Abstract
To understand the role of a gene in the development of colitis, we compared the responses of wild-type mice and gene-of-interest deficient knockout mice to colitis. If the gene-of-interest is expressed in both bone marrow derived cells and non-bone marrow derived cells of the host; however, it is possible to differentiate the role of a gene of interest in bone marrow derived cells and non- bone marrow derived cells by bone marrow transplantation technique. To change the bone marrow derived cell genotype of mice, the original bone marrow of recipient mice were destroyed by irradiation and then replaced by new donor bone marrow of different genotype. When wild-type mice donor bone marrow was transplanted to knockout mice, we could generate knockout mice with wild-type gene expression in bone marrow derived cells. Alternatively, when knockout mice donor bone marrow was transplanted to wild-type recipient mice, wild-type mice without gene-of-interest expressing from bone marrow derived cells were produced. However, bone marrow transplantation may not be 100% complete. Therefore, we utilized cluster of differentiation (CD) molecules (CD45.1 and CD45.2) as markers of donor and recipient cells to track the proportion of donor bone marrow derived cells in recipient mice and success of bone marrow transplantation. Wild-type mice with CD45.1 genotype and knockout mice with CD45.2 genotype were used. After irradiation of recipient mice, the donor bone marrow cells of different genotypes were infused into the recipient mice. When the new bone marrow regenerated to take over its immunity, the mice were challenged by chemical agent (dextran sodium sulfate, DSS 5%) to induce colitis. Here we also showed the method to induce colitis in mice and evaluate the role of the gene of interest expressed from bone-marrow derived cells. If the gene-of-interest from the bone derived cells plays an important role in the development of the disease (such as colitis), the phenotype of the recipient mice with bone marrow transplantation can be significantly altered. At the end of colitis experiments, the bone marrow derived cells in blood and bone marrow were labeled with antibodies against CD45.1 and CD45.2 and their quantitative ratio of existence could be used to evaluate the success of bone marrow transplantation by flow cytometry. Successful bone marrow transplantation should show a vast majority of donor genotype (in term of CD molecule marker) over recipient genotype in both the bone marrow and blood of recipient mice.
Keywords: Immunology, Issue 68, Genetics, Cellular Biology, Physiology, Bone marrow transplantation, colitis, mice, irradiation
Protocol
1. Before-you-start Technical Considerations
We recommend using both C57/BL6 wild-type mice and knockout mice for experiment because mice with corresponding CD molecules can be purchased from major mouse vendors.
Since it is difficult to track the wild-type mice and knockout mice derived donor cells after bone marrow transplantation, it is necessary to use CD45.1 mice to represent wild-type mice. (CD45.1 C57/SJL WT mice Jackson Laboratories stock #002014).
Most mouse colonies are CD45.2. Our knockout mice belong to CD45.2 genotype (alternatively a CD45.2 WT mice source: Jackson Laboratories stock #000664). The determination of CD45.1 or CD45.2 genotype can be performed using flow cytometry of bone marrow and peripheral blood as mentioned in section 7 in this protocol.
Mice after irradiation have compromised immunity. Keep mice in pathogen free facility.
Operation of irradiator requires special security clearance and training. To save time in training and approval processes, it is best to collaborate with a laboratory with qualified technician who is able to operate the irradiator.
Many institutions have flow cytometry laboratories with experienced technicians. To minimize problems with flow cytometry operation, it is better to discuss your flow cytometry experiment plan with the experienced technician before you start.
Mice were assigned in this manner (10 mice per group):
| Group | Bone marrow donor to recipient | Treatment | |
| A | BM exchange | CD45.1 WT to CD45.2 KO | DSS colitis |
| B | Water normal control | ||
| C | BM exchange | CD45.2 KO to CD45.1 WT | DSS colitis |
| D | Water normal control | ||
| E | Sham | CD45.1 WT to CD45.1 WT | DSS colitis |
| F | Water normal control | ||
| G | Sham | CD45.2 KO to CD45.2 KO | DSS colitis |
| H | Water normal control |
* A brief illustration of experimental protocol is shown in Figure 1.
2. Recipient Mice Irradiation
Mice are bred and kept in pathogen-free facility. Place mice in autoclaved irradiation pie with filter. Place one mouse in each slot of irradiation pie. Place the pie into the irradiator and make sure the turntable and pie are turning.
Turn on the air-pump for ventilation. Close the irradiator door and lock it.
Irradiate the mice with 1,000 rad (which is equivalent to 10 Gy) around 10 min (depending on radiation source and half-life). From this time point, the irradiated mice are immuno-compromised and have weak immunity against infection. After irradiation, take the pie out and place in a sterile container for transportation to biosafety cabinet in animal facility. Avoid exposing the mice to external environment to minimize chance of infection.
In biosafety cabinet, transfer the mice into autoclaved mouse cages with autoclaved bedding (4 mice per cage. Add new sterile water with sulfatrim suspension - 3.12 ml per 100 ml water).
Wrap the water bottle with aluminum foil as the antibiotics are light sensitive. Shake to mix the sulfatrim solution bottle well before loading it to the cage.
3. Donor Bone Marrow Extraction
Sacrifice mice with carbon dioxide or isoflurance and then immerse in 1:200 Lysol solution. Spray the mice with 70% ethanol to wet the skin and dissect open bones.
Soak the dissection instruments in 70% ethanol for sterilization.
Perform the dissection in a biosafety cabinet. Use the sterile dissection instruments to expose the humerus, femur, tibia and fibula bones, free from attaching muscles and ligaments.
Cut open both ends of bones to show the red bone marrow. Hold the bones with forceps. Flush the red bone marrow out with cold PBS with 3 ml syringe and 25G needles to a Petri dish. Flush from both ends of bones to yield more bone marrow cells.
Use 6 ml syringes and 18G1/2 needles to break red bone marrow plugs by repeated aspiration and ejection. Transfer bone marrow with 50 ml Falcon tubes.
Spin the bone marrow down in 1,800 rpm for 5 min at 4 °C. Discard supernatant, resuspend pellet by vortexing for 5-10 sec.
Dilute 10X RBC lysis buffer with sterile water. Add 1X RBC lysis buffer (5 ml per donor mouse), vortex again for 5 sec and keep exactly 3 min at room temperature.
After 3 min, fill the tubes with 20 ml cold PBS to stop the lysis and mix. Pour the content through a 40 μm cell strainer directly into a new 50 ml Falcon tube.
Rinse the original tubes with 5 ml of PBS and transfer to cell strainer as well. Top up to 50 ml with PBS and mix.
4. Counting Bone Marrow Donor Cells
Add 100 μl of Trypan blue to an Eppendorf tube and 100 μl of bone marrow suspension to an Eppendorf tube and mix. Pipette the stained cell mixture to a hemocytometer.
The amount of cells is calculated by total living bone marrow cell count in the Falcon tubes = unstained cell number in 16 squares x 2 (due to Trypan blue dilution) x 10,000 x 50 ml
Spin down the bone marrow cells in 50 ml Falcon tubes at 2,000 rpm for 5 min at 4 °C.
Remove the supernatant. Based on the total cell counts, resuspend the pellet with PBS to 1 x 108 cells per ml
5. Infusion to Recipient Mice
Warm the recipient mice on a heat pad and under a warming lamp. Put the recipient mice in restrainer. Inject 1 x 107 cells per mouse in 100-200 μl intravenously.
Injection of donor bone marrow must be done between 4-24 hr after irradiation.
Keep up to 4 injected mice per cage. Maintain the injected recipient mice in autoclaved cages with antibiotics-treated water for the first 4 weeks in pathogen free animal facility and let the mice regain immunity. Change cages, antibiotic-treated water and food every 4 days to maintain hygiene.
6. Induction of Colitis and Evaluation of Colitis
4 weeks after irradiation and bone marrow transplantation, switch to regular water without antibiotics and maintain the mice for 2 more weeks to regain their normal gut microflora.
6 weeks after bone marrow injection, measure initial body weight. Some mice are given 5% dextran sulfate in drinking water ad libitum to induce colitis. Some mice are given regular drinking water only as normal control groups.
5 days later, withdraw peripheral blood, transfer to heparin coated tubes (Vacutainer) and keep in ice. Pool the blood from 4 mice to 1 tube. Combine 300 μl of blood with 300 μl of cell staining buffer = 600 μl. Divide the blood to 5 Eppendorf tubes, each with 100 μl.
Sacrifice the mice with carbon dioxide or isoflurane. Evaluate macroscopic damage scores (please refer to another JOVE video publication for details 1). Measure body weight change. Measure bowel length and thickness with digital caliper. Observe stool texture (hard, soft or bloody). Determine occult blood in stool with Hemooccult. Dissect colon tissues and fix in formalin for H&E staining.
Dissect 1 femur bone per mouse, extract bone marrow and flush the bone marrow with cold PBS via 25G needle and 1 ml syringe. Pool 4 bone marrow plugs to 1 tube and keep in ice.
Pour the bone marrow to Petri dish, shear the bone marrow plug with 18G needle and 5 ml syringe several times until no bone marrow plug is present.
Spin down with 1,500 rpm for 5 min at 4 °C. Remove supernatant and resuspend with 600 μl cell staining buffer. Divide the resuspended bone marrow to 5 Eppendorf tubes, each with 100 μl.
7. Quality Inspection of Bone Marrow Transplantation by Flow Cytometry
Label each sample tubes of blood or bone marrow #1-5:
Prepare the antibody mixture for each respective tube in the dark.
#1 No antibody #2 30 μl FITC isotype control + 30 μl PE isotype control + 15 μl CD16/32 blocking #3 30 μl FITC CD45.1 Ab + 15 μl CD16/32 blocking #4 30 μl PE CD45.2 Ab + 15 μl CD16/32 blocking #5 30 μl FITC CD45.1 Ab + 30 μl PE CD45.2 Ab + 15 μl CD16/32 blocking
| Add nothing to blood or bone marrow sample #1 |
| Add 5 μl of antibody mixture #2 to each blood or bone marrow sample #2 |
| Add 3 μl of antibody mixture #3 to each blood or bone marrow sample #3 |
| Add 3 μl of antibody mixture #4 to each blood or bone marrow sample #4 |
| Add 5 μl of antibody mixture #5 to each blood or bone marrow sample #5 |
Keep in dark in ice for 30 min.
Add 2 ml 1X RBC lysis buffer to each tube. Keep in dark on ice for 15 min.
Spin down the cells at 1,500 rpm, 5 min at 4 °C. (GH 3.8 rotor, 1,500 rpm = 350 x g) Remove supernatant. Resuspend the pellet with 500 μl cell staining buffer in the dark, then vortex briefly.
Check the CD45.1 (representing WT) and CD45.2 (representing KO) in the immunostained blood and bone marrow samples by flow cytometry. Select FITC to represent WT CD45.1 and PE to represent KO CD45.2.
Analyze the flow cytometry results by FlowJo software. Calculate the ratio of FITC:PE ratio or PE:FITC ratio to determine whether donor genotype is dominant genotype in blood and bone marrow.
Representative Results
If the gene-of-interest plays a significant role in immune cells during development of colitis, the mice receiving bone marrow of different genotype through bone marrow transplantation (WT to KO or KO to WT) should have an altered response to DSS colitis. One of the most important parameters for determining the severity of colitis is the H&E staining of the colonic tissues. Colonic tissue structure changes and signs of inflammation can be quantitatively evaluated by H&E histology scoring system. Criteria for H&E histology scoring for DSS colitis model can found here 2. Alternatively, chemical induced colitis can also be induced by trinitrobenzene sulfonic acid (TNBS). Methods of TNBS colitis induction and H&E histology scoring criteria can be found in a previous publication 3. Histology score difference between groups can be analyzed by student's t-tests.
The mice may have significantly ameliorated or worsened colitis when compared to mice with sham bone marrow transplantation (WT to WT or KO to KO). If the gene-of-interest plays a significant role in colitis via bone marrow derived cells, the mice with exchanged bone marrow should respond significantly different from the mice with sham bone marrow transplantation.
For DSS colitis model, histology change may be evaluated by histology scoring (see Figure 4). Significant change of histology score may indicate the development of colitis mediated by the gene-of-interest in bone marrow derived cells. For example, cathelicidin is an anti-microbial and anti-inflammatory peptide gene (gene-of-interest in our case) 4. Without bone marrow transplantation, cathelicidin KO mice generally developed worse colitis than wild-type mice did in response to DSS. Transfusion of wild-type (WT) bone marrow to cathelicidin knockout (KO) mice leads to ameliorated colitis while transfusion of bone marrow from cathelicidin knockout (KO) mice to wild-type (WT) mice leads to worsened colitis when exposed to DSS (Figure 2).
To verify expression of gene-of-interest, mRNA expression of gene-of-interest (e.g. cathelicidin) from donor wild-type bone marrow should be detectable in the colonic (or other) tissues of the cathelicidin deficient knockout recipient mice after bone marrow transplantation. It is also interesting to know whether the expression of gene-of-interest in wild-type recipient mice is reduced after donation of knockout mice bone marrow.
Successful engraftment of donor bone marrow is represented by dominant ratio of donor CD isotype over recipient CD isotype in both peripheral blood cells and bone marrow of recipient mice. Bone marrow shows best labeling of CD45.1 and CD45.2 in flow cytometry as blood has a lot of unstained cells (Figure 2 and 3).
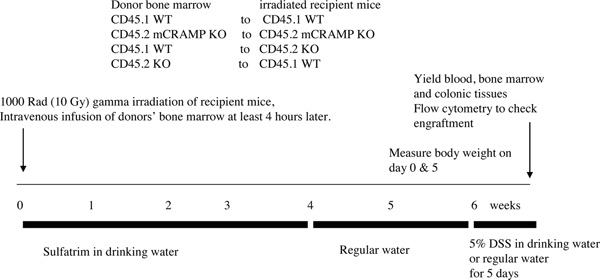 Figure 1. The experimental protocol of bone marrow transplantation.
Figure 1. The experimental protocol of bone marrow transplantation.
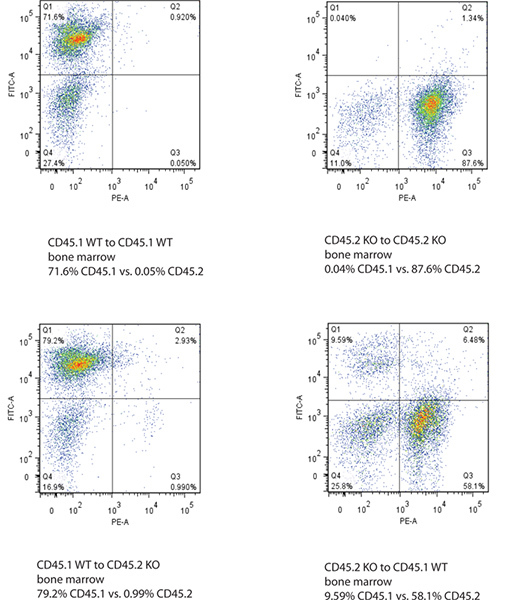 Figure 2. Flow cytometry data of bone marrow of recipient mice after bone marrow transplantation. The Y-axis shows FITC-labeled CD45.1 signal and X-axis shows PE-labeled CD45.2 signal. Successful bone transplantation is defined by the change of CD45.1 or CD45.2 genotype in both bone marrow and blood of the recipient mice. Click here to view larger figure.
Figure 2. Flow cytometry data of bone marrow of recipient mice after bone marrow transplantation. The Y-axis shows FITC-labeled CD45.1 signal and X-axis shows PE-labeled CD45.2 signal. Successful bone transplantation is defined by the change of CD45.1 or CD45.2 genotype in both bone marrow and blood of the recipient mice. Click here to view larger figure.
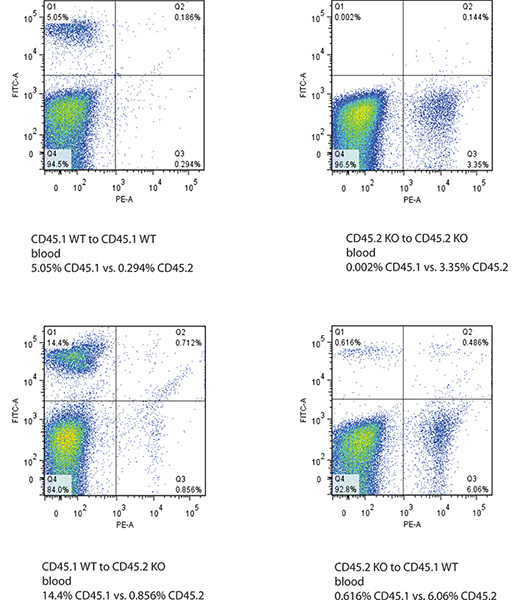 Figure 3. Flow cytometry data of blood of recipient mice after bone marrow transplantation. The Y-axis shows FITC-labeled CD45.1 signal and X-axis shows PE-labeled CD45.2 signal. Successful bone transplantation is defined by the change of CD45.1 or CD45.2 genotype in both bone marrow and blood of the recipient mice. Click here to view larger figure.
Figure 3. Flow cytometry data of blood of recipient mice after bone marrow transplantation. The Y-axis shows FITC-labeled CD45.1 signal and X-axis shows PE-labeled CD45.2 signal. Successful bone transplantation is defined by the change of CD45.1 or CD45.2 genotype in both bone marrow and blood of the recipient mice. Click here to view larger figure.
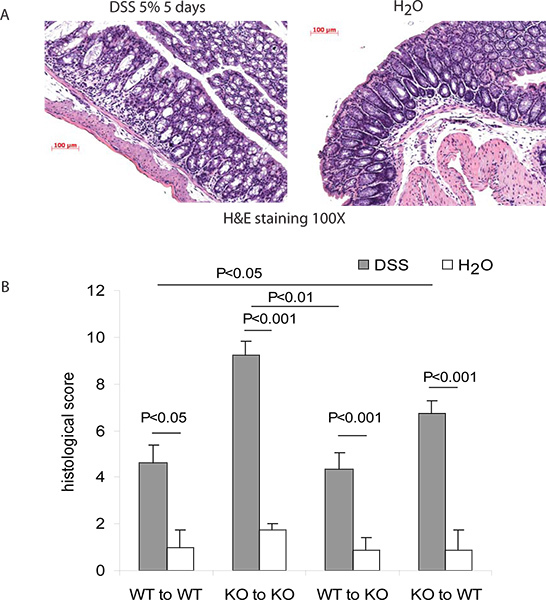 Figure 4. Evaluation of colitis in mice after bone marrow transplantation. (A) Sample H&E images of colons with normal histology and DSS colitis. (B) Histology scores. Successful induction of DSS colitis can be confirmed by significant increase of histology score by day 5 of DSS treatment. After the exchange of bone marrow to different genotype, the histology score should change significantly. This suggests the altered course of colitis by the gene-of-interest expressing in the bone marrow derived cells. Data are represented by mean ± standard error of means.
Figure 4. Evaluation of colitis in mice after bone marrow transplantation. (A) Sample H&E images of colons with normal histology and DSS colitis. (B) Histology scores. Successful induction of DSS colitis can be confirmed by significant increase of histology score by day 5 of DSS treatment. After the exchange of bone marrow to different genotype, the histology score should change significantly. This suggests the altered course of colitis by the gene-of-interest expressing in the bone marrow derived cells. Data are represented by mean ± standard error of means.
Discussion
This bone marrow transplantation approach is suitable for immunology research of colitis, infection, cancer, obesity and other diseases. This bone marrow transplantation experiment is needed when the gene-of-interest is expressed in both bone marrow derived and non-bone marrow derived cells and the gene-of-interest is suspected to mediate disease by cells from either population. For example, antimicrobial peptide cathelicidin is shown to modulate acute colitis. But it is expressed in both epithelial cells and immune cells (such as macrophages). Then we used bone transplantation to define which population of cells modulates acute colitis in mice. The colitis severity was significantly altered after the change of bone marrow cathelicidin genotypes via bone marrow transplantation. Then we can conclude that cathelicidin expressed in bone marrow derived cells plays a significant role in modulating acute colitis in response to DSS.
Bone marrow derived cells typically include red blood cells, white blood cells and platelets. Bone marrow transplantation changes the genotype of these cells but not others. Laterally speaking, this experiment can only differentiate the role of gene-of-interest in bone marrow derived cells versus non-bone marrow derived cells. But this experiment cannot further define which population of bone marrow derived cells mediates the colitis as all red blood cells, white blood cells and platelets are derived from bone marrow. The fate of bone marrow derived cells migrated to colons during colitis is an active and controversial area of research. After bone marrow transplantation and induction of murine colitis, it is likely that some of bone marrow derived cells exist as lymphoid cells and myofibroblasts in the colons 5. Another report suggested that bone marrow derived cells serve as endothelial progenitor cells for neovascularization in recovery process 6. There is also evidence showing bone marrow derived cells are associated with epithelial cell differentiation in patients with colitis 7. We cannot exclude the possibility that some of bone marrow derived cells may differentiate to cells other than typical immune cells and play modulating roles in colitis development. Nevertheless, bone marrow derived monocyte/macrophage population is important for the protection against chemical induced colonic mucosal damage 8,9. This report is consistent with our finding that cathelicidin expressed from bone marrow derived cells modulates DSS colitis while cathelicidin is secreted from monocytes/macrophages 4.
On the other hand, there are many ways to track the success of bone marrow transplantation. The flow cytometry analysis of CD45.1 and CD45.2 genotypes can provide a quantitative method to determine the proportion of donor bone marrow stem cell derived cells in the recipient mice. Bone marrow derived cells can differentiate into multiple kinds of cells 10. When flow cytometry analysis is not feasible, it is possible to use male (XY chromosome) donor mice and female (XX chromosome) recipient mice 11. The donor mice will carry unique Y chromosomes in the body of XX only female recipient mice. Y chromosome can be identified by fluorescent in situ hybridization 11. In addition, immunohistochemistry of CD45.1, CD45.2 and/or the gene-of-interest in the tissues may be necessary to visualize the donor bone marrow derived cells.
But CD45 flow cytometry analysis and Y chromosome in situ hybridization procedures are usually done after the mice were sacrificed. To monitor the location of donor cells in the recipient mice being used to study chronic diseases like cancer without sacrificing the mice, it is possible to use green fluorescent protein transgenic mice as donor mice 12. Therefore, the donor bone marrow derived cells can be tracked in the body of the recipient mice under non-invasive high resolution optical imaging under transient anesthesia and this can be done repeatedly.
Not all mice have successful bone marrow transplantation 13. We observed ~10-20% of mice die in the first 2 weeks after irradiation due to anemia or infection. Therefore, more mice than needed should be prepared at the start of experiment. For example, you should prepare 10 mice per group at the start of experiment if you expect 8 mice per group available at the end of the colitis experiment. Also, make sure you remove the dead mice as soon as possible. The speed of immune reconstitution is correlated to the number to hematopoietic stem cells in bone marrow infused into the recipient mice 14. Therefore, it is crucial to have sufficient number of live bone marrow cells (1 x 107 cells per mouse) infused into recipient mice for successful bone marrow transplantation.
Recipient mice after irradiation have compromised immunity. Donor mice dissection and bone marrow preparation procedures should be done in the same standard as cell culture experiments. Use of sterile instruments and containers, aseptic handling techniques should be applied. Throughout all experiments, PBS contains 1% Penicillin-streptomycin and 10 U/ml heparin should be used during the handling of bone marrows. All reagents are cell culture grade. Additional technical discussion can be found in reference 13.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was funded by Pilot and Feasibility Study grant from UCLA-CURE Center, the Crohn's and Colitis Foundation of America Career Development Award (#2691) and National Institute of Health NIDDK K01 (DK084256) funding to Hon Wai Koon.
Bone marrow irradiation operation was assisted by Bernard Levin and Scott Kitchen of UCLA Center for AIDS Research Mouse/Human Chimera Core facility. Flow cytometry operation was assisted by UCLA Vector Core facility.
References
- Kim JJ, Shajib MS, Manocha MM, Khan WI. Investigating Intestinal Inflammation in DSS-induced Model of IBD. J. Vis. Exp. 2012. p. e3678. [DOI] [PMC free article] [PubMed]
- Ungaro R. A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:1167–1179. doi: 10.1152/ajpgi.90496.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagliuolo I. Protective effects of neurokinin-1 receptor during colitis in mice: role of the epidermal growth factor receptor. Br. J. Pharmacol. 2002;136:271–279. doi: 10.1038/sj.bjp.0704697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koon HW. Cathelicidin signaling via the Toll-like receptor protects against colitis in mice. Gastroenterology. 2011;141:1852–1863. doi: 10.1053/j.gastro.2011.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY. marrow cells in murine colitis: multi-signal analysis confirms pericryptal myofibroblast engraftment without epithelial involvement. PLoS One. 2011;6:e11. doi: 10.1371/journal.pone.0026082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X. New cell therapy using bone marrow-derived stem cells/endothelial progenitor cells to accelerate neovascularization in healing of experimental ulcerative colitis. Curr. Pharm. Des. 2011;17:1643–1651. doi: 10.2174/138161211796197007. [DOI] [PubMed] [Google Scholar]
- Valcz G. Lymphoid aggregates may contribute to the migration and epithelial commitment of bone marrow-derived cells in colonic mucosa. J. Clin. Pathol. 2011;64:771–775. doi: 10.1136/jclinpath-2011-200022. [DOI] [PubMed] [Google Scholar]
- Takaba J, Mishima Y, Hatake K, Kasahara T. Role of bone marrow-derived monocytes/macrophages in the repair of mucosal damage caused by irradiation and/or anticancer drugs in colitis model. Mediators Inflamm. 2010;2010:634145. doi: 10.1155/2010/634145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhautdin B. Protective role of macrophage-derived ceruloplasmin in inflammatory bowel disease. Gut. 2012. [DOI] [PMC free article] [PubMed]
- Seta N, Kuwana M. Derivation of multipotent progenitors from human circulating CD14+ monocytes. Exp. Hematol. 2010;38:557–563. doi: 10.1016/j.exphem.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Crain BJ, Tran SD, Mezey E. Transplanted human bone marrow cells generate new brain cells. J. Neurol. Sci. 2005;233:121–123. doi: 10.1016/j.jns.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Finnberg NK. High-resolution imaging and antitumor effects of GFP(+) bone marrow-derived cells homing to syngeneic mouse colon tumors. Am. J. Pathol. 2011;179:2169–2176. doi: 10.1016/j.ajpath.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Struuck R, Dysko RC. Principles of bone marrow transplantation (BMT): providing optimal veterinary and husbandry care to irradiated mice in BMT studies. J. Am. Assoc. Lab Anim. Sci. 2009;48:11–22. [PMC free article] [PubMed] [Google Scholar]
- Chen BJ, Cui X, Sempowski GD, Domen J, Chao NJ. Hematopoietic stem cell dose correlates with the speed of immune reconstitution after stem cell transplantation. Blood. 2004;103:4344–4352. doi: 10.1182/blood-2003-07-2534. [DOI] [PubMed] [Google Scholar]


