Abstract
Study Objectives:
Sighs are thought to have a role in regulating breathing control. They may preceed a central apnea (sigh-CA) or a pause (sigh-P), particularly in quiet sleep. Recent techniques characterizing cardiorespiratory synchronization (CRS) provide sensitive measures of cardiorespiratory coupling, which is an important factor in breathing control. We speculated that the strength of CRS and direction of cardiorespiratory coupling (DC), would differ between sigh-P and sigh-CA; before and after a sigh; and with maturation.
Design:
Prospective study. CRS and DC were calculated from the respiratory signal and heart rate before and after sighs recorded during overnight polysomnography.
Setting:
Sleep laboratory.
Participants:
The data were selected from 15 subjects of a prospective cohort of 34 healthy infants at ages 2 weeks, 3 months and 6 months.
Interventions:
N/A.
Measurements and results:
Both CRS and respiratory modulation on heart rate (RMH) (negative DC index) were decreased around sigh-CA compared with sigh-P at all ages. Short-term CRS decreased after both sigh-P and sigh-CA in infants aged 2 weeks and 3 months. Long term CRS did not change before and after sigh-P or sigh-CA. CRS and RMH were increased at 3 months and 6 months compared to 2 weeks.
Conclusions:
A sigh was not found to be associated with apparent resetting of breathing control in healthy infants less than 6 months of age. Cardiorespiratory coupling appears to be a leading marker of changes in breathing control, preceding central apnea associated with a sigh.
Citation:
Nguyen CD; Dakin C; Yuill M; Crozier S; Wilson S. The effect of sigh on cardiorespiratory synchronization in healthy sleeping infants. SLEEP 2012;35(12):1643-1650.
Keywords: Breathing control, central apnea, directional coupling, synchrogram, maturation
INTRODUCTION
A sigh, or a spontaneous augmented breath, is a common phenomenon in human breathing. It is observed throughout life, but occurs more frequently in infancy,1 and even more often in preterm infants2 than in adults. A sigh may be followed by breathing instability, in the form of a central apnea associated with an oxygen desaturation. Previous studies reported that 26% of all apneas in term infants were preceded by a sigh,3 while this number increased up to 40% in infants at increased risk for sudden death.4 It has been speculated that both a sigh and the associated apnea may be mediated via a common neurogenic mechanism.6
A sigh is thought to play a role in resetting breathing control7 within the neuroregulatory feedback loop. Other potential roles include lung recruitment during spontaneous breathing8–10 and resetting autonomic tone.11 While the physiological mechanisms involved in the control of breathing with regard to hypoxic and hypercapnic challenges12 are relatively well established, the integrative mechanisms controlling breathing patterns such as sigh are less well understood. The physiologic breathing control parameters precipitating a spontaneous sigh, the effect of this event on stability of respiratory system and the consequences thereof are unclear.
Previous work has investigated the role of a sigh by characterizing the raw respiratory signal around these events. Many linear models and quantification techniques have been proposed from the control engineering perspective, to measure the stability and variability of the respiratory signal around these events.7,13,14 Fleming et al. measured the oscillatory responses of breath-to-breath ventilation after a sigh in infants aged a few days old to 7 months, by linear control variables (damping ratio and oscillatory frequency).13 These results suggested that the respiratory system becomes more stable and responsive with age. This was in agreement with findings from a linear model approach.14 Baldwin et al. applied moving window coefficient of variation, autocorrelation functions, de-trended fluctuation analysis, and phase space plot techniques to capture the respiratory dynamics preceding and following a sigh in full-term infants.7 Short-range variability of respiratory control was reported to improve after a sigh, while long-range variability remained constant. The phase space plot also demonstrated a stable respiratory system with greater variability after a sigh.
The analysis of the respiratory signal has some inherent limitations. The raw respiratory signal is affected by noise, relating to recording instrumentation, calibration, and body movement. This affects the accuracy of the signal quantification and derived measures based on amplitude. Furthermore, an important factor in the control of breathing is the close interdependence of breathing and heart rate, as demonstrated in respiratory sinus arrhythmia, which is related to reflex activity between pulmonary afferents and vagal outflow.15 Cardiorespiratory interaction is vital to provide ideal gas exchange within a narrow range and in a manner responsive to varying physiological and metabolic demands. Hence, the separate analysis of each system as a discrete measure may not be appropriate in this context.
In this study, we propose a new approach to investigate a sigh associated with a pause or a central apnea by characterizing cardiorespiratory interaction (coupling) around these events in quiet sleep. In this sleep stage, the coupling between respiratory rate and heart rate is particularly prominent,15 and breathing is tightly regulated without the respiratory and metabolic variability introduced by response to awake or active sleep related stimuli. Cardiorespiratory coupling may be evaluated by measures of cardiorespiratory synchronization (CRS) and directionality of cardiorespiratory coupling. There is evidence showing that infant cardiorespiratory coupling changes with age16,17 and has a role in supporting oxygenation.18 Inverted directionality of the cardiorespiratory coupling was also reported in an infant with life threatening events.19 The CRS may therefore provide more useful information of cardiorespiratory dynamics than respiratory parameters alone, with information about autonomic responses and cardiorespiratory regulation.
The factors leading to a sigh, their effect on cardiorespiratory coupling, and the consequences on oxygenation are not well understood. Sigh behavior during maturation in healthy infants may provide an insight into the control of breathing in those at risk of inadequate control dynamics, such as those at risk of sudden infant death syndrome. We hypothesized that, if the role of a sigh is to reset breathing control mechanisms, then cardiorespiratory synchronization and directionality of cardiorespiratory coupling would differ before and after a sigh, and may differ between a sigh followed by a central apnea and a sigh followed by a pause. We also hypothesized that there would be a maturational effect on cardiorespiratory synchronization measures around a sigh event.
MATERIALS AND METHODS
Subjects and Study Design
The data were obtained from a prospective cohort of 34 healthy Caucasian infants (16 females and 18 males), born by normal delivery or planned caesarean section at term (38 to 42 weeks) with normal birth weight (10th to 90th percentile)20 and Apgar score. This study was conducted from March 2006 to January 2009 and under the approval of the Mater Health Services Human Research Ethics Committee (Number 952C).
Full overnight polysomnography (PSG) recordings were performed on each infant at ages of 2 weeks, 3 months, and 6 months. The data of each infant were selected if they had both sigh events at all studies: sigh followed by a pause (sigh-P) and sigh followed by central apnea (sigh-CA) events. The PSG was recorded on the EMBLA system (Embla N7000 system, EMBLA, 2009) including electroencephalogram (EEG), submental electromyogram (EMG), electrocardiogram (ECG), electrooculogram (EOG), uncalibrated respiratory inductance plethysmography (RIP), arterial oxygen saturation by pulse oximetry (SpO2), transcutaneous CO2 and nasal airflow, with digital video recording. Oxygen saturation (SpO2) was measured by Masimo pulse oximeter–Radical oximeter 7 (Masimo Corporation, Irvine, CA). Each PSG recording was performed overnight from 20:00 to 04:00.
Sleep Stage and Event Scoring
This study was manually scored following international standards by a trained, experienced scorer. For infants at 2 weeks old, the sleep stages were scored according to Anders et al.21 For infants aged ≥ 3 months, scoring was according to the AASM manual for Scoring Sleep22 as recommended by Grigg-Damberger et al.23
For event scoring, a sigh was defined as a brisk increase in thoracoabdominal excursion with amplitude at least twice than that of preceding 10 breaths. This may be followed by a pause in breathing but without oxygen desaturation, defined in this study as a sigh followed by a pause or sigh-P (Figure 1A). A sigh-CA was defined as a sigh immediately followed by a central apnea (defined according to AASM 2007 as ≥ 2 missed breaths and associated with oxygen desaturation SpO2 ≥ 3%22) (Figure 1B).
Figure 1.
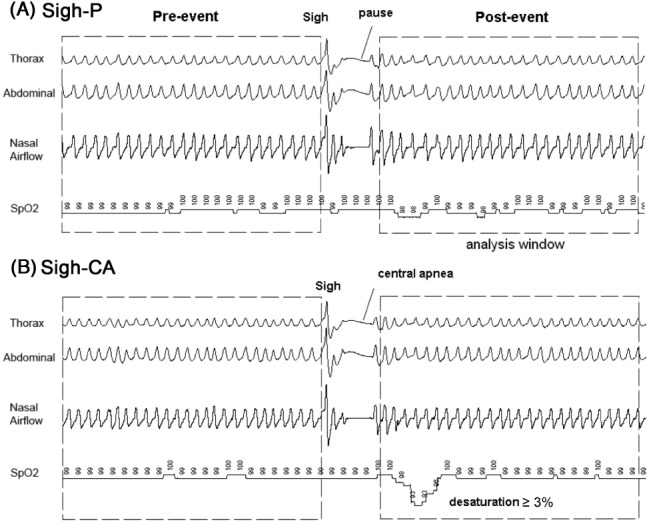
Pneumogram derived from thorax and abdominal (RIP) channels and oral-nasal airflow with associated blood oxygen saturation profile (SpO2). Sigh followed by pause (sigh-P) and sigh followed by post sigh central apnea (sigh-CA) are indicated in (A) and (B). A central apnea is defined according to AASM 2007 as ≥ 2 missed breaths and associated with oxygen desaturation SpO2 ≥ 3%,
Data Analysis
In this study, we measured cardiorespiratory synchronization strength and directionality of the cardiorespiratory coupling in the context of the phase synchronization approach. The data were analyzed in terms of short-range and long-range periods to characterize the variability and stability of the system, respectively. Phase synchronization was selected from a variety of techniques used to analyze cardiorespiratory synchronization (CRS). This is based on the concept of synchronization between 2 weakly coupled oscillating systems, and has been reported as a sensitive measurement of CRS, showing discrimination between different regimes of cardiorespiratory dynamics.24,25 It has been applied to young healthy athletes,26 healthy adults,27 heart transplant patients,28 infants during sleep,16 and anesthetized rats,29 and proved to be able to detect weak CRS in noisy, nonstationary, and short data.
Cardiorespiratory Synchronization Strength
The interaction of cardiovascular signals (ECG) and respiratory signals were studied within the context of a phase dynamic approach30 by a synchrogram technique.26 A synchrogram is a graphical tool representing the phase of the respiration signal at the peak of the R-wave,25,26 allowing several synchronization scenarios or n:m integer ratios of cardiac cycles to breaths to be visually identified in one plot. We extracted the R wave from the raw ECG by an automatic algorithm.31 The synchrogram technique requires narrow banded input signals; therefore a second order Savitzky-Golay filter was applied to respiratory signal to remove high frequency noise.25
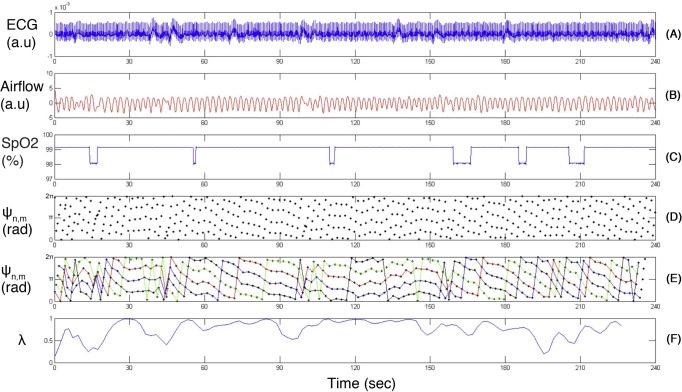
Several methods have been proposed to automatically quantify the synchrogram and measure the degree of synchronization.16,28,32–34 In this study, we exploited the method to generate the synchronization index λ.16,32 The cardiorespiratory systems are synchronized when λ = 1 and completely de-synchronized when λ = 0. In this study, window length M was set to 5. To obtain stable values of λ, the average of 10 different values of θ, equally distributed over interval [0, 2π] were used. The synchrogram with the chosen number of breathing cycles m = 1 was generated.
Direction of Cardiorespiratory Coupling
In addition to characterizing the degree of coupling, the directionality of cardiorespiratory coupling was quantified by the “evolution map approach” (EMA)35 with directional coupling index d. This aims to quantify the mutual influence of the phase of the respiratory signal on the time derivative of the ECG signal. The d index varies from 1, if there is a unidirectional coupling (heart rate→respiration) to −1 in the opposite case (respiration→heart rate) and −1 < d < 1 in the case of bidirectional coupling. The more “negative” value of d indicates the stronger respiratory drive. This directional coupling index d is able to provide reliable results for time series signals with considerable length.
Short-Range Synchronization
Strength of CRS: Using the synchrogram generated from the ECG and respiratory signals of 50-breath series before and after a sigh, the main synchronization ratio was calculated by measuring the maximum frequency of number of R peaks, which occur in every breathing cycle (Fig 2E). The strength of CRS (λ) was calculated for each 10-breath analysis window.
Figure 2.
Single channel ECG, oral-nasal airflow and associated blood oxygen saturation from sample data set with a generated synchrogram: (A) ECG; (B) Oral-nasal airflow; (C) Blood oxygen saturation (SpO2); (D) Generated synchrogram with breathing cycle m = 1; (E) Generated synchrogram with corresponding horizontal lines. Cardiorespiratory synchronization strength (λ) is indicated in (F). Ψn,m (rad): Phase difference between heart rate and respiratory signals.
Long-Range Synchronization
Strength of CRS: Using the synchrogram generated from the ECG and respiratory signals of 80-breath series before and after each selected sigh, the long-range synchronization ratio was calculated, with the strength of CRS (λ) being the average of λ from every 10-breath analysis window.
Directionality of coupling: d was measured from the ECG and respiratory signals of 80-breath series before and after each selected sigh.
Statistical Analysis
CRS strength (λ) and directionality index (d) were evaluated using generalized estimating equation (GEE) analysis36 and unbalanced nonparametric Friedman's test.37 These were chosen due to the unbalanced number of sigh events in each subject at each study. GEE analysis was performed by the GEE toolbox (STATA 8, StataCorp LP, College Station, TX). The unbalanced Friedman's test was implemented by a custom-written MATLAB software (The Mathworks Inc.).
RESULTS
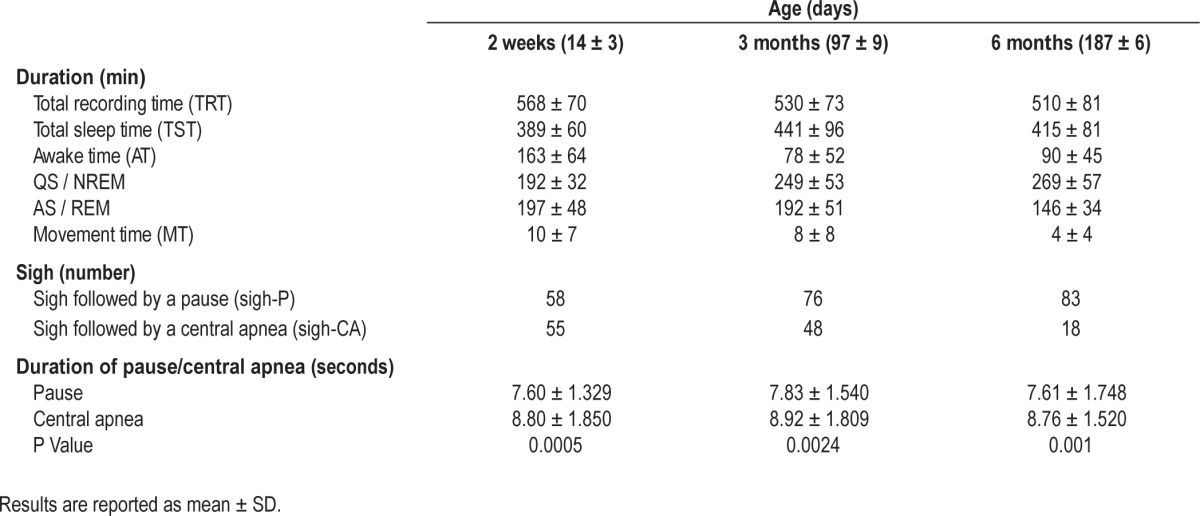
Of the prospective cohort of 34 infants, 25 infants underwent studies at all 3 ages. Both sigh-P and sigh-CA events were identified from overnight sleep studies at all 3 ages in 15 infants (7 females and 8 males). A total of 217 sigh-P and 121 sigh-CA events, which were artifact-free and non-overlapping, were identified during quiet sleep (Table 1). At 6 months old, the number of sigh-CA events was low (18 events). The duration of central apneas associated with sighs was observed to be longer than the duration of pauses associated with sighs in all 3 ages (Table 1).
Table 1.
Polysomnography parameters, sigh events and duration of pauses/central apneas in 15 healthy infants at different maturation states
Long-range sigh-P vs. sigh-CA
Cardiorespiratory synchronization strength (λ) and directional coupling (d) of 80-breath series were used to compare long-range synchronization of sigh-P and sigh-CA before and after each selected sigh. The more “negative” d index indicates the stronger respiratory modulation on heart rate (RMH).
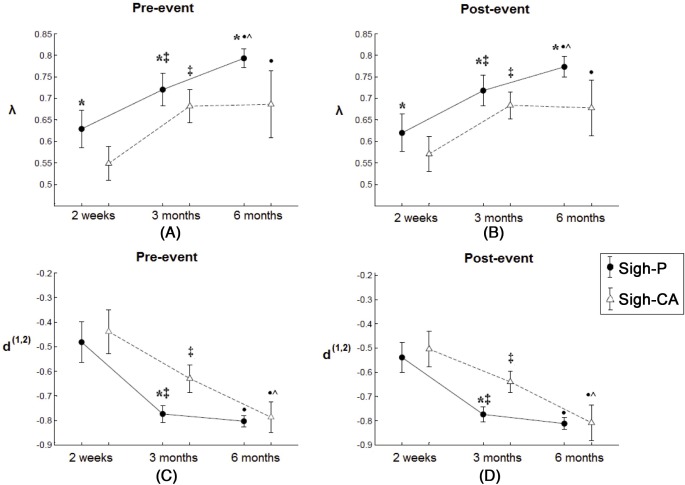
Synchronization strength index (λ): (Figure 3): λ was higher before and after a sigh for sigh-P compared with sigh-CA at each age (P < 0.02).
Figure 3.
Long-range cardiorespiratory synchronization strength (λ) and directional coupling index (d(1,2)) (mean and 95% CI) preceding (pre-event) and following (post-event) sigh-P/sigh-CA of 15 healthy infants at 2 weeks, 3 months and 6 months old. (A)-(B): cardiorespiratory synchronization strength, (C)-(D): direction of coupling. *Significant difference between sigh-P and sigh-CA events of the same age, ‡significant difference of sigh-P/sigh-CA events between 2 weeks and 3 months, •significant difference of sigh-P/sigh-CA events between 2 weeks and 6 months, ^significant difference of sigh-P/sigh-CA events between 3 months and 6 months.
Directional coupling index (d): At 3 months, d was lower before and after a sigh for sigh-P compared with sigh-CA. No significant differences were observed at 2 weeks or 6 months of age (Figure 3).
The Effect of Sigh on Synchronization Strength and Direction of Coupling
Short-Range Synchronization
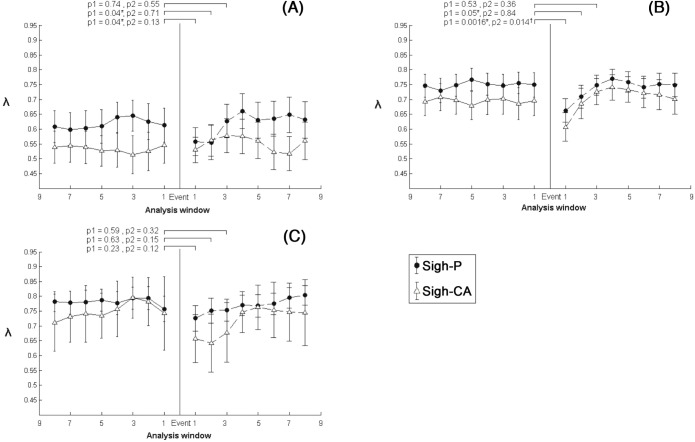
At 2 weeks old, λ deceased in the 1st and the 2nd analysis windows after a sigh-P (equivalent to 15 breaths, P = 0.04; Figure 4A). From the 3rd analysis window onward, λ increased and returned to the value before the sigh-P (P = 0.74). No difference was found after sigh-CA events. At 3 months old, λ deceased in the 1st and the 2nd analysis windows after a sigh-P (P = 0.0016, P = 0.05), and decreased in the 1st analysis window after a sigh-CA (P = 0.014; Figure 4B). Thereafter, λ increased and returned pre-sigh level for both sigh-P and sigh-CA. At 6 months old, no difference was found following a sigh (Figure 4C).
Figure 4.
Short-range cardiorespiratory synchronization strength (λ) (mean and 95% CI) preceding (pre-event) and following (post-event) sigh-P/sigh-CA of 15 healthy infants at 2 weeks, 3 months, and 6 months old. (A) 2 weeks, (B) 3 months, and (C) 6 months. Each analysis window contained 10 breaths with 50% overlap between windows. *Significant difference between pre- and post- sigh-P, †significant difference between pre- and post- sigh-CA.
Long-Range Synchronization
Synchronization strength index (λ): There was no difference in λ before and after a sigh for either sigh-P or sigh-CA at any age. Directional coupling index (d): There was also no difference in d before and after a sigh for either sigh-P or sigh-CA at any age.
Maturational effects
The long-range λ and d before and after a sigh (for both sigh-P and sigh-CA), were compared at the different ages (Figure 3). Both before and after a sigh, and for both sigh types, λ was higher at 3 months than λ at 2 weeks (P < 0.001 for all parameters). In addition, d was lower at 3 months than 2 weeks, indicating greater RMH (P < 0.0001 for all parameters). At 6 months of age, λ was higher than at 3 months for sigh-P, both before and after a sigh (P < 0.0001), but did not change for sigh-CA. d was lower (greater RMH) at 6 months compared with values at 3 months, but did not change for sigh-P.
DISCUSSION
In this study, the cardiorespiratory interactions around a sigh were characterized in infants, in order to investigate respiratory control dynamics around a sigh followed by a pause and a sigh followed by an apnea, and the effects of maturation. This study found that: a sigh followed by a central apnea was associated with a reduction in cardiorespiratory synchronization (CRS) and respiratory modulation on heart rate both before and after the sigh, in comparison with a sigh followed by a pause; short-range CRS was reduced after a sigh (in both sigh followed by a pause and sigh followed by a central apnea) in infants under 6 months of age, while long-range CRS did not change; and that there was a progressive increase in CRS and respiratory modulation on heart rate with age.
The methods employed for this study have a number of strengths: the integrated analysis of both heart rate and respiratory signals; the relatively robust phase dynamic approach; and the prospective nature of the infant cohort. Previous studies investigating the roles of a sigh have often focused on analyzing the characteristics of a single input, either the heart rate11 or the respiratory signal.7,13,38 The respiratory signal, in particular, is difficult to calibrate and sensitive to noise. The phase dynamic approach minimizes the inherent difficulties of calibration of the respiratory signal by analyzing the phase and not the amplitude.30 This approach has been proved to be robust to noise, being most suitable to detect cardiorespiratory synchronization in noisy and short time series data. Another advantage of this study was that data were obtained from a prospective cohort of infants from the neonatal period, allowing for observation of neonatal respiratory adaption and maturation of cardiorespiratory control in infancy.
The comparison between a sigh preceeding a central apnea and a sigh preceeding a pause found that both CRS and respiratory modulation on heart rate were lower around the former. This suggests that a central apnea may be more likely to follow a sigh in the context of reduced cardiorespiratory coupling. While all the infants studied were healthy, a correlation has been shown between the frequency of a sigh followed by a central apnea and disease states, such as brainstem dysfunction.41 Likewise, a sigh followed by a central apnea was speculated to be a potential marker of disordered respiratory activity during quiet sleep and may be more frequent in infants subsequently succumbing to SIDS.42
The temporary reduction in short-range CRS following both sigh types was not in keeping with a role of a sigh in resetting respiratory control, whereby a sigh may be expected to result in an increase in CRS. Indeed, the reduction in short-range CRS suggests that a sigh had a detrimental effect on cardiorespiratory coupling in infants who were less than 6 months old. This finding supports the result of previous studies that a sigh in infants may be followed by apparent breathing control difficulties such as apnea or hypoventilation, rather than an improvement in ventilation as seen in adults.38 Qureshi et al.38 also speculated that a sigh might have the potential to destabilize breathing in infants who are at risk of inadequate control of breathing, such as preterm infants, those with neurological impairment and those at risk for SIDS.38 Conversely, Baldwin et al. interpreted an increase in the variability of breathing after a sigh, to reflect greater adaptivity of the neurorespiratory control system.7 The lack of a long-range effect of a sigh on either cardiorespiratory synchronization or direction of coupling at any age demonstrated in this study is in keeping with the findings of Baldwin et al., who showed no change in the long-range respiratory variability after a sigh, and that the breathing control system returned to the same basin of attraction within phase space after a sigh in all subjects.
A maturational effect was demonstrated in this study, with a progressive increase in cardiorespiratory synchronization and respiratory modulation on heart rate with age. The greatest change was observed between 2 weeks and 3 months old. There was also a maturational change in short-term reduction in cardiorespiratory synchronization after a sigh, which was found at 2 weeks and 3 months of age, but not at 6 months of age. A maturational change in respiratory control has been previously reported in infants.16,35 Fleming et al. found that the ventilatory response following a sigh was stable but sluggish at 4 days old; from 4 days to 3-4 months, the response was unstable; and from 3-4 months to 7 months, a more stable response with rapid recovery was observed.13 Rosenblum et al. demonstrated that the coupling direction between cardiovascular and respiratory systems changed from a symmetric bidirectional interaction to a nearly unidirectional one (from respiration to cardiovascular systems) within the first 6 months of life.35 The same group also found cardiorespiratory synchronization increased with maturation of the newborn.16 These studies were of regular breathing, and did not evaluate the changes around a sigh.
The findings in this study are not in keeping with a role for a sigh in resetting respiratory control mechanisms in infants. Indeed, a sigh appears to occur at the expense of respiratory control as expressed by cardiorespiratory coupling measures in infants. A possible alternative role for a sigh in lung recruitment would appear physiologically reasonable, given the compliant chest wall of infancy and the consequent active maintenance of end expiratory lung volumes,43 which is compromised during sleep related reduction in muscle tone.44 The diminishing presence of fetal hemoglobin in healthy infants may also offer protection from any minor fluctuations of blood gases associated with transient destabilization of respiratory control over the first 3 months of life, when this effect is the greatest.45–47
There were some limitations in this study. Firstly, the CRS technique quantifies the cardiorespiratory interaction around a sigh, but is not designed to identify the mechanisms of cardiorespiratory coupling or explain the mechanism associated with a sigh. It is thought that the origin of a sigh comes from a stimulation of peripheral chemoreceptors in response to hypoxia and hypercapnia, or an inspiratory augmenting reflex from activation of lung and chest wall receptors in response to reduced lung compliance.48,49 It has been shown that a sigh is abolished by carotid body denervation48 and sectioning of the vagus nerve.49 Secondly, in this study, we aimed to capture the rapid change of cardiorespiratory synchronization; thus, only the synchronization ratio n:1 (n heartbeat in 1 breathing cycle) was considered. The “evolution map approach” (EMA) used to measure direction of cardiorespiratory coupling requires moderate length windows to operate. Numerical experiments indicated that typically about 10,000-100,000 data points are necessary to generate reliable and unbiased results in the presence of noise.50 Hence, we only applied EMA to detect coupling direction in long-range data (80-breath series, which were approximately equivalent to 30,000 data points at 200 Hz sampling rate). This study was also limited by the small number of sighs followed by central apneas in the cohort at 6 months of age, in keeping with the expected maturational reduction in frequency. Therefore, any statistical comparisons with these events need to be interpreted with care. This low number of sighs followed by central apneas also limited the number of infants with eligible sigh events.
CONCLUSIONS
A sigh was not found to be associated with apparent resetting of breathing control in healthy infants up to 6 months of age, using the methods described. Indeed, a sigh was associated with the temporary reduction of cardiorespiratory coupling less than 6 months of age. Cardiorespiratory coupling appears to be a leading marker of changes in breathing control, preceding central apnea associated with a sigh. The cardiorespiratory interaction in infants with respiratory disease, the ability to detect and quantify cardiorespiratory control and potentially predict a sigh followed by a central apnea, are areas for future research. Cardiorespiratory synchronization may form the base of a clinical measure of breathing control maturity in infants.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Work for this study was performed at: Department of Respiratory and Sleep Medicine, Mater Children's Hospital, Brisbane, QLD, Australia and School of Information Technology – Electrical Engineering, The University of Queensland, Brisbane, QLD Australia. The Prospective cohort study was funded by a grant from the Mater Children's Hospital Golden Casket Research fund. We thank Mater Children's Hospital, Dept. Respiratory and Sleep Medicine for research resources and technical support. We thank A. Bradley and K. Gibbons for statistical advice. C. Nguyen thanks M. Harris, S. Suresh, and P. Terrill for fruitful discussions and suggestions.
REFERENCES
- 1.Alvarez JE, Bodani J, Fajardo CA, Kwiatkowski K, Cates DB, Rigatto H. Sighs and their relationship to apnea in the newborn infant. Biol Neonate. 1993;63:139–46. doi: 10.1159/000243923. [DOI] [PubMed] [Google Scholar]
- 2.Hoch B, Bernhard M, Hinsch A. Different patterns of sighs in neonates and young infants. Biol Neonate. 1998;74:16–21. doi: 10.1159/000014006. [DOI] [PubMed] [Google Scholar]
- 3.Curzidascalova L, Plassart E. Respiratory and motor events in sleeping infants - their correlation with thoracic-abdominal respiratory relationships. Early Hum Dev. 1978;2:39–50. doi: 10.1016/0378-3782(78)90051-8. [DOI] [PubMed] [Google Scholar]
- 4.Hoppenbrouwers T, Hodgman JE, Arakawa K, et al. Sleep apnea as part of a sequence of events - comparison of 3 months old infants at low and increased risk for sudden infant death syndrome (SIDS) Neuropadiatrie. 1978;9:320–37. doi: 10.1055/s-0028-1091492. [DOI] [PubMed] [Google Scholar]
- 5.Kahn A, Blum D, Rebuffat E, et al. Polysomnographic studies of infants who subsequently died of sudden infant death syndrome. Pediatrics. 1988;82:721–7. [PubMed] [Google Scholar]
- 6.Weintraub Z, Alvaro R, Mills S, Cates D, Rigatto H. Short apneas and their relationship to body movements and sighs in preterm infants. Biol Neonate. 1994;66:188–94. doi: 10.1159/000244107. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin DN, Suki B, Pillow JJ, Roiha HL, Minocchieri S, Frey U. Effect of sighs on breathing memory and dynamics in healthy infants. J Appl Physiol. 2004;97:1830–9. doi: 10.1152/japplphysiol.00298.2004. [DOI] [PubMed] [Google Scholar]
- 8.Poets CF, Rau GA, Neuber K, Gappa M, Seidenberg J. Determinants of lung volume in spontaneously breathing preterm infants. Am J Respir Criti Care Med. 1997;155:649–53. doi: 10.1164/ajrccm.155.2.9032208. [DOI] [PubMed] [Google Scholar]
- 9.Thach BT, Taeusch HW. Sighing in newborn human infants: role of inflation-augmenting reflex. J Appl Physiol. 1976;41:502–7. doi: 10.1152/jappl.1976.41.4.502. [DOI] [PubMed] [Google Scholar]
- 10.Hummler H, Gerhardt T, Gonzalez A, Claure N, Everett R, Bancalari E. Increased incidence of sighs (augmented inspiratory efforts) during synchronized intermittent mandatory ventilation (SIMV) in preterm neonates. Pediatr Pulmonol. 1997;24:195–203. doi: 10.1002/(sici)1099-0496(199709)24:3<195::aid-ppul5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Franco P, Verheulpen D, Valente F, et al. Autonomic responses to sighs in healthy infants and in victims of sudden infant death. Sleep Med. 2003;4:569–77. doi: 10.1016/s1389-9457(03)00107-2. [DOI] [PubMed] [Google Scholar]
- 12.Haddad GG, Abman SH, Chernick V. Chernick-Mellins basic mechanisms of pediatric respiratory disease. 2nd ed. Hamilton, Ontario: BC Decker; 2002. [Google Scholar]
- 13.Fleming PJ, Goncalves AL, Levine MR, Woollard S. The development of stability of respiration in human infants: changes in ventilatory responses to spontaneous sighs. J Physiol-London. 1984;347:1–16. doi: 10.1113/jphysiol.1984.sp015049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Revow M, England SJ, Obeirne H, Bryan AC. A model of the maturation of respiratory control in the newborn-infant. IEEE Trans Biomed Eng. 1989;36:414–23. doi: 10.1109/10.18747. [DOI] [PubMed] [Google Scholar]
- 15.Harper R, Gozal D. Sleep and respiratory control. In: Haddad GG, Abman SH, Chernick V, editors. Chernick-Mellins basic mechanisms of pediatric respiratory disease. 2nd ed. BC Decker; 2002. p. 412. [Google Scholar]
- 16.Mrowka R, Patzak A, Rosenblum M. Quantitative analysis of cardiorespiratory synchronization in infants. Int J Bifurcat Chaos. 2000;10:2479–88. [Google Scholar]
- 17.Mrowka R, Cimponeriu L, Patzak A, Rosenblum MG. Directionality of coupling of physiological subsystems: age-related changes of cardiorespiratory interaction during different sleep stages in babies. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1395–401. doi: 10.1152/ajpregu.00373.2003. [DOI] [PubMed] [Google Scholar]
- 18.Elder DE, Larsen PD, Galletly DC, Campbell AJ. Cardioventilatory coupling in preterm and term infants: Effect of position and sleep state. Respir Physiol Neurobiol. 2010;174:128–34. doi: 10.1016/j.resp.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Wallois F, Aarabi A, Kongolo G, Leke A, Grebe R. Inverse coupling between respiratory and cardiac oscillators in a life-threatening event in a neonate. Auton Neurosci. 2008;143:79–82. doi: 10.1016/j.autneu.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States: Adv Data; 2000. pp. 1–27. [PubMed] [Google Scholar]
- 21.Anders T, Emde R, Parmalee A. A manual of standardized terminology, techniques and criteria for scoring of states of sleep and wakefulness in newborn infants. UCLA Brain Information Service; 1971. [Google Scholar]
- 22.Iber C, Ancoli-Israel S, Chesson A, Quan SF, Iber C. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. IL: American Academy of Sleep Medicine Westchester; 2007. [Google Scholar]
- 23.Grigg-Damberger M, Gozal D, Marcus CL, et al. The visual scoring of sleep and arousal in infants and children. J Clin Sleep Med. 2007;3:201–40. [PubMed] [Google Scholar]
- 24.Burykin A, Buchman TG. Cardiorespiratory dynamics during transitions between mechanical and spontaneous ventilation in intensive care. Complexity. 2008;13:40–59. [Google Scholar]
- 25.Schafer C, Rosenblum MG, Abel HH, Kurths J. Synchronization in the human cardiorespiratory system. Physical Review E. 1999;60:857–70. doi: 10.1103/physreve.60.857. [DOI] [PubMed] [Google Scholar]
- 26.Schafer C, Rosenblum MG, Kurths J, Abel HH. Heartbeat synchronized with ventilation. Nature. 1998;392:239–40. doi: 10.1038/32567. [DOI] [PubMed] [Google Scholar]
- 27.Lotric MB, Stefanovska A. Synchronization and modulation in the human cardiorespiratory system. Physica A. 2000;283:451–61. [Google Scholar]
- 28.Toledo E, Rosenblum MG, Schäfer C, Kurths J, Akselrod S. Int Symposium on Nonlinear Theory and Its Applications. Lausanne: Presses Polytechniques et Universitaires Romandes, 1998; 1998. Quantification of cardiorespiratory synchronization in normal and heart transplant subjects; pp. 171–4. [Google Scholar]
- 29.Stefanovska A, Haken H, McClintock PVE, Hozic M, Bajrovic F, Ribaric S. Reversible transitions between synchronization states of the cardiorespiratory system. Phys Rev Lett. 2000;85:4831–4. doi: 10.1103/PhysRevLett.85.4831. [DOI] [PubMed] [Google Scholar]
- 30.Pikovsky A, Rosenblum M, Kurths J, Hilborn RC. Synchronization: a universal concept in nonlinear science. Am J Phys. 2002;70:655. [Google Scholar]
- 31.Hamilton PS, Tompkins WJ. Quantitative investigation of QRS detection rules using the Mit/Bih arrhythmia database. IEEE Trans Biomed Eng. 1986;33:1157–65. doi: 10.1109/tbme.1986.325695. [DOI] [PubMed] [Google Scholar]
- 32.Tass P, Rosenblum MG, Weule J, et al. Detection of n: m phase locking from noisy data: Application to magnetoencephalography. Phys Rev Lett. 1998;81:3291–4. [Google Scholar]
- 33.Galletly DC, Larsen PD. Cardioventilatory coupling in heart rate variability: methods for qualitative and quantitative determination. Br J Anaesth. 2001;87:827–33. doi: 10.1093/bja/87.6.827. [DOI] [PubMed] [Google Scholar]
- 34.Seidel H, Herzel H. Analyzing entrainment of heartbeat and respiration with surrogates. IEEE Eng Med Biol Mag. 1998;17:54–7. doi: 10.1109/51.731321. [DOI] [PubMed] [Google Scholar]
- 35.Rosenblum MG, Cimponeriu L, Bezerianos A, Patzak A, Mrowka R. Phys Rev E . 2002. Identification of coupling direction: Application to cardiorespiratory interaction; p. 65. [DOI] [PubMed] [Google Scholar]
- 36.Zeger SL, Liang KY. Longitudinal Data-analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 37.Kroon JD, Laan PVD. A generalization of Friedman's rank statistic. Stat Neerl. 1983;37:1–14. [Google Scholar]
- 38.Qureshi M, Khalil M, Kwiatkowski K, Alvaro RE. Morphology of sighs and their role in the control of breathing in preterm infants, term infants and adults. Neonatology. 2009;96:43–9. doi: 10.1159/000201738. [DOI] [PubMed] [Google Scholar]
- 39.Penzel T. Is heart rate variability the simple solution to diagnose sleep apnoea? Eur Respir J. 2003;22:870–1. doi: 10.1183/09031936.03.00102003. [DOI] [PubMed] [Google Scholar]
- 40.Eckberg DL. Sympathovagal balance - A critical appraisal. Circulation. 1997;96:3224–32. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- 41.Yasaki E, Saito Y, Nakano K, et al. Characteristics of breathing abnormality in Leigh and its overlap syndromes. Neuropediatrics. 2001;32:299–306. doi: 10.1055/s-2001-20405. [DOI] [PubMed] [Google Scholar]
- 42.Saito Y, Ezure K, Kobayashi M, Ito M, Saito K, Osawa M. A review of functional and structural components of the respiratory center involved in the arousal response. Sleep Med. 2002;3(Suppl 2):S71–4. doi: 10.1016/s1389-9457(02)00170-3. [DOI] [PubMed] [Google Scholar]
- 43.Allen J, Gripp K. Development of the thoracic cage. In: Haddad GG, Abman SH, Chernick V, editors. Chernick-Mellins basic mechanisms of pediatric respiratory disease. 2nd ed. Vol. 51. 2002. p. 124. [Google Scholar]
- 44.Wilson SJ, O'Brien C, Harris MA, Masters IB. Measuring tidal volume and functional residual capacity change in sleeping infants using a volume displacement plethysmograph. Eur Respir J. 1998;12:1186–90. doi: 10.1183/09031936.98.12051186. [DOI] [PubMed] [Google Scholar]
- 45.Beaven GH, Ellis MJ, White JC. Studies on human foetal haemoglobin II. Foetal haemoglobin levels in healthy children and adults and in certain haematological disorders. Brit J Haematol. 1960;6:201–22. doi: 10.1111/j.1365-2141.1960.tb06236.x. [DOI] [PubMed] [Google Scholar]
- 46.Colombo B, Kim B, Atencio RP, Molina C, Terrenato L. The pattern of fetal haemoglobin disappearance after birth. Br J Haematol. 1976;32:79–87. doi: 10.1111/j.1365-2141.1976.tb01877.x. [DOI] [PubMed] [Google Scholar]
- 47.Huehns E, Beaven G. Developmental changes in human haemoglobins. Clin Dev Med. 1971;37:175–203. [Google Scholar]
- 48.Bartlett D. Origin and regulation of spontaneous deep breaths. Respir Physiol. 1971;12:230–8. doi: 10.1016/0034-5687(71)90055-7. [DOI] [PubMed] [Google Scholar]
- 49.Glogowska M, Richardson PS, Widdicombe JG, Winning AJ. Role of vagus nerves, peripheral chemoreceptors and other afferent pathways in genesis of augmented breaths in cats and rabbits. Respir Physiol. 1972;16:179–96. doi: 10.1016/0034-5687(72)90050-3. [DOI] [PubMed] [Google Scholar]
- 50.Rosenblum MG, Pikovsky AS. Detecting direction of coupling in interacting oscillators. Phys Rev E. 2001;6404:045202. doi: 10.1103/PhysRevE.64.045202. [DOI] [PubMed] [Google Scholar]