Abstract
Proper neuron to glia interaction is critical to physiological function of the central nervous system (CNS). This bidirectional communication is sophisticatedly mediated by specific signaling pathways between neuron and glia1,2 . Identification and characterization of these signaling pathways is essential to the understanding of how neuron to glia interaction shapes CNS physiology. Previously, neuron and glia mixed cultures have been widely utilized for testing and characterizing signaling pathways between neuron and glia. What we have learned from these preparations and other in vivo tools, however, has suggested that mutual signaling between neuron and glia often occurred in specific compartments within neurons (i.e., axon, dendrite, or soma)3. This makes it important to develop a new culture system that allows separation of neuronal compartments and specifically examines the interaction between glia and neuronal axons/dendrites. In addition, the conventional mixed culture system is not capable of differentiating the soluble factors and direct membrane contact signals between neuron and glia. Furthermore, the large quantity of neurons and glial cells in the conventional co-culture system lacks the resolution necessary to observe the interaction between a single axon and a glial cell.
In this study, we describe a novel axon and glia co-culture system with the use of a microfluidic culture platform (MCP). In this co-culture system, neurons and glial cells are cultured in two separate chambers that are connected through multiple central channels. In this microfluidic culture platform, only neuronal processes (especially axons) can enter the glial side through the central channels. In combination with powerful fluorescent protein labeling, this system allows direct examination of signaling pathways between axonal/dendritic and glial interactions, such as axon-mediated transcriptional regulation in glia, glia-mediated receptor trafficking in neuronal terminals, and glia-mediated axon growth. The narrow diameter of the chamber also significantly prohibits the flow of the neuron-enriched medium into the glial chamber, facilitating probing of the direct membrane-protein interaction between axons/dendrites and glial surfaces.
Keywords: Neuroscience, Issue 68, Molecular Biology, Cellular Biology, Biophysics, Microfluidics, Microfluidic culture platform, Compartmented culture, Neuron to glia signaling, neurons, glia, cell culture
Protocol
1. Assembly of the Microfluidic Culture Chamber (MCP)
MCP (Figure 1) are open chambers designed for compartmented cultures of different types of cells 4. It typically has two compartments that are connected through the central channels (3 μm in diameter). Assembly of MCP with glass-bottomed dishes is necessary for preparing cultures and subsequent imaging analysis.
First, coat sterile glass-bottomed dishes with Polyornithine (Sigma-Aldrich, 1 mg/ml) dissolved in sodium tetraborate (Sigma-Aldrich, 10 mM pH 8.5) and were incubated overnight at 37 °C.
On the following day, the coated glass-bottomed dishes were washed three times with ddH2O and dried under the sterile fume hood. The glass-bottomed dishes were then further coated with laminin (Sigma-Aldrich, 1 mg/ml) and dried under UV light for 1 hour. Coated dishes are ready to use or can be stored at -20 °C until use. These coating are necessary for plating neurons and astrocytes in the co-culture.
To assemble the culture platform, microfluidic culture platforms were placed on top of the coated glass-bottomed dishes with central connecting channels on its bottom side to form a tight seal. Regular neuron or astrocyte culture mediums were added (from the cell plating areas) on both sides (Figure 1) into the assembled MCP and incubated for 2-4 hours at 37 °C, to ensure there is no leakage between MCP and the glass-bottomed dish. We have tested with medium before plating cells to ensure there is no leak. The assembly of MCP on the glass-bottomed dish should be freshly prepared before use.
2. Preparation of Neuronal Culture and Induction of Neuronal Axons in Assembled MCP
Cortical neuronal cell cultures were freshly prepared from embryonic 14-16 day-old mouse brains. The neuron culture medium is composed of neurobasal medium, 2% B27 neurobasal supplement, 2 mM glutamine by adding 1% of 100x GlutaMAX, and 1% Penicillin-streptomysin. Following dissection of the mouse brain, the meninges was removed from the cortex under the dissecting microscope. The tissue was then minced with a razor blade to make small tissue blocks in order to increase the surface area exposed to trypsin. Tissue blocks were trypsinized (Sigma-Aldrich, 0.05% Trypsin) for 10 min in a 37 °C water bath, and then dissociated gently by trituration with a fire-polished Pasteur pipette. Dissociated cells were filtered through a 70 μm strainer to collect clear neuronal cell suspension.
Neurons (2-3 x 106/ml, 150 μl) were plated on the cell plating areas on right side (Figure 1) of the assembled MCP so that neurons can attach in the cell retention area (Figure 1). Freshly prepared neurons were plated with neuron plating medium which is supplemented with 5% fetal bovine serum (Hyclone) into the regular neuron culture medium. Because only the neurons that attach in the cell retention area are able to grow axons into the other side of the assembled MCP through central channels, it is important to plate high density of neurons in the cell plating area to ensure enough neurons are attached to the cell retention area. A representative image of high density of neuronal axons is shown in Figure 2A.
On the following day, the neuron plating medium was replaced by the regular neuron culture medium. Changing medium was accomplished by carefully aspirating the medium from the cell plating area of the chamber, but not from the cell retention area of the assembled MCP, in order to avoid air bubbles in the cell retention area and the central connecting channels. Air bubbles will severely inhibit the axon outgrowth and subsequent entry into the central channels in the MCP. Glial-cell derived neurotrophic factor (GDNF, 10-20 ng/ml) was also added on the other (left) side of the chamber on the same day to facilitate the induction of axon outgrowth5 from the neuronal side and cross through central channels of the assembled MCP (Figure 2A). Fair amount of spontaneous neurite outgrowth without GDNF is also observed from the neuronal side and cross through central channels of the assembled MCP. Axons that enter into the central channel are often observed 2-3 days after plating. Once the axons enter into the central channel, they usually enter the other side within one day. Axons are normally intact for at least one week after entering the other side.
3. Addition of Cultured Astrocytes to MCP to Establish a Compartmentalized Co-culture System
Primary astrocyte cultures were prepared from the P1-3 mouse pup brain. The brain dissociation procedure is similar to the neuronal cell isolation procedure described above. Astrocytes were first plated into 10 cm dish that were pre-coated with Polyornithine (Sigma-Aldrich, 1 mg/ml). The astrocyte culture medium (DMEM, 10% fetal bovine serum, 1% Penicillin-streptomysin) was changed every day for the next two days to remove the debris. After that, the medium was changed every three days.
Astrocytes become 90% confluent and form a monolayer after 7 days. Confluent astrocytes were trypsinized and 150 μl of re-suspended astrocytes (1x106 /ml) were re-plated into the cell plating area of the left side of the assembled MCP when axons are about to enter or have just entered into the left side of the assembled MCP. Astrocytes were usually plated 4-5 days after the neurons were plated. GDNF was first removed before the re-plating of the astrocytes into the left side of the MCP. Re-plated astrocytes were attached in the cell retention area, as shown by the GFAP immunostaining (Figure 1). Only minimal flow of medium between both sides of the chambers (determined by fluorescence dyes) was found in the MCP, as previously shown4,6 .
Astrocytes become 90% confluent and form a monolayer after 7 days. Confluent astrocytes were trypsinized and 150 μl of re-suspended astrocytes (1 x 106 / ml) were re-plated into the cell plating area of the left side of the assembled MCP when axons are about to enter or have just entered into the left side of the assembled MCP. Astrocytes were usually plated 4-5 days after the neurons were plated. GDNF was first removed before the re-plating of the astrocytes into the left side of the MCP. Re-plated astrocytes were attached in the cell retention area, as shown by the GFAP immunostaining (Figure 1). Only minimal flow of medium between both sides of the chambers (determined by fluorescence dyes) was found in the MCP, as previously shown4,6 .
After the re-plating of the astrocytes, axons that entered the left side of the assembled MCP directly interact with the astrocytes by either direct axonal contact or release of soluble factors. Immunostaining of astroglial plasma membrane glutamate transporter GLT1 and neuronal βIII-tubulin was performed to visualize the interaction between the axons and the astrocytes, as shown in Figure 2D-F.
Representative Results
Time-lapse imaging analysis of axon-induced GLT1 promoter activation in astrocytes
The compartmented neuron and astrocyte co-culture system allows only the neuronal processes, especially the axons, to selectively interact with astrocytes. Following the successful establishment of axon and astrocyte (or other glial cells) co-culture in the assembled MCP, different types of axon-glia interactions can be studied such as; axon-induced activation of astroglial gene promoter activation, astrocyte-induced axon guidance, axon-induced astroglial Ca2+ response, and axon-induced myelin synthesis in oligodendrocytes. All of these applications take advantage of the availability of powerful fluorescent reporters available for glial cells or dyes loaded to these cells.
Here we describe axon-induced transcriptional activation of astroglial glutamate transporter 1 (GLT1) promoter by using bacterial artificial chromosome (BAC) GLT1 eGFP transgenic mice and this compartmentalized co-culture system. In the BAC GLT1 eGFP transgenic mice, an eGFP fluorescence reporter was overexpressed under the control of the GLT1 genomic promoter7. The expression of the eGFP reporter correlates with endogenous GLT1 promoter activation protein expression and functional activity7 thus permitting an assessment of the GLT1 promoter activity in single astrocytes in situ. Astroglial GLT1 expression is highly dependent upon neuronal stimulation6,8 . Primary astrocytes express minimum levels of GLT1; however, GLT1 expression levels (both mRNA and protein) are strongly induced in astrocytes that are co-cultured with neurons9. The evidence is shown here by significantly increased GLT1 immunoreactivity in compartmentalized co-culture system (Figure 3). In conventional neuron and astrocyte co-cultures, high density of neurons make it less ideal to observe the effect of neuronal axons on the transcriptional activation of GLT1 promoter.
To monitor the axon-dependent GLT1 promoter activation, wild type neurons were cultured in the right side of the assembled MCP and the axons were induced following the application of the GDNF on the left side of the assembled MCP. Astrocytes derived from the BAC GLT1 eGFP mice were cultured in the left side of the assembled MCP after the axons have been induced and just entered into the left side of the assembled MCP. A total of 6 days (24 hr to 140 hr) time-lapse images were collected to monitor the eGFP fluorescence intensity change in real time and the axon growth in the MCP. As shown in Figure 4A, very minimum eGFP fluorescence expression in astrocytes was observed 24 hr following the plating of the astrocytes. Significant increase of eGFP intensity was observed 48 hr after co-culture (from 24 hr to 68 hr) when axons are well into the astrocyte side and interact directly contacting with astrocytes (Figure 4 B-C). On the other hand, eGFP fluorescence intensity was gradually decreased once axons were retracted and degenerated by kainite (200 μM) induced neuronal cell death on the neuronal (right) side of the assembled MCP (Figure 4D-F). The dynamic change of eGFP fluorescence intensity in response to the axons clearly demonstrated that astroglial GLT1 promoter activity is modulated by the axon interaction.
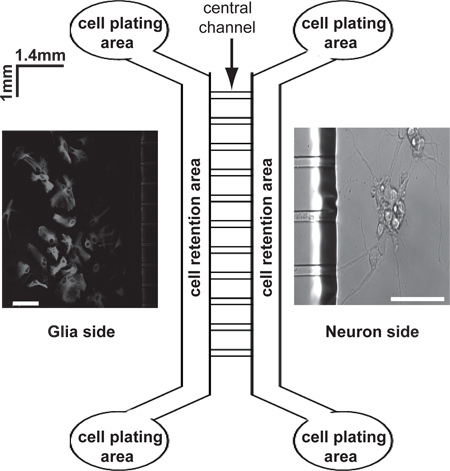 Figure 1. Schematic diagram of microfluidic neuron and astrocyte co-culture platform. Microfluidic culture platform (MCP) has two compartments that are connected through the central channels. Cells can be plated from the cell plating area on each side and neuronal axons are induced from the cell retention area and pass through the central channels to enter the other side (insert scale bar 50 μm).
Figure 1. Schematic diagram of microfluidic neuron and astrocyte co-culture platform. Microfluidic culture platform (MCP) has two compartments that are connected through the central channels. Cells can be plated from the cell plating area on each side and neuronal axons are induced from the cell retention area and pass through the central channels to enter the other side (insert scale bar 50 μm).
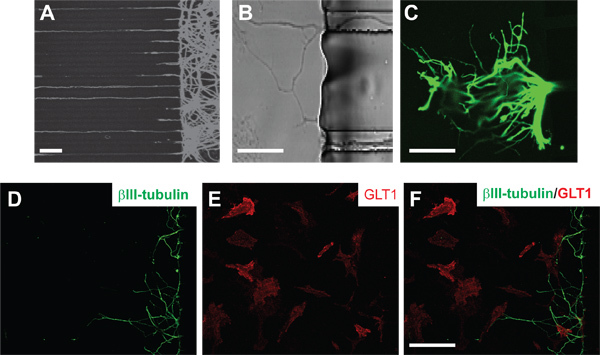 Figure 2. Induction of neuronal axons and interaction of axons with astrocyte in MCP MCP. (A) High density of neurons in the cell retention area of the assembled MCP, revealed by βIII-tubulin immunostaining. Scale bar: 50 μm (B) Magnified view of axons crossing the central channels and entering into the other side of the MCP. Scale bar: 100 μm (C) Axon growth cone revealed by the bIII-tubulin staining when axons enter into the other side of the MCP. Scale bar: 100 μm (D) Axon bundles that grow through the central channel and enter into the other side of the MCP. (E) Astrocytes revealed by the GLT1 immunostaining in the glial side of the MCP. (F) Merged image displays contact between axons and GLT1 positive astrocytes. Scale bar: 50 μm.
Figure 2. Induction of neuronal axons and interaction of axons with astrocyte in MCP MCP. (A) High density of neurons in the cell retention area of the assembled MCP, revealed by βIII-tubulin immunostaining. Scale bar: 50 μm (B) Magnified view of axons crossing the central channels and entering into the other side of the MCP. Scale bar: 100 μm (C) Axon growth cone revealed by the bIII-tubulin staining when axons enter into the other side of the MCP. Scale bar: 100 μm (D) Axon bundles that grow through the central channel and enter into the other side of the MCP. (E) Astrocytes revealed by the GLT1 immunostaining in the glial side of the MCP. (F) Merged image displays contact between axons and GLT1 positive astrocytes. Scale bar: 50 μm.
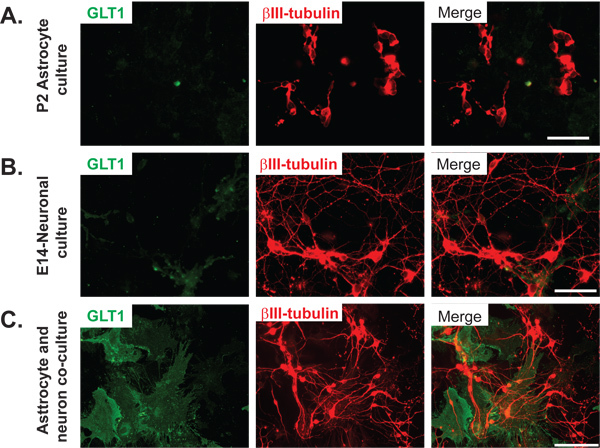 Figure 3. Neuron-dependent induction of GLT1 expression in primary astrocytes. GLT1 immunoreactivity in (A) primary astrocyte cultures, Scale bar: 50 μm (B) primary neuronal cultures, and (C) primary neuron and astrocyte co-cultures. Scale bar: Neurons are shown by immunostaining of βIII-tubulin. Significant increase of GLT1 immunoreactivity was observed in neuron and astrocyte co-cultures.
Figure 3. Neuron-dependent induction of GLT1 expression in primary astrocytes. GLT1 immunoreactivity in (A) primary astrocyte cultures, Scale bar: 50 μm (B) primary neuronal cultures, and (C) primary neuron and astrocyte co-cultures. Scale bar: Neurons are shown by immunostaining of βIII-tubulin. Significant increase of GLT1 immunoreactivity was observed in neuron and astrocyte co-cultures.
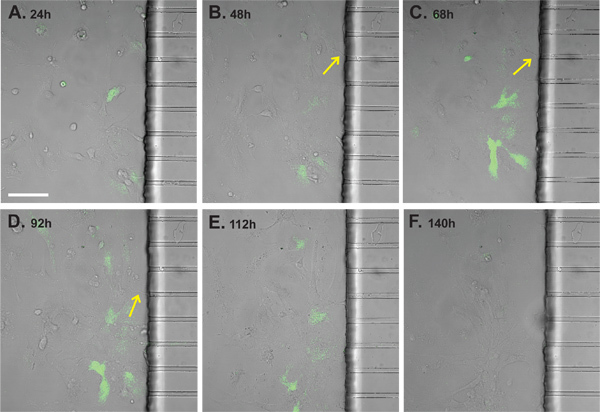 Figure 4. Time-lapse images of axon-induced GLT1 promoter activation in MCP with BAC GLT1 eGFP astrocytes and wild type neurons. Dynamic changes of eGFP intensity and the growth of axons were collected through a 6d time-lapse recording following astrocyte plating. (A) 24h (B) 48h (C) 68h (D) 92h (E) 112h (F) 140h. Entry of axons (highlighted by the yellow arrows) into the astrocyte side was observed from 48h to 92h which correlate with increased eGFP fluorescence intensity. Decrease of eGFP fluorescence intensity occurred from 112h to 140h after kainite (200 μM) was applied on the neuronal side at 92h to induce neuronal cell death and axon degeneration. Scale bar: 50 μm.
Figure 4. Time-lapse images of axon-induced GLT1 promoter activation in MCP with BAC GLT1 eGFP astrocytes and wild type neurons. Dynamic changes of eGFP intensity and the growth of axons were collected through a 6d time-lapse recording following astrocyte plating. (A) 24h (B) 48h (C) 68h (D) 92h (E) 112h (F) 140h. Entry of axons (highlighted by the yellow arrows) into the astrocyte side was observed from 48h to 92h which correlate with increased eGFP fluorescence intensity. Decrease of eGFP fluorescence intensity occurred from 112h to 140h after kainite (200 μM) was applied on the neuronal side at 92h to induce neuronal cell death and axon degeneration. Scale bar: 50 μm.
Discussion
The MCP based neuron and astrocytes co-culture system allows dissection of detailed neuron to astroglia signaling pathways by allowing only the axons pass the central channels and interacting with the astroglial cells. This co-culture system can be conveniently set up with conventional neuron and astrocyte culture procedures. We also described a practical application of this co-culture system by employing an eGFP based reporter for demonstrating axon-dependent GLT1 promoter activation in astrocytes.
This MCP based neuron and astroglia co-culture system offers several advantages compared to conventional co-culture methods: 1) ability to visualize and identify the explicit morphological interaction between single axons and astrocytes in real-time under high-resolution microscopy 2) a well-controlled independent microenvironment to apply pharmacological manipulation for cell-specific effect 3) analysis of promoter activation and protein expression exclusively induced/up-regulated in astrocytes that are modulated by axons contact. As the culture procedures for primary microglia10 and oligodendrocytes11 have been well established, this MCP co-culture system can be also applied to neuron to microglia and neuron to oligodendrocyte co-cultures. Meanwhile, we also recognize that this co-culture system is intended for the sensitive image analysis at the single cell level, which is complementary to the conventional approaches of analyzing large quantity of cells, such as RNA or protein analysis. In addition, our system simplifies the enormous interaction between neurons and glial cells in vivo by exemplifying interaction between single cell and axon. All of these should be considered when interpreting the results from this co-culture system. In the CNS, interactions between axons and different glial cells are widely present and are critically important for both neuron and glial functions12,13 . Development of this co-culture system will allow imaging-based dissection of these interactions.
Disclosures
No conflicts of interest declared.
Acknowledgments
We would like to thank Dr. Jeffrey Rothstein for providing BAC GLT1 eGFP mice and GLT1 antibody; Tufts Center for Neuroscience Research (NIH P30 NS047243; PI, Rob Jackson) for providing valuable core facilities; New faculty recruitment grant (NIH P30 5P30NS069254-02; PI, Phil Haydon) in Tufts Neuroscience Department.
References
- Stevens B. Neuron-astrocyte signaling in the development and plasticity of neural circuits. Neuro-Signals. 2008;16:278–288. doi: 10.1159/000123038. [DOI] [PubMed] [Google Scholar]
- Paixao S, Klein R. Neuron-astrocyte communication and synaptic plasticity. Current opinion in neurobiology. 2010;20:466–473. doi: 10.1016/j.conb.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Vahidi B, Taylor AM, Rhee SW, Jeon NL. Microfluidic culture platform for neuroscience research. Nature. 2006;1:2128–2136. doi: 10.1038/nprot.2006.316. [DOI] [PubMed] [Google Scholar]
- Wang CY. Regulation of neuromuscular synapse development by glial cell line-derived neurotrophic factor and neurturin. The Journal of biological chemistry. 2002;277:10614–10625. doi: 10.1074/jbc.M106116200. [DOI] [PubMed] [Google Scholar]
- Yang Y. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 2009;61:880–894. doi: 10.1016/j.neuron.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan MR. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. The Journal of neuroscience. 2007;27:6607–6619. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA. Neuronal regulation of glutamate transporter subtype expression in astrocytes. The Journal of neuroscience. 1997;17:932–940. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlag BD. Regulation of the glial Na+-dependent glutamate transporters by cyclic AMP analogs and neurons. Molecular pharmacology. 1998;53:355–369. doi: 10.1124/mol.53.3.355. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Novikova M, Maresz K, Shriver LP, Dittel BN. Development of a culture system that supports adult microglial cell proliferation and maintenance in the resting state. Journal of immunological. 2005;300:32–46. doi: 10.1016/j.jim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Espinosa-Jeffrey A, Wakeman DR, Kim SU, Snyder EY, de Vellis J. Culture system for rodent and human oligodendrocyte specification, lineage progression, and maturation. Current protocols in stem cell biology. 2009;Chapter 2 doi: 10.1002/9780470151808.sc02d04s10. [DOI] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Debanne D, Rama S. Astrocytes shape axonal signaling. Science signaling. 2011;4:pe11. doi: 10.1126/scisignal.2001884. [DOI] [PubMed] [Google Scholar]


