas.73.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney M. W., Strutt B. J. Genetic activity of X chromosomes in pluripotent female teratocarcinoma cells and their differentiated progeny. Cell. 1980 Sep;21(2):357–364. doi: 10.1016/0092-8674(80)90472-9. [DOI] [PubMed] [Google Scholar]
- Mintz B., Cronmiller C., Custer R. P. Somatic cell origin of teratocarcinomas. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2834–2838. doi: 10.1073/pnas.75.6.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B., Cronmiller C. METT-1: a karyotypically normal in vitro line of developmentally totipotent mouse teratocarcinoma cells. Somatic Cell Genet. 1981 Jul;7(4):489–505. doi: 10.1007/BF01542992. [DOI] [PubMed] [Google Scholar]
- Mintz B. Gene expression in neoplasia and differentiation. Harvey Lect. 1978;71:193–246. [PubMed] [Google Scholar]
- Mintz B., Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B. Teratocarcinoma cells as vehicles for introducing mutant genes into mice. Differentiation. 1979;13(1):25–27. doi: 10.1111/j.1432-0436.1979.tb01610.x. [DOI] [PubMed] [Google Scholar]
- Mintz B. Teratocarcinoma cells as vehicles for mutant and foreign genes. Brookhaven Symp Biol. 1977 May 12;(29):82–95. [PubMed] [Google Scholar]
- Papaioannou V. E., Evans E. P., Gardner R. L., Graham C. F. Growth and differentiation of an embryonal carcinoma cell line (C145b). J Embryol Exp Morphol. 1979 Dec;54:277–295. [PubMed] [Google Scholar]
- Papaioannou V. E., Gardner R. L., McBurney M. W., Babinet C., Evans M. J. Participation of cultured teratocarcinoma cells in mouse embryogenesis. J Embryol Exp Morphol. 1978 Apr;44:93–104. [PubMed] [Google Scholar]
- Papaioannou V. E., McBurney M. W., Gardner R. L., Evans M. J. Fate of teratocarcinoma cells injected into early mouse embryos. Nature. 1975 Nov 6;258(5530):70–73. doi: 10.1038/258070a0. [DOI] [PubMed] [Google Scholar]
- Parker J. C., Whiteman M. D., Richter C. B. Susceptibility of inbred and outbred mouse strains to Sendai virus and prevalence of infection in laboratory rodents. Infect Immun. 1978 Jan;19(1):123–130. doi: 10.1128/iai.19.1.123-130.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicer A., Wagner E. F., el-Kareh A., Dewey M. J., Reuser A. J., Silverstein S., Axel R., Mintz B. Introduction of a viral thymidine kinase gene and the human beta-globin gene into developmentally multipotential mouse teratocarcinoma cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2098–2102. doi: 10.1073/pnas.77.4.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuser A. J., Mintz B. Mouse teratocarcinoma mutant clones deficient in adenine phosphoribosyltransferase and developmentally pluripotent. Somatic Cell Genet. 1979 Nov;5(6):781–792. doi: 10.1007/BF01542641. [DOI] [PubMed] [Google Scholar]
- Stevens L. C. The development of transplantable teratocarcinomas from intratesticular grafts of pre- and postimplantation mouse embryos. Dev Biol. 1970 Mar;21(3):364–382. doi: 10.1016/0012-1606(70)90130-2. [DOI] [PubMed] [Google Scholar]
- Wagner E. F., Stewart T. A., Mintz B. The human beta-globin gene and a functional viral thymidine kinase gene in developing mice. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5016–5020. doi: 10.1073/pnas.78.8.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Dewey M. J., Mintz B. Teratocarcinoma cells as vehicles for introducing specific mutant mitochondrial genes into mice. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5113–5117. doi: 10.1073/pnas.75.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney J. B., 3rd Simplified typing of mouse hemoglobin (Hbb) phenotypes using cystamine. Biochem Genet. 1978 Aug;16(7-8):667–672. doi: 10.1007/BF00484723. [DOI] [PubMed] [Google Scholar]
- Wilcox F. H. Simplified procedure for electrophoresis of the major urinary protein of Mus musculus. Biochem Genet. 1975 Apr;13(3-4):243–245. doi: 10.1007/BF00486018. [DOI] [PubMed] [Google Scholar]