Abstract
Study Objective:
Sleep disorders are frequent non-motor symptoms in Parkinson disease (PD), probably due to multifactorial pathogeneses including disease progression, dopaminergic drugs, or concomitant illness. In recent years, the pedunculopontine tegmental (PPTg) nucleus has been considered a surgical target for deep brain stimulation (DBS) in advanced PD patients. As it is involved in controlling the sleep-wake cycle, we investigated the long-lasting effects of PPTg-DBS on the sleep of five PD patients implanted in both the PPTg and the subthalamic nucleus (STN) by rating two subjective clinical scales for sleep: the Parkinson's Disease Sleep Scale (PDSS), and the Epworth Sleepiness Scale (ESS).
Study Design:
Sleep scales were administered a week before surgery (T0), three months after DBS (T1), and one year later (T2). In this study, STN-DBS was kept constantly in ON, and three different patterns of PPTg-DBS were investigated: STN-ON (PPTg switched off); PPTg-ON (PPTg stimulated 24 h/day); PPTg-cycle (PPTg stimulated only at night).
Results:
In post-surgery follow-up, PD patients reported a marked improvement of sleep quality in all DBS conditions. In particular, stimulation of the PPTg nucleus produced not only a remarkable long-term improvement of nighttime sleep, but unlike STN-DBS, also produced significant amelioration of daytime sleepiness.
Conclusion:
Our study suggests that PPTg-DBS plays an important role in reorganizing regular sleep in PD patients.
Citation:
Peppe A; Pierantozzi M; Baiamonte V; Moschella V; Caltagirone C; Stanzione P; Stefani A. Deep brain stimulation of pedunculopontine tegmental nucleus: role in sleep modulation in advanced Parkinson disease patients—one-year follow-up. SLEEP 2012;35(12):1637-1642.
Keywords: PPTg, STN DBS, Parkinson disease, sleep
INTRODUCTION
In humans, the sleep cycle is mediated by various anatomical structures of the brainstem. In particular, the pedunculopontine tegmental nucleus (PPTg) and surrounding areas in the brainstem play a crucial role in modulating wakefulness and REM sleep.1–4 Sleep disorders are frequent non-motor symptoms in Parkinson disease (PD).5 In PD patients, sleep may be affected by several factors: the degenerative process, motor symptoms at night, neuropsychiatric or cognitive symptoms, and antiparkinsonian therapy. Finally, concomitant illnesses including breathing disorders during sleep, physiological aging, or personal habits may induce sleep deterioration.6
Besides, its role in sleep regulation, PPTg is also part of the mesencephalic locomotor region, which is involved in locomotion and postural control.4,7–9 Two decades of experience with deep brain stimulation (DBS) have provided an efficacious co-therapy in advanced PD patients with long-term effects on motor symptoms without remarkable postoperative clinical side effects.10 Indeed, it has been reported that bilateral internal globus pallidus (GPi) as well as nucleus subthalamicus (STN) DBS lead to relief of hypokinetic/rigid parkinsonian symptoms.11–13 These clinical effects are likely due to the reduction of the hyperactive inhibitory basal ganglia output on the thalamo-cortical pathway. The resulting increase in thalamo-cortical input might in turn reduce improvement of SMA activity. More recently, the pedunculopontine nucleus (PPN) has been proposed as a potential target for the treatment of axial symptoms in PD.14 Mazzone and colleagues15 and Plaha and Gill16 have pioneered research in this area, leading to PPTg-DBS therapy. Results on the first series of patients were published in 2007.17 As all PD patients investigated in the present study were surgically treated at the A. Alesini C.T.O. Hospital in Rome, we refer to our neurosurgical target is PPTg, coherently with Mazzone and colleagues.18
The aim of our study was to evaluate the short- and long-term effects of PPTg-DBS on sleep in advanced PD patients who had undergone bilateral subthalamic nucleus (STN) and PPTg-DBS using subjective clinical sleep scales. In particular, the study investigated the possible additional benefit of PPTg-DBS either continuously or cyclically, together with 185 Hz STN-DBS.
METHODS
Patients
Five patients with severe PD (mean age 62.8 ± 1.9 years; disease duration 11.8 ± 3.0 years; levodopa therapy duration 10.2 ± 3.8 years) were studied before and after bilateral STN and PPTg-DBS at the IRCCS Santa Lucia Foundation in Rome. Surgery had had been performed between 2005 and 2007 at the A. Alesini C.T.O. Hospital in Rome. For surgical procedures, see Stefani et al.17; 4 of the 5 were the same patients whose clinical data had been published in 2007.17
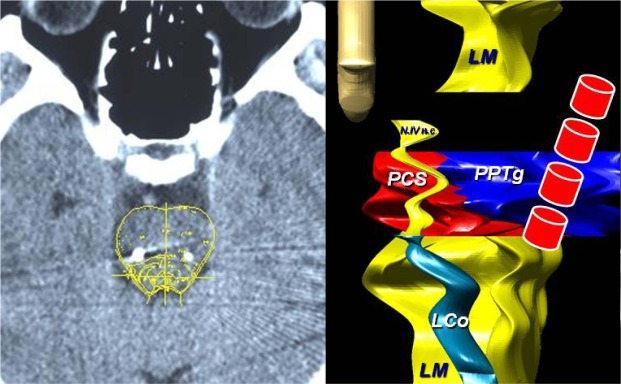
Before surgery, all patients were treated with levodopa and dopamine agonists (DA) at a mean dose of 584.4 ± 152.6 mg/day (considering DA equivalents); after surgery, antiparkinsonian therapy was reduced by 37% (mean dose 368.2 ± 56.4 mg/day). Throughout the study, PD patients' pharmacological treatment was kept constant. Their global cognitive functions were evaluated using the Mini-Mental State Examination (MMSE). Before surgery MMSE scores were all > 26/30; depression was excluded using the self-report Beck Depression Inventory (score < 10). All patients gave written informed consent to participate in the study, and the local ethics committee approved the procedures (CE/FARM 44). After surgery, cerebral TC was performed to verify electrode position (Figure 1).
Figure 1.
On the left TC scan, coronal view in one patient, showing the bilateral implantation of PTTg. On the right, Poster-anterior view of the planning system of the brainstem. The color diagram on the right side highlights the target area (PTTg) and the closest structures. LM, Median Lemniscus; L Co, Locus Ceruleus; PCS, Pedunculus Cerebellaris Superior; N. IV n.c., Nucleus IV cranial nerve. In the front line, the four electrode contacts (the 4 red cylindrical items.)
Study Protocol
Sleep scales, motor evaluations, and MMSE were rated a week before surgery (T0), 3 months after surgery (T1), and at 1-year follow-up (T2). In the 3 months between T0 and T1, all patients were on PPTg-ON and STN-ON. Throughout the study, STN-DBS was kept continuously ON, whereas 3 different patterns of PPTg-DBS were investigated: STN-ON, PPTg-ON, and PPTg-cycle. In STN-ON, only STN was ON; in PPTg-ON, the PPTg was stimulated 24 h/d; and in PPTg-cycle, PPTg was switched ON only at night (12 h: from 20:00 to 08:00). Each PPTg-DBS condition was maintained for 2 weeks to assess sleep and clinical state; the condition was then randomly changed. Patients and investigators (V.M., V.B.), who rated the clinical and sleep scales, were blind to type of stimulation and study design.
When T1 evaluations were completed, STN-DBS and PPTg-DBS were kept constant cyclically until the T2 follow-up. At that time, new motor and sleep evaluation was made using all stimulus modalities. The flowchart summarizes the study design (Figure 2).
Figure 2.
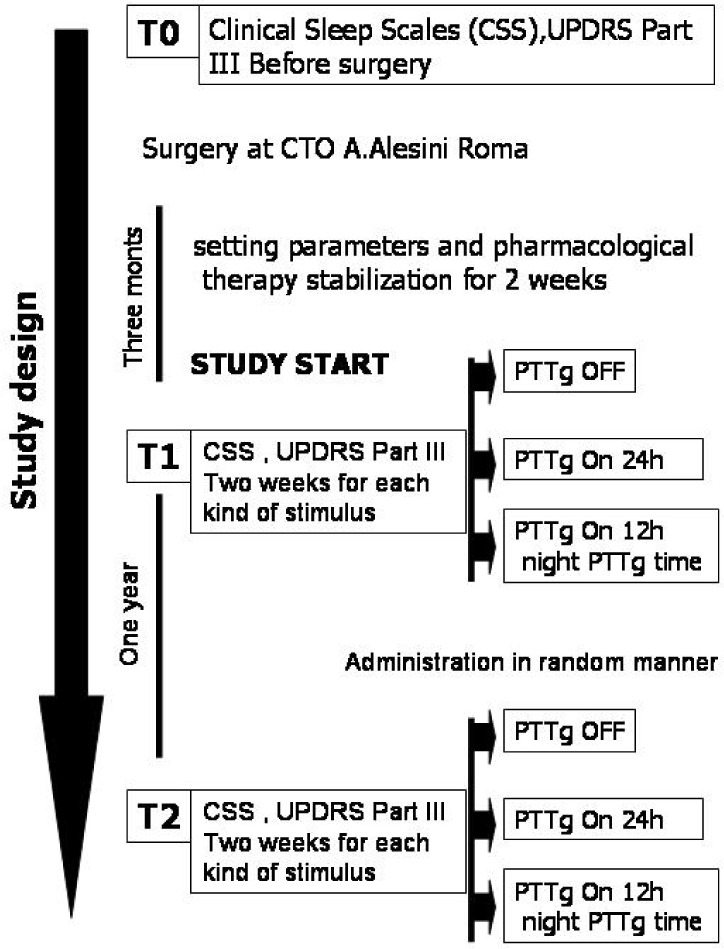
Flowchart of study design.
For STN, the stimulus parameters were the following: frequency 185 Hz, width 90 μsec, amplitude 2.5 V (range: 2.1-2.9V); for PPTg: frequency 25 Hz, width 60 μsec, amplitude 2.2 V (range: 1.8-2.2); contacts used: STN monopolar, PPTg lowest bipolar.
Two different sleep evaluation scales were used to investigate the PD patients' sleep-wake cycle: the Epworth Sleepiness Scale (ESS), an 8-item self-report questionnaire that assesses symptoms of diurnal sleepiness (scores range from 0 to 24; a score > 10 is considered pathological)19 and the Parkinson's Disease Sleep Scale (PDSS),20 a specific and comprehensive pragmatic clinical tool designed to investigate the multifactorial nature of sleep disturbances in PD. The PDSS is a 15-item visual analogue scale; scores range from zero (worst symptom condition) to 10 (symptom-free condition) for each item. The PDSS combines various sub-items that quantify specific aspects of sleep, including overall quality of nighttime sleep (item 1), sleep onset and sleep maintenance insomnia (items 2 and 3), nocturnal restlessness (items 4 and 5), distressing dreams (item 6), nocturnal psychosis (item 7), nocturia (items 8 and 9), nocturnal motor and sensory-motor symptoms (items 10-13), unsatisfactory sleep refreshment (item 14), and daytime dozing (item 15).
Clinical evaluations were made using the Unified Parkinson's Disease Rating Scale (UPDRS)-III.21
Friedman's nonparametric test was used for the statistical evaluation; if statistical significance was reached, the Wilcoxon matched-pairs test was performed at each time (T0 vs T1; T1 vs T2) and in each DBS condition (STN-ON vs PPTg-ON vs PPTg-cycle) (significance level considered: P < 0.05). The statistical analysis was performed with SPSS 15.0 for Windows (SPSS Inc, Chicago, Ill, USA).
RESULTS
Evaluation of Sleep-Wake Cycle
T0
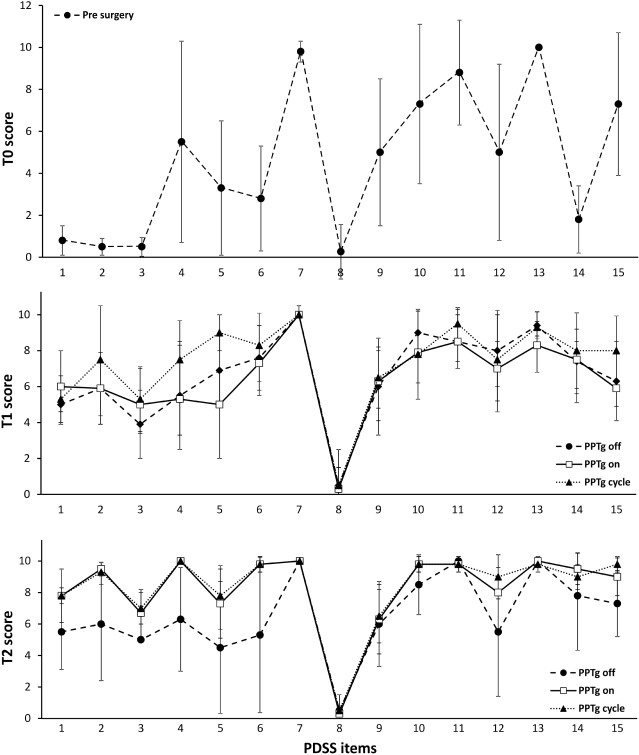
Before surgery (T0), all patients had severe sleep disturbances and diurnal somnolence, as rated by the sleep scales described above. The PDSS revealed poor quality of sleep. The mean global score of 70.0 was elevated and patients showed reduced overall quality of nighttime sleep including insomnia, restlessness, distressing dreams, psychosis, nocturia, sensory-motor disturbances, unsatisfactory sleep refreshment, and dozing (Figure 3, upper panel). The ESS showed pathological daytime sleepiness in all PD patients (Table 1).
Figure 3.
Profile of mean scores of each PDSS item rated in PD patients a week before surgery (T0) in the upper panel, 3 months (T1) after DBS in the middle panel, and 1 year (T2) after T1 in the lower panel. At T1 and T2, PDSS was assessed in all DBS conditions studied (PPTg-OFF, PPTg-ON, and PPTg-cycle).
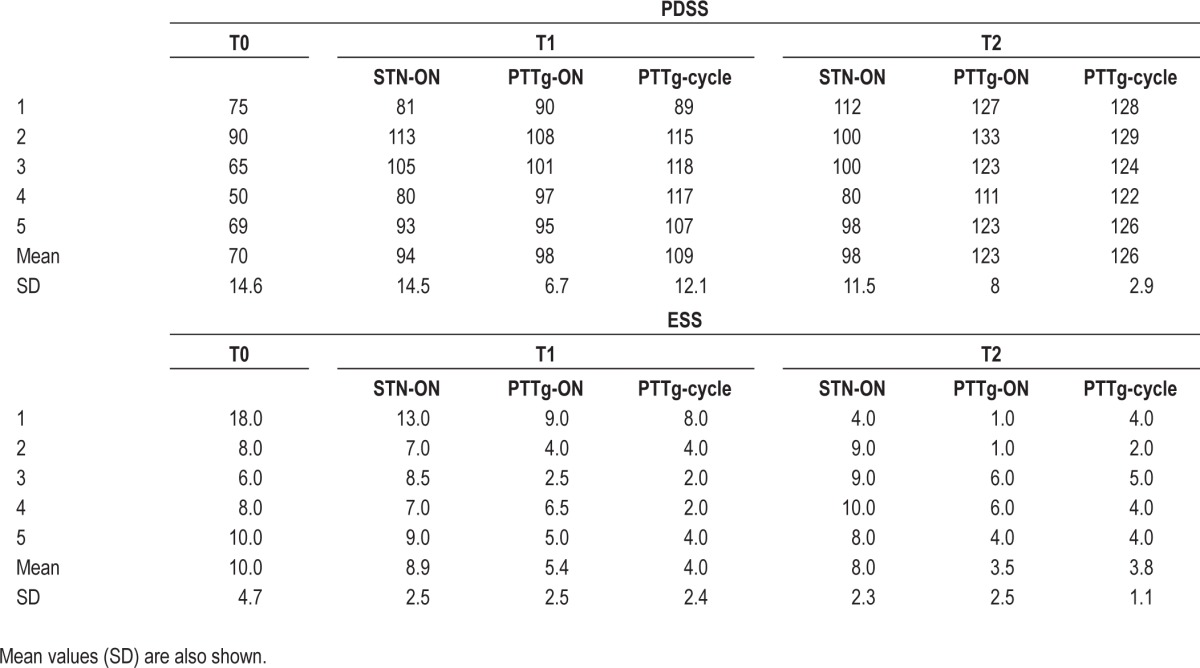
Table 1.
Each patient's total PDSS and ESS score a week before surgery (T0), 3 months (T1) after surgery, and one year (T2) afterT1. At T1 and T2, PDSS was assessed in all DBS conditions (STN-ON, PPTg-ON, and PPTg-cycle)
T1
Three months after surgery (T1), the patients' nocturnal sleep quality improved in all DBS conditions. Compared with T0, the PDSS mean global score showed a significant increase of 41% in STN-ON (P < 0.05), 35% in PPTg-ON (P < 0.05), and 57% in PPTg-cycle (P < 0.05) (Table 1). In particular, the PDSS showed improvement on items related to overall sleep quality, sleep onset and maintenance insomnia, restlessness, distressing dreams, nocturnal motor symptoms (cramps, pain), and sleep refreshment in all DBS conditions. However, when PPTg was switched ON either continuously or cyclically, further improvement of sleep onset and maintenance insomnia were observed compared with STN-ON alone; moreover, PPTg-cycle induced additional relief from nocturnal restlessness, nocturnal psychosis, and dozing compared with STN-ON and PPTg-ON (Figure 3, middle panel).
The mean score on the ESS at T1 was significantly reduced compared with T0 only in PPTg-ON (46%; P < 0.05) and PPTg-cycle (60%; P < 0.05) (Table 1). Comparison of the different PPTg stimulation modalities at T1 revealed a significant mean ESS decrease in PPTg-ON (39%; P < 0.05) and PPTg-cycle (55%; P < 0.05) compared with STN-ON, whereas PPTg-cycle induced an additional, but not significant, mean 26% mean improvement in daytime sleepiness compared with PPTg-ON (Table 1).
T2
Comparison of the T2 PDSS and the T1 PDSS results showed that both PPTg-ON and PPTg-cycle induced further amelioration of sleep quality, as shown by a global mean PDSS improvement of 31% in PPTg-ON (T1 vs T2 P < 0.05) and 15% in PPTg-cycle (T1 vs T2 P = NS). Nevertheless, the PDSS rated in STN-ON was unchanged with respect to T1. The PDSS confirmed the long-lasting benefits induced by PPTg-DBS on items related to overall sleep quality, insomnia, nocturnal motor restlessness, nocturnal psychosis, sensory-motor symptoms, and daytime sleepiness, which were already improved at T1 (Figure 3, lower panel).
The ESS documented a strong long-lasting reduction of daytime sleepiness during PPTg-cycle and PPTg-ON. Specifically, the ESS showed a mean reduction in daytime sleepiness of 56% in PPTg-ON (P < 0.05) and 53% in PPTg-cycle (P < 0.05) compared with STN-ON (Table 1).
Semi-structured interviews were administered to PD patients and their caregivers (bed partner) before surgery and the T1 and T2 follow-ups to investigate whether the patients exhibited recurrent nocturnal simple or complex motor activity during sleep. In our sample, all patients showed this activity before surgery, and as reported by the care givers, it was reduced at T1 and T2 follow-ups during PPTg-DBS in all PD patients. Two of the five patients had polysonnography (PSG); neither demonstrated REM sleep without atonia (RWSA). None of the other three patients had PSG, therefore is impossible to correctly diagnose RBD in these patients.
Assessment of Motor Function and MMSE
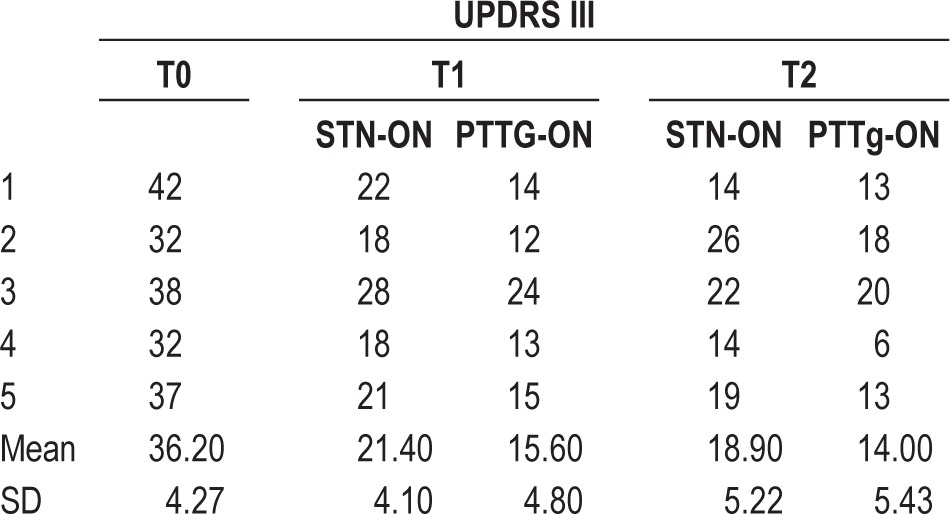
All patients underwent the UPDRS-III evaluation at T0, T1, and T2. At T1, our results showed a 27% improvement on the UPDRS-III when both PPTg and STN were stimulated (PPTg-ON) compared with STN-DBS alone (STN-ON). At T2, when both PPTg and STN were stimulated (PPTg-ON), a 26% improvement was still observed compared with STN-DBS alone (STN-ON). The PPTg-cycle condition was not considered in the clinical assessment because the stimulation was switched off during the day (Table 2).
Table 2.
Clinical data of PD patient group
The MMSE was performed before surgery and at T1 and T2 in all PD patients. No patient showed a significant decline in MMSE at T1 (28.2 ± 1.1) or T2 (27.6 ± 0.9) compared with pre-surgery (27.8 ± 1.3).
DISCUSSION
Before they were submitted to STN and PPTg-DBS, the PD patients suffered from complex nocturnal sleep disorders and diurnal somnolence that significantly improved after surgery. As regards the role of STN-DBS, our results are in agreement with those of previous studies showing the efficacy of STN-DBS on nocturnal sleep, essentially related to the reduction of nighttime motor disability and with those reporting a lack of significant changes in daytime sleepiness during STN-DBS.22–27
In this study, however, we found that, unlike STN-DBS, chronic low frequency stimulation of the PPTg induced significant improvement of both daytime sleepiness and nocturnal sleep quality in advanced PD patients. If we consider the ESS, the strong effect of PPTg-DBS on daytime sleepiness was already present at T1 and was thus confirmed as long-lasting at T2, that is, at one-year follow-up.
As concerns nocturnal sleep disturbances, a significant improvement of PDSS was observed at T1 compared with pre-surgery in all DBS conditions, but it was most evident during PPTg-cycle. At T2 (one-year follow-up), further improvement was observed in PDSS when both PPTg-DBS conditions were compared with STN-ON (STN-DBS alone), which was similar to T1 (see Table). In fact, at T2 we found a significant PDSS improvement only during continuous stimulation of the PPTg because during the PPTg-cycle, the PDSS was already strongly ameliorated at T1.
As STN was always turned ON and chronic pharmacological treatment was stable throughout the study, our results seem strictly related to stimulation of the PPTg. Considering the different PPTg stimulus modalities explored, our study shows that cyclic stimulation of the PPTg has a more prompt positive effect on PD patients' sleep quality than continuous stimulation, thus indicating the importance of stimulating this area during sleep. The different PPTg-DBS temporal stimulation patterns investigated were inspired by unpublished clinical observations of the reduced efficacy of PPTg-DBS immediately after (minutes) switching PPTg ON. In fact, we hypothesized that the sleep amelioration observed at the three-month follow-up with PPTg-cycle compared with PPTg-ON was likely due to greater physiological stimulation of PPTg activity. We may have found the same results with both forms of stimulation at one-year follow-up because of a sort of ceiling effect of the two stimulations.
In this study, PD patients' quality of sleep was investigated by rating subjective sleep scales; reporting objective data concerning changes in patient sleep architecture was not the aim of the study. Nevertheless, in two of the five patients, we investigated the effect of PPTg-DBS on sleep structure by means of PSG recordings before and after surgery in STN-ON/PPTg-OFF vs PPTg-ON/STN-OFF).28,29 The PSG data showed that, before surgery, PD patients were characterized by a sleep architecture disruption and great reduction of REM sleep. Compared with pre-surgery, both STN-DBS and PPTg-DBS induced the improved of sleep efficiency; however, STN-DBS had no impact on REM sleep, and only PPTg-DBS caused a reduction of REM latency and a relevant increase in REM sleep. Although the present data are not comparable with those of PSG, it is interesting to hypothesize a relationship in PTTg ON between reduced daytime sleepiness confirmed by improved EES performances and the increase in REM sleep observed in the two patients undergoing PSG recordings
It is known that the PPTg, which is part of the reticular ascending arousal pathway, is directly involved in the sleep-wake cycle in humans.3,30 Recently, it has also been reported that the different frequencies of the stimulus parameters strongly modified the effect of PPTg-DBS in inducing sleepiness as well as alertness.31 Our data seem to indicate that PPTg-DBS is effective in remodelling sleep a few months after surgery and highlight the long-lasting positive effect of PPTg stimulation on the sleep-cycle, supporting the contention that PPTg-DBS not only remodels the nighttime structure, but also favors wakefulness. It has been hypothesized that the neuronal plasticity induced by DBS may account for the efficacy of GPi DBS on dystonia32 and the long-lasting effects of STN-DBS on PD motor symptoms.33,34 Thus, the marked improvement of the sleep-wake cycle still observed at one-year follow-up suggests that PPTg-DBS induces a sort of recovery of the physiological activity of the neuronal pathways involved in sleep homeostasis.
However, the volume, per se, of the macro-electrode impedes the targeting of specific neuropil aggregates, which in turn render naïve the presumption of discriminating among clinical effects mediated by cholinergic and/or glutamatergic cells. Nevertheless, other research groups investigating the PPTg using different implantation sites and modalities of stimulation35–39 and different approaches (mono vs bilateral implantation, more or less mediolateral), reported gait and sleep benefits similar to those found in our studies.28,29,40 These data seem to indicate that current neurosurgical techniques do not allow stimulation of specific anatomical targets correlated with different functions in the human mesencephalic region. Previous studies emphasized the possibility that PPTg-ON does not simply activate the cerebral blood flow of the adjacent target,41 but may have a profound impact on ascending circuits by increasing the resting glucose metabolism in a large network of areas that include the orbitofrontal cortex, cingulate cortex, and prefrontal areas.42 However, as distal bipolar low-frequency stimulation was in this study, some speculations can be made. Despite obvious subjective differences in anatomical targets in terms of cranio-caudal distance from the pontomesencephalic line,18 it is likely that the parameters used may stimulate the so-called middle-caudal portion of the whole pedunculopontine nucleus (PPN) region. It is believed that this PPN subregion is mostly represented by phenotypically cholinergic elements (including “pars dissipata”) and manifests the maximal coherence with alpha band frequency at distant cortical sites,43,44 emphasizing its role in facilitating arousal and diurnal performance.
In conclusion, the results of our study suggest that PPTg-DBS leads to reorganization of the neural activity of the mesencephalic region, producing long-lasting amelioration of sleep disturbances in PD.
DISCLOSUE STATEMENT
This was not an industry supported study. Dr. Caltagirone has received research support from Novartis and Pfizer and has participated in paid speaking engagements from Novartis. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Patients were recruited at the IRCCS Santa Lucia Foundation in Rome where the research was carried out. The authors thank P. Mazzone, MD, for providing the figure of PPTg.
REFERENCES
- 1.Datta S, Spoley EE, Mavanji VK, Patterson EH. A novel role of pedunculopontine tegmental kainate receptors: a mechanism of rapid eye movement sleep generation in the rat. Neuroscience. 2002;114:157–64. doi: 10.1016/s0306-4522(02)00250-6. [DOI] [PubMed] [Google Scholar]
- 2.Mesulam MM, Geula C, Bothwell MA, Hersh LB. Human reticular formation: cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei and some cytochemical comparisons to forebrain cholinergic neurons. J Comp Neurol. 1989;283:611–33. doi: 10.1002/cne.902830414. [DOI] [PubMed] [Google Scholar]
- 3.Rye DB. Contributions of the pedunculopontine region to normal and altered REM sleep. Sleep. 1997;20:757–88. doi: 10.1093/sleep/20.9.757. [DOI] [PubMed] [Google Scholar]
- 4.Lee MS, Rinne JO, Marsden CD. The pedunculopontine nucleus: its role in the genesis of movement disorders. Yonsei Med J. 2000;41:167–84. doi: 10.3349/ymj.2000.41.2.167. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–74. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- 6.De Cock VC, Vidailhet M, Arnulf I. Sleep disturbances in patients with parkinsonism. Nat Clin Pract Neurol. 2008;4:254–66. doi: 10.1038/ncpneuro0775. [DOI] [PubMed] [Google Scholar]
- 7.Skinner RD, Garcia-Rill E. The mesencephalic locomotor region (MLR) in the rat. Brain Res. 1984;323:385–9. doi: 10.1016/0006-8993(84)90319-6. [DOI] [PubMed] [Google Scholar]
- 8.Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson's disease. Brain. 2000;123:1767–83. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- 9.Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27:585–8. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Moro E, Lozano AM, Pollak P, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson's disease. Mov Disord. 2010;25:578–86. doi: 10.1002/mds.22735. [DOI] [PubMed] [Google Scholar]
- 11.Phawa R, Wilkinson S, Smith RN, Lyons K, Miyawaki E, Koller WC. High-frequency stimulation of the globus pallidus for the treatment of Parkinson's disease. Neurology. 1997;49:249–53. doi: 10.1212/wnl.49.1.249. [DOI] [PubMed] [Google Scholar]
- 12.Limousin P, Krack P, Pollak P, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 1998;339:1105–11. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- 13.Stanzione P, Mazzone P, Peppe A, et al. Antiparkinsonian and anti-levodopa-induced dyskinesia effects obtained by stimulating the same site within the GPi in PD. Neurology. 1998;51:1776–7. doi: 10.1212/wnl.51.6.1776-a. [DOI] [PubMed] [Google Scholar]
- 14.Hamani C, Moro E, Lozano AM. The pedunculopontine nucleus as a target for deep brain stimulation. J Neural Transm. 2011;118:1461–8. doi: 10.1007/s00702-010-0547-8. [DOI] [PubMed] [Google Scholar]
- 15.Mazzone P, Lozano A, Stanzione P, et al. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson's disease. Neuroreport. 2005;16:1877–81. doi: 10.1097/01.wnr.0000187629.38010.12. [DOI] [PubMed] [Google Scholar]
- 16.Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson's disease. Neuroreport. 2005;16:1883–87. doi: 10.1097/01.wnr.0000187637.20771.a0. [DOI] [PubMed] [Google Scholar]
- 17.Stefani A, Lozano AM, Peppe A, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain. 2007;130:1596–607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- 18.Mazzone P, Sposato S, Insola A, Di Lazzaro V, Scarnati E. Stereotactic surgery of nucleus tegmenti pedunculopontini. Br J Neurosurg. 2008;22(suppl1):S33–S40. doi: 10.1080/02688690802448327. [DOI] [PubMed] [Google Scholar]
- 19.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhuri KR, Pal S, DiMarco A, et al. The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002;73:629–35. doi: 10.1136/jnnp.73.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahn S, Elton RL. UPDRS Development Committee Unified Parkinson's disease rating scale. In: Fahn S, Marsden MD, Calne DB, Goldstein M, editors. Recent development in Parkinson's disease. 153-63. Florham Park, NJ: McMillan Healthcare Information; 1987. pp. 293–304. [Google Scholar]
- 22.Arnulf I, Bejjani BP, Garma L, et al. Improvement of sleep architecture in PD with subthalamic nucleus stimulation. Neurology. 2000;55:1732–4. doi: 10.1212/wnl.55.11.1732. [DOI] [PubMed] [Google Scholar]
- 23.Hjort N, Østergaard K, Dupont E. Improvement of sleep quality in patients with advanced Parkinson's disease treated with deep brain stimulation of the subthalamic nucleus. Mov Disord. 2004;19:196–9. doi: 10.1002/mds.10639. [DOI] [PubMed] [Google Scholar]
- 24.Lyons KE, Pahwa R. Effects of bilateral subthalamic nucleus stimulation on sleep, daytime sleepiness, and early morning dystonia in patients with Parkinson disease. J Neurosurg. 2006;104:502–5. doi: 10.3171/jns.2006.104.4.502. [DOI] [PubMed] [Google Scholar]
- 25.Iranzo A. The importance of sleep medicine consultation for diagnosis of REM sleep behaviour disorder in most patients with Parkinson's disease. Sleep Med. 2002;3:537–8. doi: 10.1016/s1389-9457(02)00172-7. [DOI] [PubMed] [Google Scholar]
- 26.Monaca C, Ozsancak C, Jacquesson JM, et al. Effects of bilateral subthalamic stimulation on sleep in Parkinson's disease. J Neurol. 2004;251:214–48. doi: 10.1007/s00415-004-0305-7. [DOI] [PubMed] [Google Scholar]
- 27.Cicolin A, Lopiano l, Zibetti M, et al. Effects of deep brain stimulation of the subthalamic nucleus on sleep architecture in parkinsonian patients. Sleep Med. 2004;5:207–10. doi: 10.1016/j.sleep.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Romigi A, Placidi F, Peppe A, et al. Pedunculopontine nucleus stimulation influences REM sleep in Parkinson's disease. Eur J Neurol. 2008;15:e64–65. doi: 10.1111/j.1468-1331.2008.02167.x. [DOI] [PubMed] [Google Scholar]
- 29.Alessandro S, Ceravolo R, Brusa L, et al. Non-motor functions in parkinsonian patients implanted in the pedunculopontine nucleus: focus on sleep and cognitive domains. J Neurol Sci. 2010;289:44–8. doi: 10.1016/j.jns.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Rill E, Simon C, Smith K, et al. The pedunculopontine tegmental nucleus: from basic neuroscience to neurosurgical applications: Arousal from slices to humans: implications for DBS. J Neural Transm. 2010;118:1397–407. doi: 10.1007/s00702-010-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnulf I, Ferraye M, Fraix V, et al. Sleep induced by stimulation in the human pedunculopontine nucleus area. Ann Neurol. 2010;67:546–69. doi: 10.1002/ana.21912. [DOI] [PubMed] [Google Scholar]
- 32.Grips E, Blahak C, Capelle HH, et al. Patterns of reoccurrence of segmental dystonia after discontinuation of deep brain stimulation. J Neurol Neurosurg Psychiatry. 2007;78:318–20. doi: 10.1136/jnnp.2006.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen KZ, Zhu ZT, Munhall A, et al. Synaptic plasticity in rat subthalamic nucleus induced by high-frequency stimulation. Synapse. 2003;50:314–9. doi: 10.1002/syn.10274. [DOI] [PubMed] [Google Scholar]
- 34.Sestini S, Ramat S, Formiconi AR, Ammannati F, Sorbi S, Pupi A. Brain networks underlying the clinical effects of long-term subthalamic stimulation for Parkinson's disease: a 4-year follow-up study with rCBF SPECT. J Nucl Med. 2005;46:1444–54. [PubMed] [Google Scholar]
- 35.Wilcox RA, Cole MH, Wong D, Coyne T, Silburn P, Kerr G. Pedunculopontine nucleus deep brain stimulation produces sustained improvement in primary progressive freezing of gait. J Neurol Neurosurg Psychiatry. 2011;82:1256–9. doi: 10.1136/jnnp.2010.213462. [DOI] [PubMed] [Google Scholar]
- 36.Moro E, Hamani C, Poon YY, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson's disease. Brain. 2010;133:215–24. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- 37.Hamani C, Moro E, Lozano AM. The pedunculopontine nucleus as a target for deep brain stimulation. J Neural Transm. 2011;118:1461–8. doi: 10.1007/s00702-010-0547-8. [DOI] [PubMed] [Google Scholar]
- 38.Ostrem JL, Christine CW, Glass GA, et al. Pedunculopontine nucleus deep brain stimulation in a patient with primary progressive freezing gait disorder. Stereotact Funct Neurosurg. 2010;88:51–5. doi: 10.1159/000268742. [DOI] [PubMed] [Google Scholar]
- 39.Ferraye MU, Debû B, Fraix V, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson's disease. Brain. 2010;133:205–14. doi: 10.1093/brain/awp229. [DOI] [PubMed] [Google Scholar]
- 40.Peppe A, Pierantozzi M, Chiavalon C, et al. A Deep brain stimulation of the pedunculopontine tegmentum and subthalamic nucleus: effects on gait in Parkinson's disease. Gait Posture. 2010;32:512–8. doi: 10.1016/j.gaitpost.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Ballanger B, Lozano AM, Moro E, et al. Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson's disease: a (15)0H2O PET study. Hum Brain Mapp. 2009;30:3901–9. doi: 10.1002/hbm.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ceravolo R, Brusa L, Galati S, et al. Low frequency stimulation of the nucleus tegmenti pedunculopontine increases cortical metabolism in Parkinsonian patients. Eur J Neurol. 2011;18:842–9. doi: 10.1111/j.1468-1331.2010.03254.x. [DOI] [PubMed] [Google Scholar]
- 43.Androulidakis AG, Mazzone P, Litvak V, et al. Oscillatory activity in the pedunculopontine area with Parkinson's disease. Exp Neurol. 2008;211:59–66. doi: 10.1016/j.expneurol.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Thevathasan W, Pogosyan A, Hyam JA, et al. Alpha oscillations in the pedunculopontine nucleus correlate with gait performance in parkinsonism. Brain. 2012;135(Pt 1):148–60. doi: 10.1093/brain/awr315. [DOI] [PMC free article] [PubMed] [Google Scholar]