Abstract
Study Objectives:
Orexin peptides activate orexin 1 and orexin 2 receptors (OX1R and OX2R), regulate locomotion and sleep-wake. The dual OX1R/OX2R antagonist almorexant reduces activity and promotes sleep in multiple species, including man. The relative contributions of the two receptors in locomotion and sleep/wake regulation were investigated in mice.
Design:
Mice lacking orexin receptors were used to determine the contribution of OX1R and OX2R to orexin A-induced locomotion and to almorexant-induced sleep.
Setting:
N/A.
Patients or Participants:
C57BL/6J mice and OX1R+/+, OX1R-/-, OX2R+/+, OX2R-/- and OX1R-/-/OX2R-/- mice.
Interventions:
Intracerebroventricular orexin A; oral dosing of almorexant.
Measurements and Results:
Almorexant attenuated orexin A-induced locomotion. As in other species, almorexant dose-dependently increased rapid eye movement sleep (REM) and nonREM sleep in mice. Almorexant and orexin A were ineffective in OX1R-/-/OX2R-/- mice. Both orexin A-induced locomotion and sleep induction by almorexant were absent in OX2R-/- mice. Interestingly, almorexant did not induce cataplexy in wild-type mice under conditions where cataplexy was seen in mice lacking orexins and in OX1R-/-/OX2R-/- mice. Almorexant dissociates very slowly from OX2R as measured functionally and in radioligand binding. Under non equilibrium conditions in vitro, almorexant was a dual antagonist whereas at equilibrium, almorexant became OX2R selective.
Conclusions:
In vivo, almorexant specifically inhibits the actions of orexin A. The two known orexin receptors mediate sleep induction by almorexant and orexin A-induced locomotion. However, OX2R activation mediates locomotion induction by orexin A and antagonism of OX2R is sufficient to promote sleep in mice.
Citation:
Mang GM; Dürst T; Bürki H; Imobersteg S; Abramowski D; Schuepbach E; Hoyer D; Fendt M; Gee CE. The dual orexin receptor antagonist almorexant induces sleep and decreases orexin-induced locomotion by blocking orexin 2 receptors. SLEEP 2012;35(12):1625-1635.
Keywords: Orexin, hypocretin, mouse, sleep mechanisms, locomotion
INTRODUCTION
Orexin/hypocretin neuropeptides regulate sleep/wake behavior, locomotor activity, and other hypothalamic functions.1–4 The two orexins, orexin A and orexin B (also called hypocretin 1 and hypocretin 2), are synthesized in a small cluster of neurons in the lateral hypothalamus by cleavage of a precursor peptide, prepro-orexin.3,4 Orexin neurons are tonically active during wakefulness but show little or no activity during rapid eye movement (REM) and nonREM (NREM) and sleep.5–8 These neurons send excitatory inputs to neurons involved in maintaining wakefulness, such as noradrenergic neurons in the locus coeruleus, serotonergic neurons in the dorsal raphe, histaminergic neurons in the tuberomammillary nucleus, and cholinergic neurons in the basal forebrain, the laterodorsal and pedunculopontine tegmental nuclei.9–11 Orexins activate the G protein-coupled receptors orexin receptor 1 (OX1R) and orexin receptor 2 (OX2R). OX1R binds orexin A with higher affinity than orexin B, whereas OX2R binds both orexins with high affinity.3,12 Orexin receptors are widely coexpressed in the brain except in the locus coeruleus, where OX1R is enriched, and in the tuberomammillary nucleus, where OX2R is enriched.13
In humans, lack of orexin-producing neurons leads to the sleep disorder narcolepsy, with excessive daytime sleepiness, sleep paralysis, and cataplexy as the main symptoms.14–16 Mice lacking orexins or orexin receptors show a strong narcoleptic-like phenotype with cataplexy1,17,18 as do dogs with a defect in OX2R signaling.2 Mice deficient in OX1R (OX1R-/-, also known as Hcrtr1-/-) have mild fragmentation of sleep/wake, whereas mice deficient in OX2R (OX2R-/-, also known as Hcrtr2-/-) show a pronounced narcoleptic phenotype albeit without the strong cataplexy phenotype seen in orexin knockout (KO) mice.19,20
The orexin receptors have been proposed as targets for the treatment of sleep disorders.21,22 Recently, several OX1R/OX2R antagonists were shown to promote sleep in clinical studies.23– The first to be tested in the clinic, almorexant, reduces locomotor activity and/or increases sleep in rats, dogs, and humans.27 It is unclear whether both orexin receptors or only one of the two must be antagonized for sleep induction, although evidence suggests OX2R antagonism is likely to be key.28,29 To address this question, we characterized the effects of almorexant and the role of OX1R and OX2R in orexin-induced locomotor activity and sleep in mice by examining: (1) the effects of almorexant on orexin-induced locomotion; (2) the effects of OX1R-, OX2R-, and OX1R/OX2R-deficiency on orexin-induced locomotion; (3) the effects of almorexant on sleep; and (4) the effects of almorexant on sleep in OX1R-, OX2R-, and OX1R/OX2R-deficient mice.
METHODS
Subjects
Male mice weighing 25-35 g were single- or group-housed on wood shavings in Makrolon® type II (14 cm × 16 cm × 22 cm) and type III (15 cm × 22 cm × 37 cm) cages, respectively. Each cage contained a nest box, a piece of wood, and nesting materials made of tissue paper, and animals had access to food and water ad libitum. The housing cages were placed in a temperature- and humidity-controlled room (20-24°C, 45% humidity) with a light/dark cycle of 12:12 (lights on at 03:00, max 80 Lux). All experiments were conducted in accordance with the Veterinary Authority of Basel, Switzerland, and every effort was made to minimize the number of animals used and any pain and discomfort.
Mice heterozygous for the disrupted Hcrtr1 (OX1R+/-) or Hcrtr2 (OX2R+/-) allele, on a mixed C57BL/6J.129/SvEv background, were obtained from Deltagen (San Mateo, CA) (B6.129P2-Hcrtr1tm1Dgen, B6.129P2-Hcrtr2tm1Dgen). Mice were backcrossed to C57BL/6J for 10 generations before using. From breedings of heterozygous mice, homozygous KO (OX1R-/- and OX2R-/-) and WT (OX1R+/+ and OX2R+/+) littermates were selected by genotyping. Mice deficient for both orexin receptors (B6.129P2-Hcrtr1tm1Dgen xHcrtr2tm1Dgen, called OX1R-/-/OX2R-/-) were obtained by crossing the single receptor lines. To drastically reduce the numbers of animals bred for this study, OX1R-/-/OX2R-/- mice were generated from breedings of double homozygous animals. Thus, there were no WT littermates available for these mice. In addition, the animals used in the locomotion studies were those produced during the multiple crossings needed to obtain the double KO animals. Mice heterozygous for the disrupted orexin Hcrt (orexin-/+) allele backcrossed at least 11 generations to C57BL/6J were obtained from the University of Texas (B6-Orexintm1Ywa).1 Mice homozygous for the mutation were selected by genotyping.
Substances
Almorexant was purchased (custom synthesis) from Anthem Biosciences (Bangalore, India), and dosed by mouth in freshly prepared suspension with 0.5% methylcellulose on the day of the experiment. Orexin A was purchased from Bachem (Bubendorf, Switzerland), and dissolved in phosphate buffered saline.
Implantation of Intracerebroventricular Cannulae
Mice were anesthetized with ketamine/xylazine (110 mg/kg, 10:1, intraperitoneally) and placed into a stereotaxic frame. The skull was exposed and stainless steel guide cannulae (diameter: 0.35 mm; length: 6 mm) were bilaterally implanted to the lateral ventricles using the following coordinates30: -0.3 mm rostral from bregma, ± 1.2 mm lateral from bregma, -2.1 mm ventral from dura. The guide cannulae were fixed to the skull with dental cement and two to three anchoring screws. To prevent postsurgical pain, the analgesic buprenorphine (0.01 mg/kg, intraperitoneally) was given twice per day on the first 2 days after surgery. Behavioral tests started following full recovery (5-6 days after surgery).
Implantation of Electrocorticogram/Electroencephalogram and Electromyogram Electrodes
One hour prior to surgery, mice were administered buprenorphine (Temgesic, 0.05 mg/kg subcutaneously). Mice were anesthetized with ketamine/xylazine (110 mg/kg, 10:1, intraperitoneally) and placed in a stereotaxic frame. The skull was exposed and four miniature stainless-steel screws (SS-5/TA Science Products GmbH, Hofheim, Germany) attached to 36-gauge, Teflon-coated solid silver wires were placed in contact with the frontal and parietal cortex (3 mm posterior to bregma, ± 2 mm from the sagittal suture) through bore holes. The frontal electrodes served as reference. The wires were crimped to a small six-channel connector (CRISTEK Micro Strip Connector, International Precision Products, Bardowick, Germany) that was affixed to the skull with dental acrylic. Electromyogram (EMG) signals were acquired by a pair of multistranded stainless- steel wires (7SS-1T, Science Products GmbH, Hofheim, Germany) inserted into the neck muscles and also crimped to the headmount. After surgery, mice were singly housed and allowed to recover in their cage placed on a heating pad. Temgesic, 0.05 mg/kg, subcutaneously, was given 8h and 16h after surgery to prevent pain. After 24h, the mice were housed with their former cagemates and allowed to recover for 2 wk.
Orexin-Induced Locomotor Activity
For measuring locomotor activity, a computerized motility measurement system was used (Moti 4.25, TSE Systems, Bad Homburg, Germany). This system automatically measures locomotor activity in transparent boxes (20 cm × 32 cm × 17 cm) by counting the interruptions of horizontal infrared beams spaced 5.7-8.4 cm apart in a frame set at the cage-floor level of the boxes. All locomotor experiments were performed during the light phase, when the stimulatory effects of orexin can be detected, beginning between Zeitgeber time (ZT) 4 and ZT5. The mice were put into the motility boxes, and their spontaneous locomotor activity was recorded after a 30-min habituation period. In the first experiment, designed to study the effect of almorexant on orexin-induced activity, almorexant or vehicle (control group) was then orally administered (pretreatment) in C57BL/6 mice. Each mouse was in a single experiment. After recording baseline activity for 30 min, intracerebroventricular (ICV) injections of orexin A were performed: the mice were gently restrained by the experimenter, injectors with a diameter of 0.15 mm (connected to Hamilton syringes by tubes) were introduced into the guide cannulae, and the animals were released in a cage. A total volume of 0.3 μl solution with 3 μg orexin A was then injected at a flow rate of 0.1 μl/min, controlled by a microinfusion pump (CMA100, CMA, Stockholm, Sweden). The injector was removed after an additional 60 sec. The mice were then returned to the motility boxes and locomotor activity was recorded for a further 75 min.
In the second experiment, designed to study the effect of receptor deficiency on orexin-induced activity, orexin A was injected 60 min after putting the different KO mice or their WT littermates into the setup (30 min habituation, 30 min baseline activity with no pretreatment).
Sleep Studies
Mice were habituated to individual cages in the sound-attenuated recording chamber for 6 to 10 days with a 12:12 light:dark cycle (lights on 03:00, max 80 lux) and a constant temperature of approximately 23°C. Mice had access to food and water ad libitum and to one nesting paper and a piece of wood. Approximately 5h before the start of the experiment, mice were weighed and attached to the recording cables that connected their headmounts to a commutator (G-4-E, Gaueschi) allowing free movement in the experiment boxes. On day 1, the mice were manipulated and habituated to the oral application syringe. On day 2, they received vehicle (methylcellulose 0.5%, 10 ml/kg by mouth). On day 3, almorexant was administered by mouth. All manipulations and oral applications were performed in a time window of 5-15 min before lights off and start of the recordings. Recordings began simultaneously with lights off at 15:00 (hr 0) and continued for 23h. The experimental chamber was secured about 5 min prior to lights off and the mice were undisturbed during the recordings. The chamber was opened for 1h per day before lights off to care for the mice and perform any manipulations necessary. On day 4, mice were replaced in groups in their housing cages.
Electroencephalogram (EEG)/EMG signals were amplified using a Grass Model 78D amplifier (Grass Instrument Co., Quincy, MA), analog filtered (EEG: 0.3 to 30 Hz, EMG: 5 to 30 Hz), and acquired using Harmonie V5.2 (acquisition frequency: 200 Hz with calibration the first day, record duration: 23h). Animals were video recorded during data collection, using an infrared video camera and locomotor activity was detected using infrared sensors (InfraMot Infrared Activity Sensor 30-2015 SENS, TSE Systems) placed in the roof of the boxes. Activity signals were acquired in 10-sec intervals by the software Labmaster V2.4.4 (TSE Systems). EEG/EMG and activity channels were imported into and scored in 10-sec epochs using the rodent scoring module of Somnologica® (ResMed, Basel Switzerland) into wake, NREM sleep, and REM sleep. Epochs during which there were state transitions were scored as the state present for at least 50% of the epoch. A direct comparison between the results obtained by hand-scoring 84h of recordings with the results from the automated scoring yielded an agreement of 90.3%. This is comparable to the results obtained by others.31
Cataplexy
To specifically assess cataplexy, mice were placed into the recording cages only 1h before lights off. To further increase the chances of the mice showing cataplexy, a running wheel, fruit loops, and a ping-pong ball were added to the boxes containing nesting paper, food, water, and a piece of wood. EEG/EMG activity and video recordings began at lights off as for the sleep experiments and continued for 16h. Mice were not previously habituated to the recording boxes as cataplexy in mice is stimulated by novelty, running on wheels, and palatable food. An episode of cataplexy was defined as an abnormal transition from active wake to a sudden loss of activity, characterized by a period of at least 10 sec of EEG theta activity accompanied by muscle atonia.32 Potential episodes of cataplexy were most easily detected by viewing the videotapes at four times normal speed and any sudden cessation of movement or collapse of the mice outside their nesting area were noted. Periods without motion, when it was not possible to clearly see if the mice were grooming or feeding, were also noted as potential cataplexy. The EEG/EMG activity records were then examined. When there was strong theta activity and nuccal atonia followed by a sudden return to a wake EEG with activity, the corresponding epochs were re-scored as cataplexy. The cataplexy had to be immediately preceded and followed by active waking. The cataplectic attacks occurred anywhere in the cage. The mice typically collapsed prone or lying on the side, whereas during sleep they adopted the characteristic curled/hunched posture and were usually in the nest. Behavioral arrests that were accompanied by rapid entry into sleep with or without sleep onset REM periods were not re-scored as cataplexy but were left as sleep.
Statistical Analysis
All analyses were performed with the software Systat (versions 12 and 13, Systat Software Inc. Washington, DC) and results expressed as means ± standard error of the mean (SEM).
For the analysis of locomotor activity, analyses of variance (ANOVAs) for each experimental condition (different almorexant treatments or different genotypes) were performed. First, only the total distance traveled within the 30 min before ICV orexin A infusions were used to analyze genotype or treatment effects on baseline locomotor activity. Then, two-factor ANOVAs were performed to analyze whether the experimental condition affected ICV orexin-induced locomotor activity. As between-subject factors, the experimental condition (pretreatment with vehicle/almorexant or genotype) and the ICV injection (vehicle or orexin) were used. Time (total distance traveled 30 min before and the 75 min after ICV treatment) served as a within-subject factor. In pilot studies, these time windows were found to be optimal for the determination of orexin-induced locomotor activity. If not otherwise stated, the F and P values reported in the results are those from the interaction between ICV injection and time. The ICV orexin injections were considered to be effective if this interaction reached statistical significance.
For the sleep experiments, the time spent per hour in wake, NREM sleep, and REM sleep were analyzed by restricted maximum likelihood (REML) analysis, with time (hr), treatment (drug or genotype), and the interaction between time and treatment as fixed factors and animal as random factor. Unlike the ANOVA, this test does not require that data are normally distributed and that groups have equal variance for the results to be valid. In addition, missing values can exist in the dataset. When either the main treatment effect or the interaction was significant (P < 0.05), Fisher least significant difference (LSD) post hoc test was run to identify during which hours there was a significant difference between the vehicle and treatment days. The REML analysis was run for the entire 12hr dark period for the 100 and 300 mg/kg doses and over 4h for the 25 mg/kg dose. F and P value for the treatment effect are reported in the results when significant at P < 0.05. When the treatment was not significant, but the interaction (treatment × h) was significant then this comparison is reported. Results from the LSD test are shown on the figures as *P < 0.05, **P < 0.01, ***P < 0.001.
Functional Analysis of Almorexant on Human, Rat, and Mouse Receptors
Chinese hamster ovary (CHO) or human embryonic kidney (HEK) cells expressing mouse, rat, or human OX1R or OX2R were grown in Dulbecco minimum essential medium (DMEM)/F12/10% fetal calf serum (FCS). For passaging, medium was removed from a 50-90% confluent large cell culture bottle, cells were washed with 13 ml phosphate buffered saline (Gibco 14190), the phosphate buffered saline was removed and 3 ml Trypsin (0.5 mg/ml) added, the bottle was kept for 3 min at 37°C, 17 ml medium was added, and 0.5-2 ml of cell suspension was transferred into a new bottle with 50 ml DMEM/F12/10% FCS.
Determination of orexin A-stimulated calcium accumulation was performed over 2 days using a fluorescent imaging plate reader (FLIPR384, Molecular Devices, Sunnyvale, CA). On day 1, cells expressing either OX1R or OX2R were seeded in black 384 well clear bottom plates at approximately 8,000 cells per well in 50 μl medium and incubated overnight at 37°C. On day 2, medium was discarded and cells loaded with 50 μl of loading buffer (1 mM Fluo-4 AM, Invitrogen F14202 (Life Technologies, Zug, Switzerland), dimethyl sulfoxide in working buffer). Cells were incubated for 60 min at 37°C and the medium discarded. Cells were washed with 100 μl working buffer (Hanks' balanced salt solution, 10 mM Hepes (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 200 mM CaCl2, 0.1 % bovine serum albumin, 2.5 mM probenecid, pH 7.4) to remove the excess Fluo-4 AM and 20 μl working buffer was added. Plates were incubated 10-15 minutes at room temperature. Then the assay plate was transferred to the Molecular Devices-FLIPR384 and 10 μl almorexant was injected at three times the final concentration. The baseline calcium signal was recorded for 10 sec, then compound was injected, and the calcium signal recorded every sec for 1 min, then every 2 sec 40 times. Plates were then incubated at room temperature for 30, 60, 120, or 240 min. Calcium signals were again measured as previously mentioned; this time orexin A (15 μl) was injected at three times the final concentration. For each experiment, full orexin A concentration response curves were generated on each plate: they serve to calculate the half maximal effective concentration (EC50) for that plate and to adapt the EC80 values in the subsequent experiments, which vary according to cell line and passage number.
The concentration response curves were analyzed according to the law of mass action, for both orexin A (EC50), and almorexant (half maximal inhibitory concentration, IC50) with slope factors and maximal/minimal effects; the antagonist data are transformed according to Cheng and Prusoff33 (KI = IC50/1+ (L/EC50), where L is the agonist concentration used in the assay and EC50 its concentration for half maximal activation) and the antagonist data finally expressed as Ki (nM) and pKi values (–log M). The potency ratios of almorexant for OX2R over OX1R are represented graphically versus incubation time (min).
RESULTS
Effects of Almorexant on Orexin-Induced Locomotor Activity
Pilot studies showed that ICV injections of 3 μg orexin A induced a robust increase in locomotor activity lasting approximately 75 min in C57BL/6 mice, similar to what has been reported.34 Lower doses were less effective and the effect began to plateau at approximately 3 μg (data not shown). Therefore, we used 3 μg orexin A for the following experiments and to analyze the time window 75 min after orexin A infusions. The 30 min before orexin A infusions were taken to analyze genotype or treatment effects on baseline locomotor activity. C57BL/6 mice (n = 10-13/group) were treated with vehicle, i.e., 0, 50, 100, or 200 mg/kg almorexant by mouth 30 min prior to administration of 3 μg orexin A ICV. As expected, orexin increased locomotor activity in the control animals pretreated with vehicle (Figure 1; F(1,21) = 4.40, P = 0.049). This effect of ICV orexin was also observed in the group of mice pretreated with 50 mg/kg almorexant (F(1,19) = 4.33, P = 0.05) but not in the mice that received 100 or 200 mg/kg almorexant (F(1,21) = 0.06, P = 0.81 and F(1,18) = 0.51, P = 0.48, respectively). Thus, almorexant dose-dependently blocked the increase in locomotor activity induced by ICV orexin. In addition, all almorexant doses robustly decreased baseline locomotor activity when compared with the baseline activity in the control mice pretreated with vehicle (factor pretreatment in ICV vehicle-injected animals: F > 4.97, P < 0.03).
Figure 1.
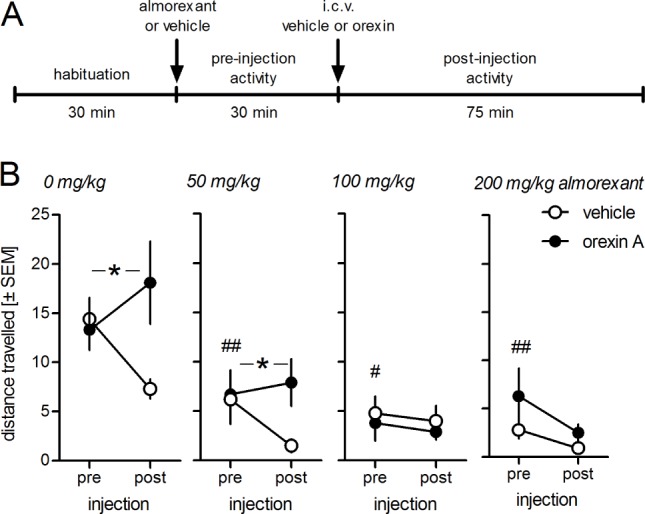
Almorexant blocks orexin-induced locomotion in C57BL/6 mice. A, Design of experiment. Each mouse was tested once and received either vehicle (0 mg/kg almorexant) or a given dose of almorexant prior to recording 30 min of baseline activity (N = 10-12/group). After recording baseline activity half the mice received 3 μg orexin A intracerebroventricular (icv) or an equal volume of vehicle and activity was recorded for a further 75 min. B, Data are expressed as means ± standard error of the mean (SEM) of the total distance traveled in the 30 min prior to icv injection of 3 μg orexin (pre) and for 75 min after icv injections (post). Almorexant at doses from 50 mg/kg by mouth significantly reduced baseline locomotor activity and abolished the stimulatory effect of orexin at 100 and 200 mg/kg. *P < 0.05 interaction icv injection × time, i.e. stimulatory effect of orexin; #P < 0.05, ##P < 0.01, baseline activity with almorexant versus baseline activity with vehicle pretreatment.
Orexin-Induced Locomotor Activity in WT, OX1R-Deficient, OX2R-Deficient, and OX1R/OX2R-Deficient Mice
Baseline locomotor activity was not affected in any of the receptor-deficient mice relative to the WT mice (Figure 2; ANOVA: F(3,30) = 0.74, P = 0.54). ICV injections of orexin A increased locomotion in WT and OX1R-deficient animals (F > 5.34, P ≤ 0.05), but had no effect in OX2R- and OX1R/OX2R-deficient mice (F < 0.11, P > 0.75). The apparently greater orexin-induced increase in locomotion in the OX1R deficient mice versus WT mice was not significant.
Figure 2.
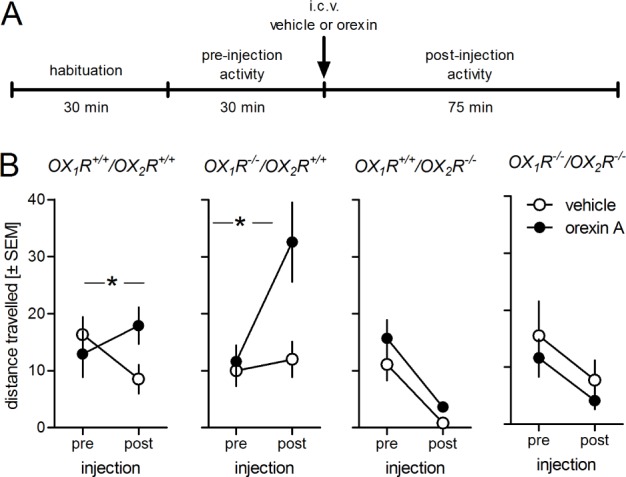
Orexin increases locomotor activity by activation of OX2R receptors. A, Design of experiment. Each mouse was tested only once (N = 7-9/group). Following a 30-min habituation, baseline activity was recorded for 30 min. Each mouse then received an intracerebroventricular (icv) orexin (3 μg) injection or an equal volume of vehicle. B, Data are expressed as mean ± standard error of the mean (SEM) of distance traveled in the 30 min prior to icv injections (pre) and in 75 min after icv injections (post). Orexin increased locomotion in wild-type mice (OX1R+/+/OX2R+/+) and in mice deficient for OX1R but had no effect on mice deficient for OX2R or lacking both orexin receptors. Baseline activity was not different in the different strains. *P < 0.05 interaction icv injection × time, i.e., stimulatory effect of orexin.
Effects of Almorexant on Sleep in Normal C57BL/6 Mice
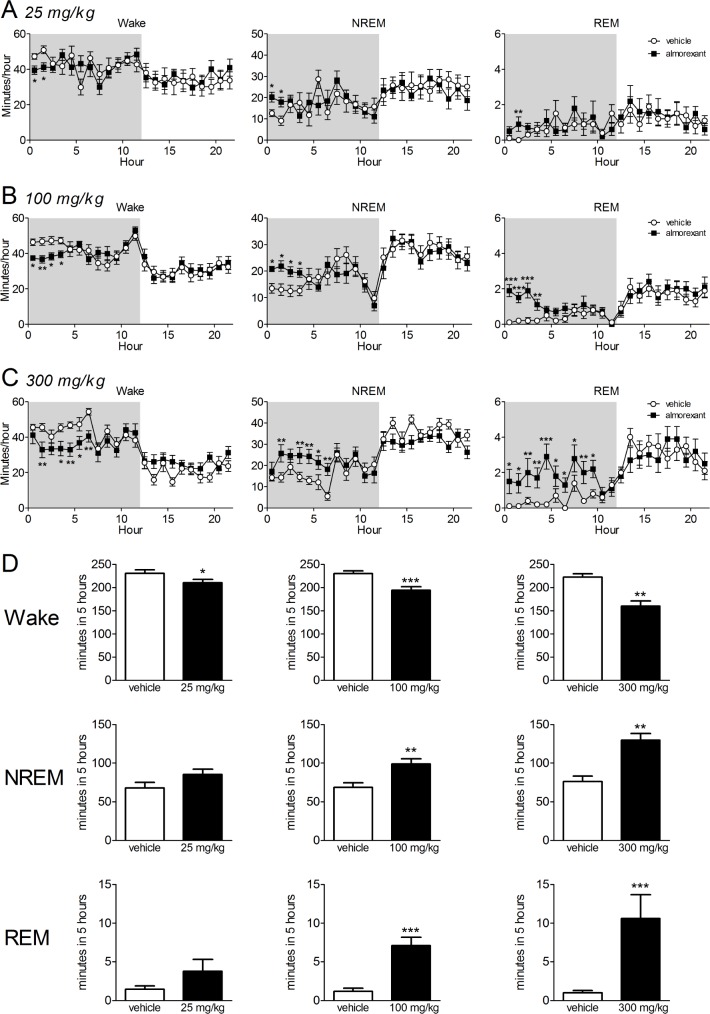
Almorexant dose-dependently reduced the time spent awake and increased the time spent in NREM and REM sleep when applied before lights off compared with the previous day when vehicle was dosed just before lights off (Figure 3). Statistical analyses confirmed significant effects of almorexant on wake at all doses tested (25 mg/kg: F(3,49) = 2.91, P = 0.044, n = 8; 100 mg/kg: F(11,276) = 2.84, P = 0.002, n = 13; 300 mg/kg: F(1,207) = 25.5, P < 0.001; n = 10). Post hoc tests revealed almorexant reduced wake for 2h at 25 mg/kg, for 4h at 100 mg/kg and for the 2nd to 7thh at 300 mg/kg (Figure 3A-C). Likewise, almorexant increased both NREM sleep (25 mg/kg: F(3,49) = 2.86, P = 0.046; 100 mg/kg: F(11,276) = 2.61, P = 0.004; 300 mg/kg: F(1,207) = 18.1, P < 0.001) and REM sleep (25 mg/kg: F(3,49) = 6.79, P = 0.012; 100 mg/kg: F(1,276) = 37.1, P < 0.001; 300 mg/kg: F(1,207) = 60.0, P < 0.001). We also quantified the amount of time spent in each state during the first 5h after lights off (Figure 3D). Using this measure, clear dose-dependent decreases in wake and increases in both NREM and REM sleep were revealed. Interestingly, the effect of 300 mg/kg almorexant on REM sleep outlasted the reduction in wake and the increase in NREM sleep by 3h (Figure 3C). This prolonged REM sleep-inducing effect was not seen at the lower doses tested.
Figure 3.
Almorexant dose-dependently reduces wake and induces sleep at the beginning of the dark (active) phase in the normal C57BL/6 mice. A-C, Almorexant at the doses indicated was given 5-10 min before the recording started (t = 0, lights off). Shaded region indicates dark period. Data are expressed as means ± standard error of the mean (SEM) of total min in the given vigilance phases in eachh after treatment. *P < 0.05, **P < 0.01, ***P < 0.001 Fisher least significant difference pair-wise comparisons vehicle versus almorexant. D, Quantification of the cumulative time spent in each stage during the first 5h on the day of vehicle treatment (clear bars) and the day of almorexant treatment (black bars) at the indicated doses. *P < 0.05, **P < 0.01, ***P < 0.001 paired t-test.
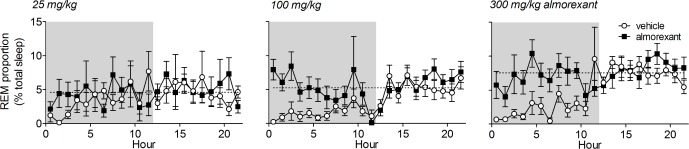
As we observed that almorexant increased both NREM and REM sleep in mice, we wished to examine whether the balance between REM sleep and NREM sleep after almorexant treatment was similar to that normally occurring in the mice. The proportion of REM sleep in the total sleep time during the dark phase was increased by almorexant (Figure 4). This was expected from the comparison of the raw data in Figure 3. Because REM sleep is more likely to occur when preceded by prolonged NREM sleep, we considered that the normal REM/NREM sleep balance would be better reflected by the REM sleep percentage of total sleep time during the light phase on the vehicle treatment day, when the mice slept about the same amount as they did with almorexant, but without drug influence. At all doses of almorexant tested, the proportion of REM sleep remained within that seen during the light phase on the vehicle day (Figure 4). Because REM sleep is under circadian control, the ideal comparison would be to hold both total sleep time and time of day constant while applying almorexant. Unfortunately the lack of effect of almorexant when dosed during the light phase in rodents precludes this.27
Figure 4.
Almorexant increases the proportion of total sleep time spent in rapid eye movement (REM) sleep during the dark phase but this remains within the proportions seen during normal sleep in the light phase on the vehicle day. Almorexant at the indicated doses was given by mouth 5-10 min before the start of the recording (t = 0). Shaded area indicates dark period. Dotted line indicates mean REM sleep proportion during the light phase on the vehicle day. REM sleep proportion = 100 × time in REM sleep/(time in REM sleep + time in nonrapid eye movement [NREM] sleep) calculated perh.
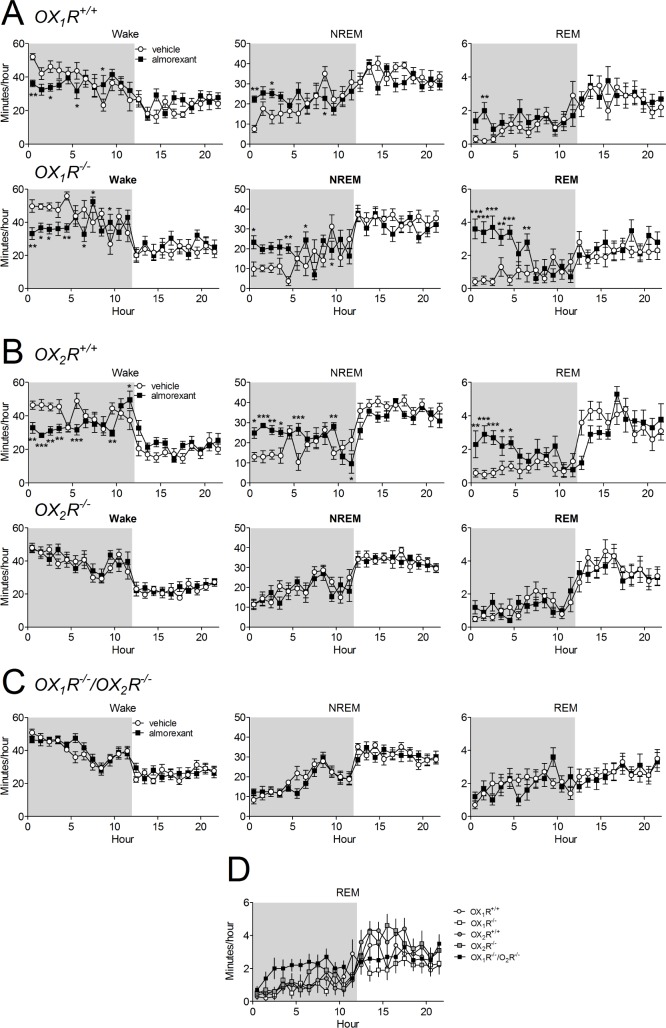
Effects of Almorexant on Sleep are Mediated by OX2Rs
As 100 mg/kg almorexant clearly induced both NREM and REM sleep in normal C57BL/6 mice, we chose to use this dose in mice deficient in orexin receptors to determine whether antagonism of OX1R, OX2R, or both receptors is necessary for sleep induction. Almorexant induced sleep in both OX1R-/- and OX1R+/+ mice (Figure 5A). Compared with the vehicle day, almorexant increased NREM sleep in both WT and KO mice (OX1R+/+: F(11,207) = 2.07, P = 0.024; OX1R-/-: F(1,207) = 9.67, P = 0.002; n = 10), increased REM sleep (OX1R+/+: F(1,207) = 4.11, P = 0.044, OX1R-/-: F(1,207) = 57.0, P < 0.001) and reduced wake in both groups (OX1R+/+: F(1,207) = 2.06, P < 0.05; OX1R-/-: F(1,207) = 13.5, P < 0.001). Inhibition of OX1Rs is therefore not necessary for sleep induction.
Figure 5.
Almorexant induces sleep by blocking OX2R receptors. Almorexant (100 mg/kg) was given 5-10 min before the start of the recording (t = 0). Shaded region indicates dark period. The effects of almorexant on sleep/wake time were compared to the vehicle in OX1R-deficient mice (A, lower panels) and their wild-type littermates (A, upper panels), in OX2R-deficient mice (B, lower panels) and their wild-type littermates (B, upper panels) and in OX1R/OX2R-deficient mice (C). D, The amount of rapid eye movement sleep on the vehicle days for each group of mice are plotted together. Data are expressed as means ± standard error of the mean (SEM) of total min in the given vigilance phase in each hour. *P < 0.05, **P < 0.01, ***P < 0.001 Fisher least significant difference pair-wise comparisons vehicle versus almorexant.
In contrast, almorexant did not change time spent in sleep or wake in OX2R-/- mice (Figure 5B; wake: F(1,230) = 0.57, P = 0.45; NREM sleep: F(1,230) = 0.68, P = 0.41; REM sleep: F(1,230) = 0.024, P = 0.88; n = 11). In the WT littermates, almorexant induced sleep and reduced wake similar to what was seen in C57BL/6 mice (Figure 5B, Figure 3B, wake: F(1,184) = 20.0, P < 0.001; NREM sleep: F(1,184) = 16.7, P < 0.001; REM sleep: F(1,184) = 31.9, P < 0.001; n = 9). Thus, inhibition of OX2Rs is sufficient for sleep induction by almorexant.
To determine if the sleep-promoting effects of almorexant are mediated solely by inhibition of orexin receptors or if there may be an off-target effect promoting sleep, we tested the effect of almorexant in mice lacking both, OX1R and OX2R (Figure 5C). There was no effect of almorexant on the amount of wake (F(1,230) = 1.01, P = 0.32, n = 11), NREM sleep (F(1,230) = 1.08, P = 0.30) or REM sleep (F(1,230) = 0.04, P = 0.80) in mice lacking both receptors, demonstrating that almorexant induces sleep via the known orexin receptors.
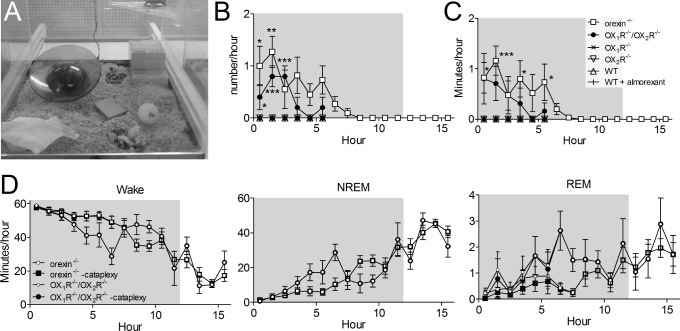
Almorexant Does Not Induce Cataplexy in Mice
Cataplexy with narcolepsy has been reported to occur in mice lacking orexin peptides and OX1R/OX2R deficient mice but is absent in OX1R and OX2R deficient mice, which show sleep attacks that differ from the cataplexy seen in the peptide KO mice.1,17,19 Episodes of cataplexy may be confused with REM sleep or quiet wake (wake without movement) when scored on an epoch-by-epoch basis, both when examined by a trained manual scorer (when the cataplexy episode is of short duration) or by the scoring software. Thus, in our analysis in Figure 5 episodes of cataplexy would have been scored as REM sleep or wake. Indeed, when we compared the amount of time spent in REM sleep in the different KO mice and the WT littermates, only the OX1R/OX2R deficient mice appeared to have more REM sleep during the dark phase in the vehicle condition, possibly reflecting some cataplexy in these mice (Figure 5D).
We first examined cataplexy in mice lacking orexin peptides. These cataplexy-prone mice were seen to have extremely few cataplectic events when they were well adapted to the recording boxes (data not shown). Because novelty, exercise, and palatable food have all been suggested to increase the frequency of cataplectic events,18,35,36 we recorded the video, EEG/EMG and activity beginning at lights off without a long habituation period in enriched recording boxes (Figure 6A). Cataplexy was detected only in mice lacking orexin peptides or lacking both orexin receptors (Figure 6B and C, orexin-/- versus WT F(1,75) = 6.5, P = 0.013, OX1R-/-/OX2R-/- F(1,45) = 10.7, P = 0.002). Because no cataplexy was detected in the mice lacking orexin peptides more than 8h after lights off, we restricted further analysis in the other mice to the 6h immediately after lights off. As previously reported, we observed no cataplexy in WT mice, in mice lacking OX1R or OX2R, nor in WT mice treated with almorexant (Figure 6B and C). In the mice lacking orexin receptors, the average duration of cataplexy was less than 1 min/hr even in these conditions designed specifically to increase cataplexy (Figure 6C). The scoring software classified 60% of the cataplexy as REM sleep, 39% as wake and 1% as NREM sleep. Figure 6D shows the effect of correcting the sleep scoring for cataplexy in the orexin-/- mice and the OX1R/OX2R deficient mice for the cataplexy. Note that the amount of wake at the beginning of the dark phase is extremely high due to the stimulatory effect of the novel environment and lack of habituation. Even in these conditions designed to stimulate cataplexy there is a rather small effect on the sleep scoring even on the maximally affected REM state. On average only about 20 sec/hr of the time classified as REM sleep was in fact cataplexy. Thus, we are confident that cataplectic episodes did not significantly distort sleep/wake scoring in our orexin/almorexant experiments.
Figure 6.
Cataplexy occurred only in orexin-/- mice and mice lacking both receptors. A, The recording chamber with addition of a running wheel, ping-pong ball and fruit loops to promote cataplexy. B, The number of cataplexy events per hour decreased with time in orexin-/- (n = 11) and OX1R-/-/OX2R-/- (n = 8). No cataplexy was detected in wild-type (WT) mice (n = 7), WT mice treated with 300 mg/kg almorexant 5-10 min before lights off (n = 7), OX1R-/- (n = 8) or OX2R-/- (n = 8) mice. C, Duration of cataplexy. D, Sleep scoring with and without cataplexy removed. Shaded region is lights off. Values are mean ± standard error of the mean (SEM). *P < 0.05, **P < 0.01, ***P < 0.001 Fisher least significant difference pair-wise comparisons versus WT.
Selectivity of Almorexant for OX2R Increases with Incubation Time
It has been reported that almorexant binds almost irreversibly to the human OX2R and dissociates rapidly from the OX1R, suggesting that it may function as an OX2R preferring antagonist in vivo.37 We decided to test whether the differences in binding kinetics of almorexant at the two receptors is reflected by its apparent potency in functional assays. Calcium accumulation in response to orexin A was estimated using FLIPR in intact cells expressing recombinant human, rat, and mouse OX1R and OX2R after incubation with almorexant for various time intervals (30-240 min). There was no change in calcium signal when almorexant was applied alone, indicating that almorexant has no apparent agonist/inverse agonist intrinsic activities. The apparent antagonist potency of almorexant at human, rat and mouse OX1Rs was constant irrespective of incubation time (Table 1). In contrast, at OX2R the apparent potency increased with increasing time of incubation of almorexant (increasing pKi/decreasing Ki). When incubated for 30 min, almorexant was apparently a nonselective antagonist showing singlefold to threefold selectivity for OX2R over OX1R in mouse (Ki mOX1R/Ki mOX2R = 1.0), rat (Ki rOX1R/Ki rOX2R = 2.2) and human (Ki hOX1R/Ki hOX2R = 3.1) but when incubated for increasing times, up to 240 min, the selectivity for OX2R increased to nine to 25 times over OX1R (Figure 7).
Table 1.
Apparent antagonist potency of almorexant at mouse, rat, and human OX2R increases with increasing incubation times whereas apparent potency at OX1R remains constanta

Figure 7.
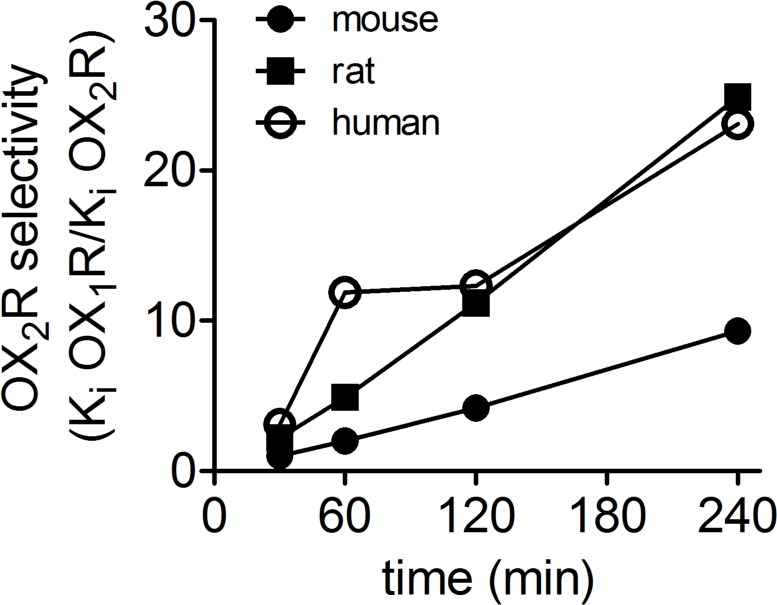
Functional selectivity of almorexant for human, rat and mouse OX2R over OX1R increases with time of incubation in vitro. The potency ratios of almorexant for OX2R over OX1R were calculated based on Ki values and represented graphically wrt incubation time (min) for the three species.
DISCUSSION
Dual OX1R/OX2R antagonists are being developed as new approaches for the treatment of insomnia.24,25,27 In the current studies, we found that the dual OX1R/OX2R antagonist almorexant dose-dependently blocked the locomotion-inducing effects of ICV orexin, reduced active wake, and induced REM sleep and NREM sleep in C57BL/6J mice. Almorexant was ineffective in mice lacking both OX1R and OX2R, suggesting that inhibition of the two known orexin receptors is sufficient to explain the sleep-promoting effects of almorexant. Almorexant failed to induce sleep in mice lacking OX2R, whereas it induced sleep in mice lacking OX1R, confirming that antagonism of OX2R is sufficient for sleep induction.28,29 The cataplectic phenotype of mice lacking orexin or both orexin receptors was confirmed in our study.1,17 In the same conditions we also confirmed that there was no cataplexy induced by almorexant.27
When interpreting our data from KO mice, it is important to keep in mind that compensatory mechanisms may be activated during development, potentially confounding the interpretation of the results. We made several attempts using autoradiography to quantify orexin receptor density in brain slices; however, due to the low abundance of the receptors and lack of sufficiently potent ligands, this has not been successful to date. At the messenger ribonucleic acid level, no difference was found between orexin receptor KO and WT mice,38 arguing against dramatic upregulation of the nondeleted orexin receptor gene in the KO mice. Whereas receptor density differences between individuals may alter behavioral effects of agonists in vivo, apparent antagonist potency is expected to be much less affected.
Our locomotor activity experiments in orexin receptor-deficient mice show that baseline activity is not affected by deficiency of only one or both orexin receptors. However, we observed that the stimulatory effect of ICV orexin injections on locomotor activity 39–42 is OX2R-mediated, because OX1R deficiency did not prevent the orexin-induced increase in locomotion. This supports published rat data demonstrating that orexin-induced locomotion cannot be blocked by coadministration of an OX1R-specific antagonist but can be mimicked by an OX2R-specific agonist.42Almorexant dose-dependently blocked orexin-induced locomotion, as well as baseline locomotor activity. Interestingly, the lowest almorexant dose (50 mg/kg) reduced baseline locomotor activity without preventing the stimulatory effect of ICV orexin whereas higher doses (100 and 200 mg/kg) were able to reduce baseline and orexin-induced locomotion. In rats and dogs, 30 mg/kg was the minimal effective dose of almorexant to reduce baseline locomotor activity.27 Based on our data in orexin receptor-deficient mice, this effect of almorexant on orexin-induced locomotion is very likely OX2R-mediated.
Although almorexant is a rather balanced OX1R/OX2R antagonist, kinetic studies demonstrate that almorexant dissociates very slowly from the human OX2R receptor but has fast and reversible kinetics at the human OX1R.37 Using a functional assay in intact cells expressing human, rat, or mouse receptors, we demonstrated that this difference in binding kinetics results in an increase of almorexant potency at OX2R with time, whereas the potency at OX1R remained constant. Thus, almorexant acts as a pseudoirreversible or very slowly equilibrating antagonist at human, rat, and mouse OX2R and a fast equilibrating antagonist at OX1Rs. Almorexant may therefore behave in vivo as an OX2R preferring antagonist rather than as a nonselective dual orexin receptor antagonist.
Orexin increases wakefulness and suppresses both NREM and REM sleep.40,43 Administration of orexin A in orexin-deficient mice and dogs also inhibits narcoleptic and cataplectic episodes.44,45 Selective activation of orexin neurons promotes wakefulness46,47 and selective inhibition promotes sleep.47 Together, these data highlight a critical role for orexin in the maintenance of wakefulness. With respect to total sleep duration, we observed no large differences between WT mice and mice with a deficiency in OX1R or OX2R or both orexin receptors under control conditions (vehicle applications). As already published for rats, dogs, and humans,27 we observed robust and dose-dependent sleep-promoting effects of almorexant and deficiency of OX2R was sufficient to block these effects. This is in line with previous studies highlighting a principal role for OX2R in sleep. For example, the OX1R antagonist GSK1059865 alone was devoid of effect on sleep, whereas the selective OX2R antagonist JNJ1037049 produced sleep in rats under conditions where target engagement was demonstrated for both compounds using functional magnetic resonance imaging.28 In addition, ICV administration of an OX2R-selective agonist, [Ala11]orexin B, promotes wakefulness and suppresses NREM sleep and REM sleep in rats.48 Mieda et al.,38 studying the effects of orexin A in WT and orexin receptor-deficient mice, reported that activation of OX2R promoted wakefulness and suppressed NREM sleep whereas OX1R activation was less effective. Both OX1R and OX2R appeared to mediate the orexin A induced suppression of REM sleep by a similar degree. Interestingly, the authors suggest that OX1Rs directly suppress REM sleep, whereas the effect mediated by OX2Rs is indirect. Thus, the normal regulation of wakefulness/NREM sleep transitions appears to depend critically on OX2R, indicating that OX2R is the main player in sleep/wake control. Combined loss of OX1R and OX2R signaling leads, however, to a more severe phenotype including sleep onset REM periods and cataplexy.19,49
In conclusion, we have demonstrated that the orexin system modulates locomotion and sleep primarily via OX2R with only a minor role for OX1R. Importantly, we provide direct evidence that almorexant directly antagonizes the in vivo actions of orexin and that antagonism of OX2R is sufficient to induce sleep in mice. In addition, we can conclude that no as-yet unidentified receptors for orexin play a major role in these behaviors as there was no effect of either ICV orexin or almorexant in mice lacking the two known orexin receptors.
DISCLOSURE STATEMENT
This study was performed at and funded by the Novartis Institutes for BioMedical Research, the research arm of Novartis AG. Ms. Mang received a student stipend from Novartis while working on this study for her Master's degree. All other authors are/were employed by NIBR, received their salaries from and potentially own stock in the company.
ACKNOWLEDGMENTS
Sabine Kauffmann performed the genotyping of the OX1R and OX2R mice. Claudia Betschart and Thomas Oertner gave valuable comments on the manuscript. The authors acknowledge the support of Jürgen Wagner, Kevin McAllister, and Graeme Bilbe.
REFERENCES
- 1.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 2.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 3.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 4.de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–20. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–70. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 7.Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619–31. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–98. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Date Y, Ueta Y, Yamashita H, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–53. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–60. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- 12.Ammoun S, Holmqvist T, Shariatmadari R, et al. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J Pharmacol Exp Ther. 2003;305:507–14. doi: 10.1124/jpet.102.048025. [DOI] [PubMed] [Google Scholar]
- 13.Marcus JN, Aschkenasi CJ, Lee CE, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 14.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 15.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 16.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalogiannis M, Grupke SL, Potter PE, et al. Narcoleptic orexin receptor knockout mice express enhanced cholinergic properties in laterodorsal tegmental neurons. Eur J Neurosci. 2010;32:130–42. doi: 10.1111/j.1460-9568.2010.07259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morawska M, Buchi M, Fendt M. Narcoleptic episodes in orexin-deficient mice are increased by both attractive and aversive odors. Behav Brain Res. 2011;222:397–400. doi: 10.1016/j.bbr.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Willie JT, Chemelli RM, Sinton CM, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–30. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 20.Mochizuki T, Arrigoni E, Marcus JN, et al. Orexin receptor 2 expression in the posterior hypothalamus rescues sleepiness in narcoleptic mice. Proc Natl Acad Sci U S A. 2011;108:4471–6. doi: 10.1073/pnas.1012456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishino S. The hypocretin/orexin receptor: therapeutic prospective in sleep disorders. Expert Opin Investig Drugs. 2007;16:1785–97. doi: 10.1517/13543784.16.11.1785. [DOI] [PubMed] [Google Scholar]
- 22.Zeitzer JM, Nishino S, Mignot E. The neurobiology of hypocretins (orexins), narcolepsy and related therapeutic interventions. Trends Pharmacol Sci. 2006;27:368–74. doi: 10.1016/j.tips.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Bettica P, Squassante L, Groeger JA, Gennery B, Winsky-Sommerer R, Dijk DJ. Differential effects of a dual orexin receptor antagonist (SB-649868) and zolpidem on sleep initiation and consolidation, SWS, REM sleep, and EEG power spectra in a model of situational insomnia. Neuropsychopharmacology. 2012;37:1224–33. doi: 10.1038/npp.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant: a novel dual orexin receptor antagonist. J Neurogenet. 2011;25:52–61. doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- 25.Bettica PU, Lichtenfeld U, Squassante L, et al. The orexin antagonist Sb-649868 promotes and maintains sleep in healthy volunteers and in patients with primary insomnia. Sleep. 2009;32:774. [Google Scholar]
- 26.Hoever P, Dorffner G, Benes H, et al. Orexin receptor antagonism, a new sleep-enabling paradigm: a proof-of-concept clinical trial. Clin Pharmacol Ther. 2012;91:975–85. doi: 10.1038/clpt.2011.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brisbare-Roch C, Dingemanse J, Koberstein R, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150–5. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- 28.Gozzi A, Turrini G, Piccoli L, et al. Plos One. 2011. Functional magnetic resonance imaging reveals different neural substrates for the effects of orexin-1 and orexin-2 receptor antagonists; p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dugovic C, Shelton JE, Aluisio LE, et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330:142–51. doi: 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: 2nd Edition 2001 Academic Press; [Google Scholar]
- 31.Pick J, Chen Y, Moore JT, et al. Rapid eye movement sleep debt accrues in mice exposed to volatile anesthetics. Anesthesiology. 2011;115:702–12. doi: 10.1097/ALN.0b013e31822ddd72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scammell TE, Willie JT, Guilleminault C, Siegel JM. A consensus definition of cataplexy in mouse models of narcolepsy. Sleep. 2009;32:111–6. [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 34.Jones DN, Gartlon J, Parker F, et al. Effects of centrally administered orexin-B and orexin-A: a role for orexin-1 receptors in orexin-B-induced hyperactivity. Psychopharmacology (Berl) 2001;153:210–8. doi: 10.1007/s002130000551. [DOI] [PubMed] [Google Scholar]
- 35.Clark EL, Baumann CR, Cano G, Scammell TE, Mochizuki T. Feeding-elicited cataplexy in orexin knockout mice. Neuroscience. 2009;161:970–7. doi: 10.1016/j.neuroscience.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espana RA, McCormack SL, Mochizuki T, Scammell TE. Running promotes wakefulness and increases cataplexy in orexin knockout mice. Sleep. 2007;30:1417–25. doi: 10.1093/sleep/30.11.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malherbe P, Borroni E, Pinard E, Wettstein JG, Knoflach F. Biochemical and electrophysiological characterization of almorexant, a dual orexin 1 receptor (OX1)/orexin 2 receptor (OX2) antagonist: comparison with selective OX1 and OX2 antagonists. Mol Pharmacol. 2009;76:618–31. doi: 10.1124/mol.109.055152. [DOI] [PubMed] [Google Scholar]
- 38.Mieda M, Hasegawa E, Kisanuki Y, Sinton CM, Yanagisawa M, Sakurai T. Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J Neurosci. 2011;31:6518–26. doi: 10.1523/JNEUROSCI.6506-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorpe AJ, Cleary JP, Levine AS, Kotz CM. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology (Berl) 2005;182:75–83. doi: 10.1007/s00213-005-0040-5. [DOI] [PubMed] [Google Scholar]
- 40.Hagan JJ, Leslie RA, Patel S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–6. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura T, Uramura K, Nambu T, et al. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873:181–7. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- 42.Samson WK, Bagley SL, Ferguson AV, White MM. Orexin receptor subtype activation and locomotor behaviour in the rat. Acta Physiologica. 2010;198:313–24. doi: 10.1111/j.1748-1716.2009.02056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piper DC, Upton N, Smith MI, Hunter AJ. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur J Neurosci. 2000;12:726–30. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 44.Mieda M, Willie JT, Hara J, Sinton CM, Sakurai T, Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci U S A. 2004;101:4649–54. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujiki N, Yoshida Y, Ripley B, Mignot E, Nishino S. Effects of IV and ICV hypocretin-1 (orexin A) in hypocretin receptor-2 gene mutated narcoleptic dogs and IV hypocretin-1 replacement therapy in a hypocretin-ligand-deficient narcoleptic dog. Sleep. 2003;26:953–9. doi: 10.1093/sleep/26.8.953. [DOI] [PubMed] [Google Scholar]
- 46.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–4. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS One. 2011;6:e20360. doi: 10.1371/journal.pone.0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akanmu MA, Honda K. Selective stimulation of orexin receptor type 2 promotes wakefulness in freely behaving rats. Brain Res. 2005;1048:138–45. doi: 10.1016/j.brainres.2005.04.064. [DOI] [PubMed] [Google Scholar]
- 49.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–81. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]