Abstract
In organs, the correct architecture of vascular and ductal structures is indispensable for proper physiological function, and the formation and maintenance of these structures is a highly regulated process. The analysis of these complex, 3-dimensional structures has greatly depended on either 2-dimensional examination in section or on dye injection studies. These techniques, however, are not able to provide a complete and quantifiable representation of the ductal or vascular structures they are intended to elucidate. Alternatively, the nature of 3-dimensional plastic resin casts generates a permanent snapshot of the system and is a novel and widely useful technique for visualizing and quantifying 3-dimensional structures and networks.
A crucial advantage of the resin casting system is the ability to determine the intact and connected, or communicating, structure of a blood vessel or duct. The structure of vascular and ductal networks are crucial for organ function, and this technique has the potential to aid study of vascular and ductal networks in several ways. Resin casting may be used to analyze normal morphology and functional architecture of a luminal structure, identify developmental morphogenetic changes, and uncover morphological differences in tissue architecture between normal and disease states. Previous work has utilized resin casting to study, for example, architectural and functional defects within the mouse intrahepatic bile duct system that were not reflected in 2-dimensional analysis of the structure1,2, alterations in brain vasculature of a Alzheimer's disease mouse model3, portal vein abnormalities in portal hypertensive and cirrhotic mice4, developmental steps in rat lymphatic maturation between immature and adult lungs5, immediate microvascular changes in the rat liver, pancreas, and kidney in response in to chemical injury6.
Here we present a method of generating a 3-dimensional resin cast of a mouse vascular or ductal network, focusing specifically on the portal vein and intrahepatic bile duct. These casts can be visualized by clearing or macerating the tissue and can then be analyzed. This technique can be applied to virtually any vascular or ductal system and would be directly applicable to any study inquiring into the development, function, maintenance, or injury of a 3-dimensional ductal or vascular structure.
Keywords: Medicine, Issue 68, Resin cast, 3-dimensional, portal vein, intrahepatic bile duct, vascular, ductal
Protocol
1. Prepare Cannula
Warm a 1-inch long section of PE10 tubing with your fingertips and stretch it so that the tubing becomes thin. Note: the size of the vessel or duct to be cannulated will determine the degree of stretching required. The cannula must be well-stretched for bile duct casts but may only be required to be moderately stretched to fit within the larger portal vein.
Cut the stretched tubing at a diagonal to generate a beveled tip.
Cut the other edge of the tubing to create a 5-6 inch segment.
Insert a 32 gauge, ½ inch hypodermic needle into the non-beveled edge of the tubing.
2. Prepare Syringe with PBS
Fill a 3 ml syringe with PBS.
Screw the syringe onto the hypodermic needle with attached pulled PE10 tubing previously prepared.
Flick syringe and push syringe plunger to ensure that PBS has filled the needle and tubing and there are no air bubbles in the syringe.
Place the prepared syringe with PBS into a syringe-holder shaped out of modeling clay. This holder will keep the syringe steady while the tubing is inserted into the duct.
3. Other Set-up
Weigh 0.1 g of catalyst into a small container, e.g., one well of a 24-well plate.
Pull 1 ml resin into a 3 ml syringe. Note: relative amounts of catalyst and resin may be adjusted to alter curing time. Decreasing amount of catalyst may, however, increase cast shrinkage and lead to a less desirable cast. Note: when handling resin, take precautions to avoid contact with resin or inhalation of the resin fumes. Always wear gloves and perform steps involving resin in a well-ventilated area, such as a chemical fume hood
Cut a piece of 5.0 silk suture of approximately 5 inches to be used as a ligature.
4. Prepare Mouse
Sacrifice adult mouse. Lay mouse on its back and open up the abdominal cavity.
Lay mouse on dissecting scope platform. Position a 15 ml conical tube perpendicularly underneath the mouse to increase visibility and accessibility to liver.
Wet a cotton swab with PBS and use it to flip the liver upwards and away from you to expose the dorsal view of the liver along with the extrahepatic portal vein or common bile duct.
For portal vein casts, you can prevent blood clotting by flushing the vein with room temperature PBS. Attach a needle to a 3 ml syringe filled with PBS and insert needle into extrahepatic portal vein as far from the liver as you can. Gently push PBS through into the portal vein. Make a nick in the common cardinal vein to drain blood. Use a wet cotton swab to massage liver, enhancing blood drainage. This step is not recommended for the bile duct and may not be necessary for all vascular systems. Note: this step can also be performed with 4% paraformaldehyde instead of PBS. Use of 4% paraformaldehyde may increase vascular integrity and result in a more precise cast, specifically of smaller vascular structures.
5. Cannulate Extrahepatic Portal vein or Common Bile Duct
Use curved forceps to pass the surgical suture ligature underneath the portal vein or bile duct, approximately ¼ inch from the liver.
Tie the suture into a loose common knot. DO NOT tighten the knot.
If you are working with an open-ended system, it may be helpful to tie and tighten a ligature at the other end of the system. If you have opened the common cardinal vein to drain the blood from the portal vein, tie a ligature around it to increase pressure within the portal vein and prevent resin leakage into the central hepatic vein.
Use spring scissors to make a small cut in the portal vein or bile duct, approximately ¼ inch below the suture knot. This cut should penetrate no more than halfway through the diameter of the portal vein or bile duct.
Using #5 forceps, hold the portal vein or bile duct at the site of the cut and insert beveled end of the tubing into the bile duct.
Push tip of tubing past the pre-tied ligature and towards the liver.
Test for cannulation accuracy by injecting PBS through the cannula and watching to see if it enters the portal vein or bile duct.
Tighten the ligature to hold the cannula in place.
6. Resin Cast the Portal Vein or Intrahepatic Bile Duct
Add pre-measured 1 ml resin to pre-measured 0.1 g catalyst and mix thoroughly. Avoid generating air bubbles.
Draw resin-catalyst mixture back into the 3 ml syringe. Remove any air bubbles by gently flicking the syringe and expelling bubbles. Note: for casting small structures, it may be helpful to use a vacuum chamber to remove very small air bubbles from the resin and enhance cast quality. To allow for time to perform this step, it will be necessary to decrease the catalyst/resin ratio from that suggested above in order to slow resin curing.
Carefully replace PBS-containing syringe with resin-containing syringe, leaving the 32 gauge needle attached to the cannula.
Slowly and gently push resin into the portal vein or bile duct until resistance increases and liver is filled.
Use wet cotton swab to gently massage liver and encourage an even fill.
Remove cannula from the structure and quickly re-tighten the ligature to prevent resin leakage.
Allow mouse to lay flat at room temperature for 20 min while the resin cures, then remove liver.
Proceed with either clearing or macerating the liver.
7. Clear Liver for Visualization in situ
Fix the liver in 4% paraformaldehyde overnight at 4 °C, rocking.
Wash the liver in PBS for at least four hr, rocking. At this point, you may separate the liver lobes if desired.
Dehydrate in 50% methanol and then in 100% methanol for at least four hr each, rocking at room temperature.
Clear the liver in a 1:2 solution of benzyl alcohol and benzyl benzoate (BABB) for at least 24 hr, rocking. Longer wash times may be needed. If the liver does not clear completely after 2-3 days, move back to 100% methanol for 24 hr and repeat BABB clearing.
Submerge liver in BABB in a glass Petri dish for imaging in situ.
8. Macerate Liver Tissue
Remove liver from mouse and put into a 50 ml conical tube with water. Wait 1 hr for complete resin curing. Separate liver lobes if desired.
Pour off water and move liver to a glass bottle. Submerge in 15% KOH. Leave overnight at room temperature. Do not rock.
Carefully pour off KOH and wash cast in distilled water. Note: the cast is very fragile and should be handled with extreme care. Avoid disrupting the cast when adding or removing fluid from the tube.
When resin cast is clean, gently lay on a Kimwipe to dry, then transfer to a container to be stored.
9. Representative Results
A successful cast of the portal vein or bile duct will show a continuous network of progressively smaller branches extending from the hilum to the liver periphery.
Figure 1 shows a portal vein cast as visualized in situ after BABB clearing. Figure 1A shows the cast of the entire left liver lobe and the shape and size of the cast. Figure 1B shows a close-up view of the same cast, demonstrating the penetration of the resin into the smallest portal vein branches at the liver periphery.
Figure 2 shows a portal vein cast that has undergone KOH maceration. Figure 2A shows the entire left liver lobe and Figure 2B is a close up of the small peripheral branches.
This technique has also been successfully applied to the intrahepatic bile duct and published using both the BABB clearing and KOH maceration techniques1,2,7.
Common problems include incomplete fills, bubbles in the resin, and overflow into surrounding systems or tissues. Figure 3 demonstrates how to recognize an incomplete fill (Figure 3A), bubbles (Figure 3A), and an overflow of resin from the portal vein to the central vein (Figure 3B).
All images are taken using a Leica MZ 16 FA stereoscope and QImaging RETIGA 4000R camera.
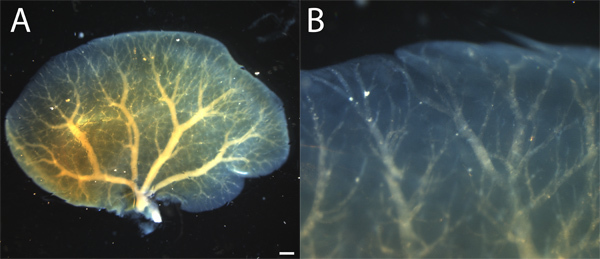 Figure 1. BABB-cleared resin cast of hepatic left lobe portal vein. A. Resin cast shows a hierarchical branching structure extending from the hilum to the liver periphery. Resin cast appears in yellow color within blue-tinted liver lobe. B. A close up shows that resin fill extends to small branches in the liver periphery. Resin is whitish through cleared liver. Scale bar = 1 mm.
Figure 1. BABB-cleared resin cast of hepatic left lobe portal vein. A. Resin cast shows a hierarchical branching structure extending from the hilum to the liver periphery. Resin cast appears in yellow color within blue-tinted liver lobe. B. A close up shows that resin fill extends to small branches in the liver periphery. Resin is whitish through cleared liver. Scale bar = 1 mm.
 Figure 2. Tissue-macerated resin cast of hepatic left lobe portal vein. A. Resin cast of whole liver lobe shows many branches of varying sizes. B. Close-up shows small branches in periphery of liver. Feathering appearance of small branches represents resin filling of sinusoidal spaces and may or may not be visible on resin casts and will increase the apparent branch density if present. Resin is white in color. Scale bar = 1 mm.
Figure 2. Tissue-macerated resin cast of hepatic left lobe portal vein. A. Resin cast of whole liver lobe shows many branches of varying sizes. B. Close-up shows small branches in periphery of liver. Feathering appearance of small branches represents resin filling of sinusoidal spaces and may or may not be visible on resin casts and will increase the apparent branch density if present. Resin is white in color. Scale bar = 1 mm.
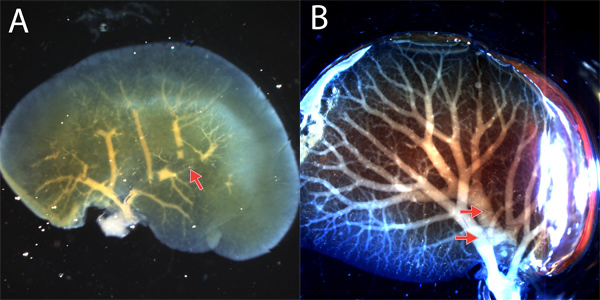 Figure 3. Common problems with resin casts. A. An incomplete fill of the left lobe portal vein is apparent by some or all cast branches failing to extend to the liver periphery. This cast also displays bubbles, visible as breaks in the continuous cast (arrow). B. Due to the proximity of the portal vein and central vein branches in some places, the portal vein resin will often enter and fill the central vein. This can be recognized when there are two distinct hilar branches (arrows) and two branching structures that have different patterns and overlap. Discriminating between technical errors and true morphological alterations can be done in two ways. First, the cast structure should be compared to the structure expected based on 2-dimensional analysis or other methods. Second, it is important to perform several replicates, and when possible, quantify the structure and perform statistical analysis to determine reproducibility and if there is a statistically significant morphological alteration between and within different experimental and control arms.
Figure 3. Common problems with resin casts. A. An incomplete fill of the left lobe portal vein is apparent by some or all cast branches failing to extend to the liver periphery. This cast also displays bubbles, visible as breaks in the continuous cast (arrow). B. Due to the proximity of the portal vein and central vein branches in some places, the portal vein resin will often enter and fill the central vein. This can be recognized when there are two distinct hilar branches (arrows) and two branching structures that have different patterns and overlap. Discriminating between technical errors and true morphological alterations can be done in two ways. First, the cast structure should be compared to the structure expected based on 2-dimensional analysis or other methods. Second, it is important to perform several replicates, and when possible, quantify the structure and perform statistical analysis to determine reproducibility and if there is a statistically significant morphological alteration between and within different experimental and control arms.
Discussion
We have described specific examples for how the portal vein and intrahepatic bile duct systems of the liver can be cast, but this technique can be applied to virtually any other ductal or vascular system with slight adaptations. Previous work has demonstrated the feasibility of this technique in multiple species, including mouse1,2,7-9, duckling10,11, rabbit12,13, dog14, and pig15, and in many organs, including nasal gland14, heart16, bladder12,13, and liver1,2,7,8. These casts can be used to compare multiple systems (for example, the portal vein and bile duct) within the same tissue, the same structure across different developmental stages5, or the effect on a structure of genetic or environmental alterations or influences1-4,6,7. The wide application of this technique across species and organs makes resin casting a highly valuable tool for studying the morphology of vascular and ductal structures and has the potential to greatly increase our knowledge of the normal function of these structures as well as how they are affected, or how they affect an organ, in animal disease models.
The use of resin to create 3-dimensional casts provides a permanent snapshot of a ductal or vascular system and has several advantages over similar techniques. First, the cast can be analyzed as a stand-alone structure after maceration, offering an advantage over India ink or similar ink injections. Second, the permanence of the structure increases the ease of analysis. Last, the ability of the Mercox II resin to withstand the BABB clearing procedure allows for a unique ability to visualize a structure in situ even within large or dense tissues.
There are several types of resin available with different characteristics. Mercox II has been chosen for this technique for its many advantages; Mercox II has low viscosity, allowing it to penetrate small peripheral portal vein and bile duct branches, it has quick and complete curing at room temperature, essential for analyzing the stand-alone macerated cast, it has minimal shrinkage when cured, and it withstands BABB tissue clearing. Another type of resin, Microfil (Flowtech, Carver, MA), has been used in several published studies8,9,15. Comparatively, Microfil does not cure completely at room temperature and is best visualized through tissue clearing. Microfil casts of the portal vein have previously been analyzed through 10% formalin fixation and subsequent clearing with ethanolmethyl salicylate8.
Resin additives can provide additional methods of cast visualization; addition of colored dyes, fluorescent molecules, or microspheres can enhance analysis. Consideration must be taken, however, to ensure that the dye molecules chosen are small enough to penetrate the intended vascular or ductal structure, that they do not significantly alter the properties of the resin, that they are able to withstand either tissue clearing or maceration, and that they are compatible with the desired method of analysis. Choosing resin additives will depend greatly on the specific application: for example, microspheres (Molecular Probes) are too large to pass through the hepatic sinusoids, making them unsuitable for the study of normal sinusoidal architecture but a perfect tool to investigate the development of portosystemic shunts, which are large enough to accommodate microspheres, in a genetically altered mouse model8.
Finally, resin cast analysis can be performed in either cleared or macerated casts. BABB-cleared casts are useful to measure the structure of a system as it relates to the organ within which it resides. KOH-macerated casts can be analyzed in multiple ways. Previous studies have used scanning electron microscopy (SEM) to visualize microcirculation networks and vessel structure12,13,17. Casts can also be quantified for branch number, diameter, and volume with use of microcomputed tomography (microCT)1,15,18, 19,20.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH) to S.S.H. (R01-DK078640), from the Howard Hughes Medical Institute (HHMI) through the HHMI/Vanderbilt University Certificate Program in Molecular Medicine to T.J.W. (GRDOT56006779), the Vanderbilt Diabetes Research and Training Center (P30-DK020593) and the Vanderbilt Digestive Disease Research Center (P30-DK058404) providing Core Services.
References
- Sparks EE, Perrien DS, Huppert KA, Peterson TE, Huppert SS. Defects in hepatic Notch signaling result in disruption of the communicating intrahepatic bile duct network in mice. Dis. Model Mech. 2011;4:359–367. doi: 10.1242/dmm.005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderpool C. Genetic interactions between hepatocyte nuclear factor-6 and notch signaling regulate mouse intrahepatic bile duct development in vivo. Hepatology. 2012;55:233–243. doi: 10.1002/hep.24631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EP, Ulmann-Schuler A, Staufenbiel M, Krucker T. Altered morphology and 3D architecture of brain vasculature in a mouse model for Alzheimer's disease. Proceedings of the National Academy of Sciences. 2008;105:3587–3592. doi: 10.1073/pnas.0709788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Steenkiste C. Vascular corrosion casting: analyzing wall shear stress in the portal vein and vascular abnormalities in portal hypertensive and cirrhotic rodents. Lab Invest. 2010;90:1558–1572. doi: 10.1038/labinvest.2010.138. [DOI] [PubMed] [Google Scholar]
- Dickie R, Cormack M, Semmler-Behnke M, Kreyling WG, Tsuda A. Deep pulmonary lymphatics in immature lungs. Journal of Applied Physiology. 2009;107:859–863. doi: 10.1152/japplphysiol.90665.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DM, McEntee GP, McGeenery KF, Fitzpatrick JM. Microvasculature of the pancreas, liver, and kidney in cerulein-induced pancreatitis. Archives of Surgery. 1993;128:293–295. doi: 10.1001/archsurg.1993.01420150049009. [DOI] [PubMed] [Google Scholar]
- Sparks EE, Huppert KA, Brown MA, Washington MK, Huppert SS. Notch signaling regulates formation of the three-dimensional architecture of intrahepatic bile ducts in mice. Hepatology. 2010;51:1391–1400. doi: 10.1002/hep.23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson TR. Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9884–9889. doi: 10.1073/pnas.0504391102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmeryckx B, Emmerechts J, Bovill EG, Hoylaerts MF, Lijnen HR. Effect of ageing on the murine venous circulation. Histochem. Cell Biol. 2012. [DOI] [PubMed]
- Hossler FE, Olson KR. Microvasculature of the nasal salt gland of the duckling, Anas platyrhynchos: quantitative responses to osmotic adaptation and deadaptation studied with vascular corrosion casting. J. Exp. Zool. 1990;254:237–247. doi: 10.1002/jez.1402540302. [DOI] [PubMed] [Google Scholar]
- Hossler FE, West RF. Venous valve anatomy and morphometry: studies on the duckling using vascular corrosion casting. Am. J. Anat. 1988;181:425–432. doi: 10.1002/aja.1001810411. [DOI] [PubMed] [Google Scholar]
- Hossler FE, Monson FC. Structure and blood supply of intrinsic lymph nodes in the wall of the rabbit urinary bladder--studies with light microscopy, electron microscopy, and vascular corrosion casting. Anat. Rec. 1998;252:477–484. doi: 10.1002/(SICI)1097-0185(199811)252:3<477::AID-AR16>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hossler FE, Monson FC. Evidence for a unique elastic sheath surrounding the vesicular arteries of the rabbit urinary bladder--studies of the microvasculature with microscopy and vascular corrosion casting. Anat. Rec. 1998;252:472–476. doi: 10.1002/(SICI)1097-0185(199811)252:3<472::AID-AR15>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Hossler FE, Kao RL. Microvasculature of the urinary bladder of the dog: a study using vascular corrosion casting. Microsc. Microanal. 2007;13:220–227. doi: 10.1017/S1431927607070249. [DOI] [PubMed] [Google Scholar]
- Wischgoll T, Choy JS, Kassab GS. Extraction of morphometry and branching angles of porcine coronary arterial tree from CT images. Am J Physiol. Heart. Circ. Physiol. 2009;297:H1949–H1955. doi: 10.1152/ajpheart.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossler FE, Douglas JE, Douglas LE. Anatomy and morphometry of myocardial capillaries studied with vascular corrosion casting and scanning electron microscopy: a method for rat heart. Scan Electron Microsc. 1986. pp. 1469–1475. [PubMed]
- Hossler FE, Douglas JE. Vascular Corrosion Casting: Review of Advantages and Limitations in the Application of Some Simple Quantitative Methods. Microsc. Microanal. 2001;7:253–264. doi: 10.1017.S1431927601010261. [DOI] [PubMed] [Google Scholar]
- Mondy WL. Micro-CT of corrosion casts for use in the computer-aided design of microvasculature. Tissue Eng. Part C Methods. 2009;15:729–738. doi: 10.1089/ten.TEC.2008.0583. [DOI] [PubMed] [Google Scholar]
- Masyuk TV, Ritman EL, LaRusso NF. Quantitative Assessment of the Rat Intrahepatic Biliary System by Three-Dimensional Reconstruction. The American Journal of Pathology. 2001;158:2079–2088. doi: 10.1016/S0002-9440(10)64679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyuk TV, Ritman EL, LaRusso NF. Hepatic Artery and Portal Vein Remodeling in Rat Liver: Vascular Response to Selective Cholangiocyte Proliferation. The American Journal of Pathology. 2003;162:1175–1182. doi: 10.1016/S0002-9440(10)63913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


