Abstract
Nucleic acid-based vaccination is a topic of growing interest, especially plasmid DNA (pDNA) encoding immunologically important antigens. After the engineered pDNA is administered to the vaccines, it is transcribed and translated into immunogen proteins that can elicit responses from the immune system. Many ways of delivering DNA vaccines have been investigated; however each delivery route has its own advantages and pitfalls. Skin tattooing is a novel technique that is safe, cost-effective, and convenient. In addition, the punctures inflicted by the needle could also serve as a potent adjuvant. Here, we a) demonstrate the intradermal delivery of plasmid DNA encoding enhanced green fluorescent protein (pCX-EGFP) in a mouse model using a tattooing device and b) confirm the effective expression of EGFP in the skin cells using confocal microscopy.
Keywords: Bioengineering, Issue 68, Biomedical Engineering, Genetics, Medicine, DNA, vaccine, immunization method, skin tattooing, intradermal delivery, GFP
Protocol
1. Plasmid DNA Purification
Transform the eukaryotic plasmid DNA encoding EGFP (pCX-EGFP) into DH5α E.coli competent cells. The empty pCX vector may also be used as a negative control.
Culture and harvest the DH5α E.coli cells and purify the pDNA according to the Qiagen EndoFree Plasmid Purification Handbook.
Filter pDNA solution through a 0.22 μm PVDF sterile filter, and store it at -20 °C until use.
2. Tattoo System Preparation
Connect the handheld unit and the control pedal to the power supply unit per the manufacturer's instructions.
Set the needle oscillation frequency to approximately 100 Hz. For the tattooing device we chose (Stealth Rotary Tattoo System), the dial on the power supply should be set to 4 volts. Refer to user manuals or technical support for other tattooing systems.
Sterilize the needle array before use. We recommend using one needle array per animal. The needles can be reused after being cleaned with soapy water and autoclaved.
Install the needle array into the handheld unit and loosely attach the plastic grip.
Adjust the needle depth to an appropriate setting by moving the plastic grip up or down. We used a needle depth of approximately 0.5 mm for our mouse experiment.
Tighten the plastic grip when the needle depth is properly set, and the tattoo system is ready for use.
3. Animal Shaving
Choose the site for the tattooing treatment. The site should be a relatively firm, flat, and fleshy area of the skin, for example, the side of the animal's hindleg.
Anesthetize a balb/c mouse with a mixture of ketamine (90 mg/kg) and xylazine (5 mg/kg) according to the animal's body weight. For other animals, refer to the anesthesia guidelines of your institution.
Check the animal's reflexes by pinching its foot to confirm the animal is properly anesthetized.
Use the electrical trimmer to trim down the hair at the animal's hindlegs slowly and carefully. Remove the hair from the planned tattooing site and the surrounding area.
Remove the residual shorter hair with the disposable safety razor. Be careful not to cut the animal's skin. Note that depilatory cream may be used as an alternative hair-removal method; however, we prefer shaving over depilatory cream.
Remove stray cut hairs from the skin with compressed air if necessary.
4. Delivery of Plasmid DNA by Tattooing
If an immunization protocol has been established previously, the dose and volume of DNA solution to be applied should be calculated accordingly. We recommend using as small a volume as practical to make the tattooing process more manageable. In this EGFP demonstration, we used 7.5 μl of DNA solution at a concentration of 0.25 mg/ml, which was applied onto a tattooing area of approximately 1 cm2. For immunization experiments, higher pDNA concentrations may be required to induce strong immune responses1,2.
Apply the DNA solution by pipetting directly onto the tattooing site. Alternatively, load the DNA solution into the plastic grip by pipetting, so the liquid is suspended between the needle and the tip of plastic grip. Refer to the video for a demonstration. We have observed no noticeable difference between the two methods. However, loading the solution into the plastic grip may be advantageous on vertical skin surfaces, for example.
Start the needle oscillation and place the needle array with light pressure on the animal's skin at the tattooing site. Then move the oscillating needle gently and slowly in a linear fashion to deliver plasmid DNA over the entire tattooing area. Maintain a 90-degree angle between the needle and the skin to avoid lacerations to the skin.
Observe the skin. Abrasion and inflammation are normal. If bleeding happens, stop the tattooing process and decrease the needle depth.
Continue the needle movement for approximately 1 min, and then stop the tattooing process.
Use a clean cotton swab to apply a thin layer of topical analgesic on the tattooing site. Topical analgesics such as Silver Sulfadiazine Cream (SSD Cream) or Neosporin ointment can help alleviate the pain or distress of the animals, and therefore it is advised to apply them before the anesthesia wears off. Observe the animals the next day for signs of pain or distress (change of gait, inactivity). Reapply analgesic cream if the signs persist.
5. Confirmation of Antigen Expression
48 hr after the tattooing treatment (note that different pDNA constructs may require different expression times), the mouse is euthanized by CO2 narcosis. For other animals, refer to the euthanasia guidelines of your institution.
Dissect the skin at the tattooing site, and fix the tissue in 4% paraformaldehyde at 4 °C overnight.
Rinse the tissue with 70% ethanol, and transfer it into 1X PBS for imaging.
Examine the whole tissue using a confocal microscope to check for EGFP signals. Alternatively, the skin tissue can be embedded in paraffin wax and examined in sections under a fluorescent microscope.
Representative Results
The expression of EGFP with an excitation peak at 488 nm and emission peak at 509 nm can be observed in mouse skin cells. From a 1.875 μg dose of DNA, containing approximately 3×1017copies of the plasmid, we typically observed 10-20 EGFP signals in the 1 cm2 tattooed area. This relatively low number of transfected cells is consistent with the results of a previous study3. The EGFP expression (Figure 1) provides the evidence that EGFP plasmid was delivered into the animal's skin cells using the DNA tattooing technique.
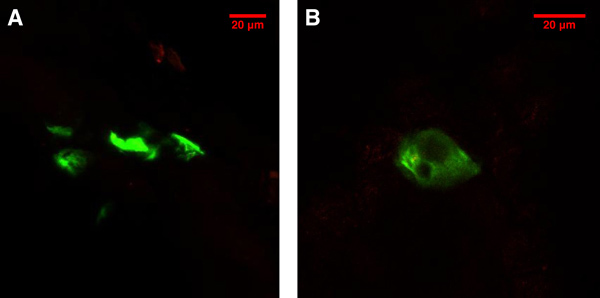 Figure 1. EGFP expression in the skin cells 48 hr after the tattooing treatment on the hindleg of a balb/c mouse viewed with a confocal microscope. A) A projection of EGFP signals from multiple focal planes. B) EGFP expression in a single cell.
Figure 1. EGFP expression in the skin cells 48 hr after the tattooing treatment on the hindleg of a balb/c mouse viewed with a confocal microscope. A) A projection of EGFP signals from multiple focal planes. B) EGFP expression in a single cell.
Troubleshooting
1. No antigen expression is detected (e.g. no EGFP positive control signals).
Increase the concentration of plasmid DNA solution by 2- to 5-fold. We recommend a starting concentration of 0.2 mg/ml. Concentrations as high as 5 mg/ml have been reported for DNA tattooing experiments3.
Avoid severe damage to the skin, as mouse skin at the tattooing site is easily cut by the razors and needles.
Make sure the DNA construct is appropriate for your experiment.
Perform gel electrophoresis to make sure the majority of plasmid DNA is in supercoiled or closed circular form.
2. Severe bleeding or damage to the skin occurs at the tattooing site.
Decrease the needle depth.
Maintain a 90-degree angle between the needle array and the skin.
Decrease the force applied during the tattooing treatment.
Decrease the speed of the tattooing movement.
3. The background signal is too high when imaging EGFP.
Use a brand-new disposable safety razor to remove as much hair as possible before tattooing. Sometimes it is necessary to shave again before the dissection.
Discussion
DNA vaccination is considered safer than traditional vaccination strategies as it does not require manipulation of, or expose the vaccines to, live or attenuated pathogens4. However, the result of DNA vaccination depends heavily on the delivery route. Skin is abundant in antigen-presenting cells, such as Langerhans Cells and dendritic cells1, and thus an ideal site for immunization in terms of immunogenicity and ease of access5,6. As a result, intradermal vaccination strategy is one of the most popular choices for DNA vaccines. As shown in this video, DNA tattooing is a simple yet promising way to administer a DNA vaccine intradermally. Interestingly, the inflammatory responses caused by the tattooing process could also serve as a natural and potent adjuvant1,2. It has been reported that the DNA tattooing can elicit up to a 100-fold increase in T-cell responses in monkeys, as compared to T-cell responses in animals immunized via the intramuscular route7,8. Compared to other dermal delivery methods, such as the gene gun, DNA tattooing holds several advantages. First, DNA tattooing does not require expensive equipment and carriers, i.e. gold particles. This would be a huge advantage in terms of vaccine distribution, especially for developing countries. Secondly, it is known that high air pressure can cause pDNA damage due to shear force, which could decrease the antigen expression level. A study has shown that DNA tattooing damages less than 3% of total pDNA9. Finally, DNA tattooing can cover a large area of skin, which could potentially elicit a stronger immune response. Tattooing devices can also be used to deliver peptide/protein vaccines, and have been proven to induce both humoral and cell-mediated immune responses10. We now use skin tattooing routinely in our animal experiments in a DNA prime-protein boost vaccination protocol (three DNA primes followed by two protein boosts over a period of 16 weeks), and we have successfully induced in mice strong immune responses against HIV-1 gp120.
Disclosures
No conflicts of interest declared.
Acknowledgments
We would like to thank all members of the Kong Lab and Dr. Yan Deng at Microscopy Core, Office of Collaborative Science, NYUMC for their assistance and technical support. This work was supported by a pilot grant from the New York University Center for AIDS Research (CFAR, NIH grant AI027742).
References
- Bins AD. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat Med. 2005;11:899–904. doi: 10.1038/nm1264. [DOI] [PubMed] [Google Scholar]
- Pokorna D, Rubio I, Muller M. DNA-vaccination via tattooing induces stronger humoral and cellular immune responses than intramuscular delivery supported by molecular adjuvants. Genet. Vaccines Ther. 2008;6:4. doi: 10.1186/1479-0556-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg JH. Optimization of intradermal vaccination by DNA tattooing in human skin. Hum. Gene Ther. 2009;20:181–189. doi: 10.1089/hum.2008.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MA. DNA vaccines: a review. J. Intern. Med. 2003;253:402–410. doi: 10.1046/j.1365-2796.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- Koide Y, Nagata T, Yoshida A, Uchijima M. DNA vaccines. Jpn. J. Pharmacol. 2000;83:167–174. doi: 10.1254/jjp.83.167. [DOI] [PubMed] [Google Scholar]
- Peachman KK, Rao M, Alving CR. Immunization with DNA through the skin. Methods. 2003;31:232–242. doi: 10.1016/s1046-2023(03)00137-3. [DOI] [PubMed] [Google Scholar]
- Verstrepen BE. Improved HIV-1 specific T-cell responses by short-interval DNA tattooing as compared to intramuscular immunization in non-human primates. Vaccine. 2008;26:3346–3351. doi: 10.1016/j.vaccine.2008.03.091. [DOI] [PubMed] [Google Scholar]
- Potthoff A. Immunogenicity and efficacy of intradermal tattoo immunization with adenoviral vector vaccines. Vaccine. 2009;27:2768–2774. doi: 10.1016/j.vaccine.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Quaak SG. DNA tattoo vaccination: effect on plasmid purity and transfection efficiency of different topoisoforms. J. Control Release. 2009;139:153–159. doi: 10.1016/j.jconrel.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Pokorna D. Vaccination with human papillomavirus type 16-derived peptides using a tattoo device. Vaccine. 2009;27:3519–3529. doi: 10.1016/j.vaccine.2009.03.073. [DOI] [PubMed] [Google Scholar]


