Abstract
Background:
The cause of nocturnal awakenings in patients with chronic insomnia is rarely researched. This study prospectively assessed the etiology of nocturnal awakenings (subjectively and objectively) among patients with insomnia at a private, community-based sleep medical center.
Methods:
Twenty adult patients with chronic insomnia enrolled between April 2008 and February 2010 met diagnostic criteria for an insomnia disorder, never previously visited a sleep specialist or underwent sleep testing, and reported no classic sleep disordered breathing symptoms. Patients completed validated scales for insomnia, sleepiness, impairment, anxiety, depression, and quality of life, a qualitative interview to assess subjective reasons for awakenings, and a diagnostic sleep study to objectively assess awakenings and their precipitants.
Results:
Subjective and objective data showed clinically meaningful insomnia, primarily sleep maintenance insomnia. The most common self-reported reasons for awakenings were: uncertain cause (50%), nightmares (45%), nocturia (35%), bedroom distractions (20%), or pain (15%). No patient identified breathing symptoms as a cause. Objectively, 531 awakenings were observed in the total sample, and 478 (90%) were preceded by sleep breathing events (apnea, hypopnea, or respiratory effort-related event). Fifty-three awakenings were caused by other factors (independent leg jerks [7], spontaneous [14], and sleep that was laboratory-induced [32]). Thirty awakenings ≥ 5 min—a duration sufficient to predispose toward an insomnia episode—were each preceded by a breathing event.
Conclusions:
Among patients with insomnia with no classic sleep breathing symptoms and therefore low probability of a sleep breathing disorder, most of their awakenings were precipitated by a medical condition (sleep disordered breathing), which contrasted sharply with their perceptions about their awakenings.
Citation:
Krakow B; Romero E; Ulibarri VA; Kikta S. Prospective assessment of nocturnal awakenings in a case series of treatment-seeking chronic insomnia patients: a pilot study of subjective and objective causes. SLEEP 2012;35(12):1685-1692.
Keywords: Awakenings, insomnia, obstructive sleep apnea, upper airway resistance
INTRODUCTION
Insomnia is the most common sleep disorder in the general population,1 and difficulty staying asleep (sleep maintenance insomnia [SMI]) is the most common presentation.2–5 For patients seeking treatment at sleep medical centers, sleep maintenance problems are prevalent.6 Yet, scant research addresses the question: “What causes patients with chronic insomnia to wake up at night?” Most studies focus on the postawakening problem of returning to sleep, which is usually attributed to hyperarousal.7
The prevailing hyperarousal theory posits patients with insomnia suffer from a state of increased alertness or arousal during waking or sleeping periods.8,9 Hyperarousal is innate or genetically predisposed (physiologically driven; e.g., increased somatic tension10) or learned through behavioral influences that promote excess alertness or arousal (psychologically driven; e.g., losing sleep over losing sleep11), both of which over-stimulate the mind with racing thoughts and ruminations that inhibit sleep. Hyperarousal creates vulnerability to nocturnal awakenings among individuals predisposed toward excess mental stimulation or conditioning influences, which then extends a brief awakening (e.g., 5 min) into a longer awakening (i.e., a period of insomnia). Thus, the theory explains the difficulty in returning to sleep (duration of an awakening), but no research explains how hyperarousal actually causes awakenings.8,9
Few researchers study or comment on subjective or objective causes of awakenings. Ohayon's work is the most authoritative.3 Among 8,937 adults surveyed from the general US population, 35.5% (n = 3,173) reported frequent nocturnal awakenings. When queried on “motives for awakening,” 75.5% (n = 6,747) reported needing to use the bathroom often or sometimes. Waking spontaneously, thirst, or noise averaged 30-40%; children, bed partner, or pain averaged 10-20%; and hunger and breathing difficulties were approximately 5%.3 Although this research was detailed, no findings were objectively confirmed with polysomnography (PSG) or tracked prospectively with sleep diaries.
Emerging research in the area of comorbidity between insomnia and sleep disordered breathing (SDB) (“complex insomnia”)12 highlight the role of sleep apnea in causing awakenings among various samples of patients with SMI, but to our knowledge there are no prospective studies among patients with insomnia without sleep breathing problems in which the etiology of their awakenings has been identified.13
To research subjective and objective causes of nocturnal awakenings, we conducted a prospective pilot study on a small series of patients with chronic insomnia without sleep breathing symptoms who were seeking treatment at a sleep medical center. We developed two hypotheses:
Subjectively, patients with chronic insomnia would express uncertainty about the etiologies for their awakenings, but they would also speculate, once prompted, on various potential psychologic and physiologic causes such as racing thoughts, stress, nightmares, nocturia, discomfort or pain, or temperature changes.
Despite attempts to exclude patients with SDB, most of the awakenings documented on a diagnostic PSG would be triggered by SDB events.
METHOD
Consent
This prospective study was approved by the Presbyterian Healthcare Services Institutional Review Board, Albuquerque, New Mexico. Research was conducted at the Sleep and Human Health Institute, a nonprofit research facility; and PSG was performed at Maimonides Sleep Arts and Sciences (MSAS) sleep center. All patients presented to MSAS with a chief complaint of insomnia. After completing the standard MSAS online intake process, including a sleep medical history, past medical history, and past psychiatric history, patients were identified as potential participants, who then received a complete description of the protocol, after which they provided verbal and written consent.
Sample and Inclusion/Exclusion Criteria
To recruit patients with insomnia without breathing symptoms is difficult. As demonstrated in a recent retrospective chart review, among 1,035 treatment-seeking patients with insomnia only 19% reported the absence of sleep breathing difficulties. Despite presenting to the sleep center with a chief complaint of chronic insomnia, 81% of the sample reported various co-occurring sleep breathing disorders, problems, or symptoms.14 In the current study, we prospectively recruited patients with insomnia without breathing symptoms linked to SDB to ensure a sample of “classic” patients with insomnia who would suffer neither from a diagnosis of obstructive sleep apnea (OSA) nor from nocturnal awakenings due to the pathophysiology of this common sleep disorder.13 To refine our sample, we included only those patients who (1) sought initial sleep evaluation at MSAS and had not previously visited a sleep specialist or undergone PSG; (2) ranked insomnia as their primary sleep complaint; (3) scored ≥ 15 on the Insomnia Severity Index (ISI)15; (4) reported sleep-related impairment; (5) met research criteria for an insomnia disorder16; (6) completed an Internet-based intake survey; (7) spoke English; and (8) were 18 yr of age or older.
A total of 1,326 patients sought care at MSAS from April 2008 to February 2010, and 512 patients (38.6%) presented with a chief complaint of chronic insomnia. Of these 512 patients, 461 (90%) also reported co-occurring sleep breathing disorders, problems, or symptoms and were excluded from the study. Also, 11 scored < 15 on the ISI, and eight had previously been evaluated or tested at another sleep facility, leaving our sample at 32 of the original 1,326 patients (2.4%). Sedative or psychotropic medication use was not an exclusion criterion. Of the 32 patients who qualified during this 22-mo period, four chose not to participate, and eight did not complete the prediagnostic interview or postdiagnostic interview due to logistical problems such as travel and work commuting issues, leaving our final sample at 20 patients.
Originally, the study was designed to recruit a control group of normal sleepers, but we were unsuccessful in doing so as has been reported by other researchers.17 In the absence of a control sample and given our focus in this study, we contrasted the number of PSG-determined awakenings in our patients with insomnia with a published normative value for age-appropriate individuals tested in a sleep laboratory on two consecutive nights.18
Intake and Measurements
The MSAS Internet-based intake survey19 includes self-reported sleep continuity questions including sleep onset latency, total sleep time, time in bed, calculated sleep efficiency, and wake time after sleep onset. Patients also completed five validated questionnaires: ISI,15 Epworth Sleepiness Scale (ESS),20 Quality of Life Enjoyment and Satisfaction Questionnaire Summary Sheet (QLESQ),21 depression and anxiety scales of the Hopkins Symptom Checklist (HSCL),22 and Functional Outcome of Sleep Questionnaire (FOSQ).23
Eligibility and Prepolysomnography Interview
The medical director (first author) reviews all intakes prior to any patient initiating care at the sleep center. At this point, he identifies potential participants by the absence of classic SDB symptoms and the apparent presence of an insomnia disorder. He further reviews the patients' intake data and determines a need for diagnostic PSG based on the American Academy of Sleep Medicine (AASM) practice parameters due to the persistent complaints of nonrestorative sleep (19 of 20; 95% of sample), unrefreshing sleep (95%), daytime fatigue (100%), or persistent insomnia on average more than 8 yr despite repeated attempts at pharmacotherapy (90% of sample).24 All 20 patients demonstrated indications for PSG.
On the PSG night, participants completed a 30-min presleep interview with the second author (a registered PSG technologist) that comprised qualitative (open-ended) and quantitative questions about difficulty falling asleep, difficulty returning to sleep post-awakenings, and the causes of nighttime awakenings. Causes for sleep onset insomnia (SOI) were elicited by this query: “If you cannot fall asleep, what is/are the reasons?” Causes for SMI were elicited with this query: “If you awaken during the night and have difficulty returning to sleep, what is/are the reasons” (Table 2).
Table 2.
Patient reported causes (% total sample [n = 20]) of sleep onset insomnia and sleep maintenance insomnia from the qualitative presleep interview

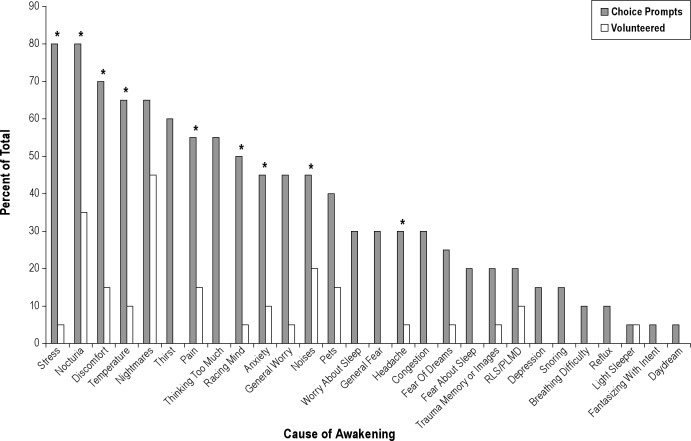
Next, for awakenings patients were able to volunteer multiple causes of awakenings based on this query: “If you awaken during the night, please explain what causes these nighttime awakenings?” Last, dichotomous choice questions probing multiple etiologies of awakenings and whether awakenings were due to mental, physical, or unknown causes were read to the patient who answered either yes or no (Figure 1).
Figure 1.
Comparisons between volunteered and dichotomous choice prompts for the causes of nightly awakenings. *P < 0.05. P value determined using McNemar test; however, McNemar test cannot be used when one comparison value is equal to zero.
Objective Testing and Posttest Interview
After the interview, all diagnostic PSG was performed at MSAS according to standard protocol. Parameters recorded were electroencephalography (EEG: C3 to A2, C4 to A1, O1 to A2, O2 to A1), left and right eye electrooculography (EOG), chin electromyography (EMG), electrocardiography (EKG 1, EKG 2), blood oxygen saturation and pulse rate via pulse oximetry probe, left and right anterior tibialis EMG, respiratory flow via nasal cannula pressure transducer and thermistor/thermocouple, chest and abdomen respiratory effort via two piezoelectric plethysmography sensor belts, and snoring via snore sensor.
All patients maintained bedtime medication regimens used at home. There was no discussion with patients about possible or discovered findings before or immediately after diagnostic tests. The medical director interpreted all PSG studies and prepared a standard clinical report for the patient and the patient's referring physician for continuity of care. After completing PSG, patients returned 1 wk later for a follow-up interview, which commenced with the patient and the second author reviewing actual data from the sleep study, wherein the patient observed the presence or absence of awakenings and their precipitants. The patient was then queried about changes in perception or lack thereof about their awakenings.
Scoring of Sleep Studies and Masking Procedures
PSG was scored manually by registered PSG technicians according to AASM criteria.25 Nocturnal awakenings were scored when 30-sec epochs consisted of greater than 50% alpha rhythm over the occipital region or, in the absence of alpha activity, eye blinks at a frequency of 0.5-5Hz, reading eye movements, or irregular conjugate rapid eye movements associated with normal or high chin muscle tone.25 Respiratory-related events were of three types: apneas were a ≥ 90% reduction in peak thermal sensor excursion lasting ≥ 10 sec; hypopneas were a ≥ 50% reduction in baseline nasal pressure signal excursion lasting ≥ 10 sec with a ≥ 3% desaturation from pre-event baseline or terminated with arousal or awakening; and respiratory effort-related arousals (RERAs) were a sequence of breaths lasting ≥ 10 sec characterized by increased respiratory effort or flattening of the nasal pressure waveform leading to an arousal or awakening in EEG activity when the sequence of breaths does not meet criteria for apnea or hypopnea.25
To ensure validity of nocturnal awakenings on PSG, sleep studies were copied prior to initial scoring and all identifiers removed. Studies were electronically encrypted and transferred to an independent entity, Quality Sleep Solutions (QSS), to be scored by a registered PSG technologist who was masked from the research protocol. Scored studies from both MSAS and QSS were compared using Sandman software (version 9.2, Embla, Ontario, Canada) inter-rater reliability function to determine scoring agreement for respiratory-related events.26 Scored awakenings were compared manually by the third author, a registered PSG technologist, who confirmed that both institutions scored the same awakenings and identified awakenings discrepant between the two. Then, all confirmed awakenings as well as those in question were presented—unscored—to the lead PSG technologist at MSAS (who was masked from the protocol) to determine whether or not each awakening and those in question met criteria. Once awakenings status was finalized, the lead sleep technologist (maintaining masking) reviewed each awakening again on unscored PSG epochs to denote the cause: laboratory-induced; leg movement; nocturia; other; respiratory event; or spontaneous.
Data Analyses
All data were analyzed with SPSS version 11.0.127 for Windows (IBM SPSS Statistics, Chicago, Ill) and included socio-demographics, intake and validated questionnaires, qualitative sleep interview responses, and objective diagnostic PSG data. The McNemar test compared differences in paired proportions on self-reported and dichotomous responses regarding causes of awakenings.28 Post hoc power analysis was performed to determine sample size necessary to achieve statistical significance of correlation coefficients.29 Statistical significance was 0.05.
RESULTS
Sample Characteristics and Sleep History
Sample characteristics including sociodemographics, sleep medications, sleep symptoms, and mental health history are shown in Table 1. Of 20 patients, 17 reported both SOI and SMI, and three reported sleep maintenance problems only. Two women reported postmenopausal status, but neither was on hormone replacement therapy. Eleven of 20 patients reported mild snoring, but dividing the sample by this variable yielded no systematic distinctions between groups on any subsequent analysis of sociodemographics, validated questionnaires, subjective and objective sleep measures, and subjective and objective causes of awakenings. Sleepiness scores were low, but tiredness scores were high. Quality-of-life scores were in the fair to good range, but impairment measured on the FOSQ was severe (Table 1).
Table 1.
Sample characteristicsa
Subjective and objective sleep indices varied widely as might be common on the first night in the sleep laboratory.30 Self-reported sleep efficiency (total sleep time/time in bed) was notably worse than in the sleep laboratory (60.00% ± 12.19 versus 81.05% ± 11.57); whereas subjective time in bed (hr) was greater than the actual period in the sleep laboratory (8.54 + 1.81 versus 7.37 + 0.94). The self-report of sleep duration (hr) was shorter than the actual period in the laboratory (5.09 + 1.18 versus 5.95 + 1.11). As further indicators of insomnia, both sleep onset latency (min) (140.80 + 112.94 versus 17.35 + 15.29) and wake after sleep onset time (min) (142.00 + 96.93 versus 65.67 + 44.34) showed dramatically higher self-report values compared with objective laboratory findings.
Qualitative Interview: Etiology of Insomnia and Awakenings
Patient responses about causes of SOI and SMI revealed a predominance of psychologic factors (e.g., racing mind, worries, stress), whereas pain was the only physical factor attaining the level of 15% of the sample. No breathing symptoms were reported as a cause of SOI or SMI (Table 2).
For causation of awakenings, we compared responses acquired without prompting to responses obtained from a dichotomous choice (yes/no) interview (Figure 1). Patients manifested limited awareness of explanations for their awakenings; whereas, once a dichotomous choice was offered, attributions rose substantially with several statistically significant increases. Few patients volunteered stress as a cause of their awakenings, yet 80% affirmed stress as a causal factor when queried. Nocturia, the second most common volunteered cause, more than doubled in attributions once queried. Although the sample dichotomized their general sense about awakenings in that 60% believed mental factors predominated and 40% believed physical factors predominated, 50% were uncertain whether their responses accurately identified causes.
Objective Awakenings
A total of 531 awakenings were scored on the 20 diagnostic PSG studies. Awakenings were common for all 20 patients as evidenced by: mean (standard deviation, SD) = 26.55 (8.38) awakenings/study; median = 27.5 awakenings/study; and range = 10 – 43 awakenings/study. This number of awakenings is notably higher than that reported as published normative values (8.40 + 5.80).18 Of these 531 awakenings (Figure 2), 7 (1%) were precipitated by independent leg jerks, 14 (3%) were spontaneous, 32 (6%) were caused by interference from the laboratory environment or sleep technician, and 478 (90%) were triggered by a respiratory event. Looking at only respiratory-related awakenings, we found 374 of the 478 (78.2%) were triggered by RERAs, 93 (19.5%) by hypopneas, and 11 (2.3%) by apneas. The mean (SD) respiratory-related awakenings per patient was 23.90 (8.16) with a median of 24.5 and a range of 7–42 awakenings. Of these 478 breathing-related awakenings, 30 lasted 5 min or longer (mean 23.95 ± 23.99 min), suggesting an increased potential for a psychophysiologic response triggering increased mental alertness and progressing to an episode of insomnia. The mean durations of these 17 respiratory effort-related events, 10 hypopnea events, and three apnea events were not statistically different from each other. Of these 30 prolonged awakenings, six resulted in a trip to the bathroom, and 18 occurred in the supine position.
Figure 2.
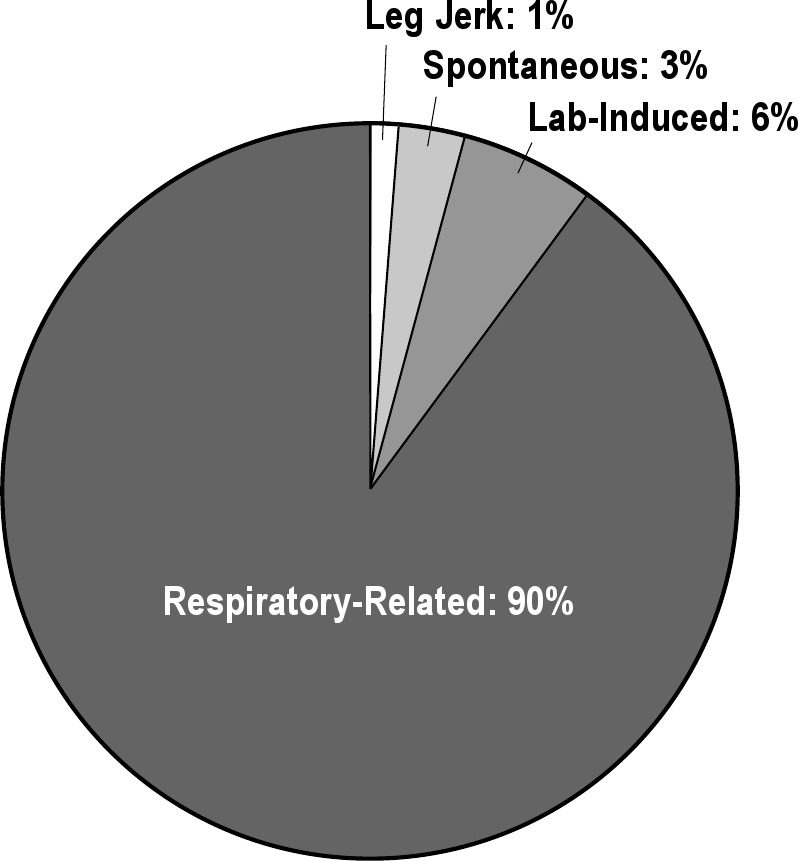
Total scored awakenings categorized by causal factor. Leg jerk: awakening preceded by independent leg jerk without any other observable precipitating event. Spontaneous: awakening without any clear-cut cause. Lab-induced: awakenings caused by entrance of PSG technician into bedroom (fix cannula, reattach lead, reposition pulse-oximeter). Respiratory-related: awakening preceded by a clear-cut breathing event (respiratory effort-related arousals, hypopnea, apnea) and without any other observable precipitating event.
Pearson correlation coefficients between insomnia severity (ISI) and respiratory variables or awakenings were largest for: total awakenings (r = 0.35; P = 0.13); total respiratory-related awakenings (r = 0.32; P = 0.18), hypopnea-related awakenings (r = 0.41; P = 0.08), and the respiratory disturbance index (RDI, r = 0.34; P = 0.14). A lack of statistical significance may be due to small sample size. Post hoc power analysis revealed a sample size of 67 patients would have been necessary to achieve statistical significance for alpha = 0.05 and power = 0.90.29
Objective Sleep Test Findings
Table 3 shows poor quality of sleep on objective findings: elevated ratios of Stage 1 and 2 nonrapid eye movement (NREM) sleep (lighter sleep); lowered ratios of rapid eye movement (REM) and Stage 3 NREM sleep (deeper sleep); frequent awakenings and awakenings per hr; and, abnormal apnea-hypopnea index (AHI) and RDI. Based on the AASM nosologic guideline of AHI ≥ 5 for the diagnosis of OSA6, 11 patients met criteria for a diagnosis (AHI = 14.39 [16.31], RDI = 35.60 [18.17]). Of the remaining nine patients, eight met AASM nosologic criteria6 (RDI ≥ 15) for upper airway resistance syndrome (UARS) (AHI = 2.44 [(1.25], RDI = 32.69 [25.24]). Using more stringent guidelines for the diagnosis of UARS (RDI > 18) proposed by Hosselet et al.,31 seven of the nine patients without OSA qualified for a diagnosis of UARS (RDI = 34.97 [26.36]). There were no significant differences between the mild snore versus nonsnore groups' AHI (14.71 ± 21.23 versus 6.05 ± 5.49 [P = 0.17]) and RDI (40.87 ± 24.26 versus 36.79 ± 19.19 [P = 0.71]).
Table 3.
Mean (standard deviation) objective sleep stage ratiosa, awakenings data, and sleep breathing data

Of potential clinical interest, we calculated the proportions derived from the total number of awakenings that were breathing-related for the 20 patients divided by the total number of their breathing events. For flow-limitation events, there were 374 ending in an awakening out of a total 2,850 flow-limitation events observed (13.1%), 93 of 918 hypopneas ended in an awakening (10.1%), and 11 of the 98 apneas observed ended in an awakening (11.2%). Thus, although most awakenings were caused by breathing events in relatively equal proportions within each category of apnea, hypopnea, or RERA, only a small proportion of breathing events led to awakenings.
Sandman 9.2 interrater reliability function analysis showed 81.76% agreement (AASM standard ≥ 80%) for scoring of breathing events between MSAS and QSS.
Posttest Interviews
After review of diagnostic studies with the second author, 17 of 20 patients (85%) accepted the possibility that SDB—a medical condition—was a contributing factor to awakenings and believed treatment of breathing disruptions would favorably affect sleep. Sixteen patients were willing to consider treatment for SDB. In recent follow-up, 12 of these 16 patients are currently being treated for SDB, including seven with oral appliance therapy and five with positive airway pressure (PAP) therapy. Of the remaining four patients, one attempted PAP therapy then ceased use, two underwent titration studies but did not fill a prescription for the device, and one completed nasal surgery. The effect of these treatments on their insomnia is not measurable due to the long interval to follow-up (range, 2-3.5 yr), the lack of a randomized control group, and the strong likelihood of numerous confounding intercurrent factors affecting outcomes.
DISCUSSION
This prospective pilot study is the first of its kind to evaluate subjective perceptions of patients with chronic insomnia about their awakenings from sleep and contrast them with objective etiologies for their awakenings. Despite our efforts to exclude patients with “complex insomnia” (comorbid insomnia and sleep breathing conditions),12 the chief objective cause for awakenings was a medical condition (a sleep breathing event) even though most patients reported unknown or psychologic precipitants as the cause. Moreover, 95% of the sample met criteria from sleep medicine nosology6 for the diagnosis of OSA or UARS.
For the 30 awakenings of clinically meaningful duration (≥ 5 min), all were preceded by breathing events. Current pathophysiology describes sleep apneic episodes as asphyxic events (hypoxic/hypercapnia), leading to sympathetic discharge involving transient increased sympathetic activity, faster heart rate, and elevated blood pressure.32,33 Could this general physiologic activation trigger or contribute to the hyperarousal activity common to patients with chronic insomnia? Once awakened by a respiratory event, the underlying hyperarousal or breathing-induced hyperarousal of patients with insomnia increases vulnerability to psychophysiologic activation in the form of waking ruminations that promote unwanted sleeplessness, but without these breathing events how would the hyperarousal theory explain insomnia episodes in this small sample of patients?
Several research groups have posited theories about hyperarousal to explain increased vulnerability to insomnia in general but not specifically to awakenings. Richardson's review34 describes four such models, including disruption of the sleep–wake homeostat; disruption of the circadian clock; failure of intrinsic sleep–wake state mechanisms (neural systems responsible for states of sleep and wakefulness); and stress response mechanisms and the related cytokine network.35,36 It is noteworthy that such theories offer clear and testable hypotheses on the nature of primary insomnia, yet none describe a specific precipitant that provokes the sleeping EEG to transition to a waking EEG for a period of 15 sec or longer.
Regardless of the specific cause of an awakening, the current findings combined with the theories discussed in the previous paragraphs agree on the singular idea that patients with insomnia awaken from sleep more easily than those who do not have insomnia, or more precisely, awaken then exhibit difficulty returning to sleep. In addition, based on clinical experience and considerable research in this field, greater vulnerability to the postawakening period often appears as or more important than the actual cause of the awakening. A 2005 study by Schwartz et al.37 exemplifies this conundrum; they studied awakenings in 50 classic apnea patients with an average RDI of 62.5 and comorbid obesity (BMI = 35.8), increased ESS scores (14.6), and no subjective or objective indications of insomnia. Comparing the number of awakenings in their group to ours, the calculated “awakening per RDI” ratio was 19% for the OSA group and 12% for our insomnia sample. Yet, even though there appear to be more awakenings in these classic OSA cases, there is no insomnia. Therefore, it may be more informative to view SDB events as a catalyst, given insomnia patients' susceptibility to transform awakenings into frank insomnia.
Randomized controlled trials (RCTs) of evidence-based treatments including PAP therapy, oral appliance therapy, or surgical interventions of the upper airway are required to determine the role of sleep breathing events in patients with chronic insomnia. To date, a few case series38–40 and a few RCTs41–43 have explored this comorbidity. The results consistently show that various SDB treatments improved insomnia in these patients. However, despite these findings, a question remains as to whether or not these patients were primarily insomnia patients or were they SDB patients with awareness restricted to their insomnia symptoms? If their insomnia improved or resolved with PAP, oral appliance therapy, surgery, or even nasal dilator strips, would it be more appropriate to categorize their insomnia as a symptom instead of a disorder? SDB treatment research must also assess effect on arousals—EEG events more common than awakenings among patients with insomnia.
The current study was undertaken with the belief that a concerted effort would enroll patients with insomnia without subjective or objective signs or symptoms of SDB. Nonetheless, the qualitative interviews revealed these patients with insomnia were perplexed by the underlying causes of their awakenings. In fairness, the cause of an awakening occurs prior to the patient waking up; therefore, how would patients with insomnia acquire useful perceptions about what disrupts their sleep? This paradox may explain the dearth of research on this behavior and why investigations have shifted toward the problem of “how do patients with insomnia respond postawakening?” Regardless, we were surprised that breathing events precipitated 90% of awakenings in contrast with our patients' listing of many other triggers in Figure 1, particularly factors such as pain or discomfort.44
This paradox also points to a larger problem in the subjective assessment of the type of patient with insomnia studied in our small sample. Our patients manifested a mean BMI < 25, reported greater clinical problems with tiredness more than sleepiness (e.g., low ESS score) (Table 1), reported no classic breathing symptoms, and presented as treatment-seeking patients with insomnia. Of clinical importance, when we assessed their risks for a sleep breathing disorder by completing the STOP-BANG questionnaire45 with their intake information, only three of 20 patients would have met criteria for PSG. Thus, only by using the AASM criteria for failure of insomnia to remit did these patients meet criteria for sleep studies (notably, persistent nonrestorative sleep and ineffective pharmacotherapy), which suggests current standards must be rigidly adhered to so that those patients requiring diagnostic PSG can be accurately identified.24
Finally, clinical research and practice are geared toward preventing awakenings or helping patients learn to return to sleep postawakening with the use of cognitive behavioral therapies46 or sedative/hypnotic agents or sedating antidepressants.47–50 The current findings suggest some proportion of patients (e.g., 75% in our sample) with chronic insomnia use prescription medications that do not directly address the inciting event that leads to their bouts of sleeplessness—a medical condition (breathing disruption) that triggered an awakening. Although such medications can depress arousability, which conceivably in our sample led to a decreased number of awakenings, these drugs are not indicated for the treatment of SDB.
The major limitation of this study is a lack of a control group of normal sleepers, which is a difficult cohort to identify and recruit for research studies.17 Thus, despite the robust findings linking breathing events to 90% of awakenings, the results of this pilot study with a small sample must be interpreted with caution. Even though there is no indication normal sleepers awaken as frequently as patients with insomnia or suffer underlying hyperarousal, the absence of objective normative values in our design prevented us from conducting the requisite analysis in comparison to patients with severe insomnia in our group. Future studies must address this obvious weakness in our protocol. In addition, more useful comparisons would examine other types of patients with insomnia, for example, those who report chronic pain as the primary cause of their sleeplessness or patients with cardiac conditions using diuretics who report nocturia as their most frequent cause of awakenings. Our sample was also skewed with 75% using prescription sedating medications regularly, which may have affected their overall sleep architecture and decreased arousal activity; however, research shows minimal respiratory depressant effects in these drugs.51 Although our findings may be generalizable to patients with insomnia seeking treatment at a sleep medical center, the typical patient with insomnia in the general population probably does not regularly use prescription sedatives.47–50 Last, an alternate interpretation of our findings is supported by emerging research showing that sleep fragmentation (common among patients with insomnia) destabilizes the human airway and creates risk for sleep breathing events.52–54 Research must address these limitations; however, we are unaware of any large-scale projects examining the clinical role of PSG in patients with chronic insomnia.55
In summary, among a small sample of patients with chronic insomnia with severe insomnia disorders, most of whom failed pharmacotherapy and all of whom manifested zero or low probability for a sleep breathing disorder, extraordinarily high rates of sleep breathing events—a medical condition—were the principal cause of their nocturnal awakenings. Replication of these findings requires investigations with larger sample sizes from diverse insomnia populations as well as research using evidence-based treatments for SDB to empirically test whether insomnia outcomes are affected by a decrease in sleep breathing events.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Krakow operates six websites that offer educational products and services, including three books on sleep disorders; he owns and operates a commercial sleep medical center, Maimonides Sleep Arts & Sciences, Ltd; he is principal investigator of the Sleep & Human Health Institute, a nonprofit sleep research institute. He received grants or consulting fees from Con Alma, Philips Respironics, GlaxoSmithKline, and Covidien. His lecture honorariums at scientific symposiums were supported in part by ResMed and Philips Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dominic Melendez, RPSGT of Quality Sleep Solutions and Jessica Sanchez, RPSGT for their participation in blindly scoring sleep studies for this prospective study.
Footnotes
A commentary on this article appears in this issue on page 1589.
REFERENCES
- 1.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 2.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 3.Ohayon MM. Nocturnal awakenings and comorbid disorders in the American general population. J Psychiatr Res. 2008;43:48–54. doi: 10.1016/j.jpsychires.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Ohayon MM, Zulley J. Correlates of global sleep dissatisfaction in the German population. Sleep. 2001;24:780–7. [PubMed] [Google Scholar]
- 5.Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey I. Sleep. 1999;22:S347–53. [PubMed] [Google Scholar]
- 6.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. pp. 51–5. [Google Scholar]
- 7.Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Med Rev. 1997;1:97–108. doi: 10.1016/s1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Hauri P, Fisher J. Persistent psychophysiologic (learned) insomnia. Sleep. 1986;9:38–53. doi: 10.1093/sleep/9.1.38. [DOI] [PubMed] [Google Scholar]
- 11.Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5–11. doi: 10.1176/appi.ajp.159.1.5. [DOI] [PubMed] [Google Scholar]
- 12.Krakow B, Melendrez D, Pedersen B, et al. Complex insomnia: insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol Psychiatry. 2001;49:948–53. doi: 10.1016/s0006-3223(00)01087-8. [DOI] [PubMed] [Google Scholar]
- 13.Wickwire EM, Collop NA. Insomnia and sleep-related breathing disorders. Chest. 2010;137:1449–63. doi: 10.1378/chest.09-1485. [DOI] [PubMed] [Google Scholar]
- 14.Krakow B, Ulibarri VA. Prevalence of sleep breathing complaints reported by treatment-seeking insomnia patients on presentation to a sleep medical center: a preliminary report. Sleep Breath. 2012 Apr 1; doi: 10.1007/s11325-012-0694-2. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 16.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 17.Fietze I, Diefenbach K. Healthy sleepers are rare: problems and success rates in establishing a control group for sleep studies. Neuropsychopharmacology. 2003;28:558–61. doi: 10.1038/sj.npp.1300082. [DOI] [PubMed] [Google Scholar]
- 18.Hirshkowitz M. Normal human sleep: an overview. Med Clin North Am. 2004;88:551–65. doi: 10.1016/j.mcna.2004.01.001. vii. [DOI] [PubMed] [Google Scholar]
- 19.Web-based intake questionnaires for the Maimonides Sleep Arts and Sciences. [Accessed June 10, 2008]. http://sleeptreatment.com/new-mexico-patients.html.
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Endicott J, Nee J, Harrison W, et al. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29:321–6. [PubMed] [Google Scholar]
- 22.Hesbacher PT, Rickels K, Morris RJ, Newman H, Rosenfeld H. Psychiatric illness in family practice. J Clin Psychiatry. 1980;41:6–10. [PubMed] [Google Scholar]
- 23.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 24.Littner M, Hirshkowitz M, Kramer, M, et al. Practice parameters for using polysomnography to evaluate insomnia: an update. Sleep. 2003;26:754–60. doi: 10.1093/sleep/26.6.754. [DOI] [PubMed] [Google Scholar]
- 25.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, Illinois: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 26.Sandman Elite Version 9.2, 2008. Nellcor Puritan Bennett, 303 Terry Fox Drive, Suite 400, Kanata, ON K2K3J1 Canada. Nellcor Puritan Bennett. 2008 [Google Scholar]
- 27.SPSS Version 11.0 for Windows, 2002. IBM SPSS Statistics, 233 S. Wacker Drive, 11th Floor, Chicago Ill, 60606. IBM SPSS Statistics. 2002 [Google Scholar]
- 28.Rabinowitz D, Betensky RA. Approximating the distribution of maximally selected McNemar's statistics. Biometrics. 2000;56:897–902. doi: 10.1111/j.0006-341x.2000.00897.x. [DOI] [PubMed] [Google Scholar]
- 29.Hulley SB. Designing clinical research. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 30.Hauri P, Olmstead E. Reverse first night effect in insomnia. Sleep. 1989;12:97–105. doi: 10.1093/sleep/12.2.97. [DOI] [PubMed] [Google Scholar]
- 31.Hosselet J, Ayappa I, Norman RG, Krieger AC, Rapoport DM. Classification of sleep-disordered breathing. Am J Respir Crit Care Med. 2001;163:398–405. doi: 10.1164/ajrccm.163.2.9808132. [DOI] [PubMed] [Google Scholar]
- 32.Cooper VL, Pearson SB, Bowker CM, Elliott MW, Hainsworth R. Interaction of chemoreceptor and baroreceptor reflexes by hypoxia and hypercapnia: a mechanism for promoting hypertension in obstructive sleep apnoea. J Physiol. 2005;568:677–87. doi: 10.1113/jphysiol.2005.094151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc. 2004;79:1036–46. doi: 10.4065/79.8.1036. [DOI] [PubMed] [Google Scholar]
- 34.Richardson GS. Human physiological models of insomnia. Sleep Med. 2007;8:S9–14. doi: 10.1016/S1389-9457(08)70003-0. [DOI] [PubMed] [Google Scholar]
- 35.Vgontzas AN, Zoumakis M, Papanicolaou DA, et al. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism. 2002;51:887–92. doi: 10.1053/meta.2002.33357. [DOI] [PubMed] [Google Scholar]
- 36.Basta M, Chrousos GP, Vela-Bueno A, Vgontzas AN. Chronic insomnia and stress system. Sleep Med Clin. 2007;2:279–91. doi: 10.1016/j.jsmc.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz DJ, Moxley P, Barker A, Longman M. On a characteristic of cortical arousals in individuals with obstructive sleep apnea. J Clin Sleep Med. 2005;1:35–40. [PubMed] [Google Scholar]
- 38.Krakow B, Lowry C, Germain A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. 2000;49:291–8. doi: 10.1016/s0022-3999(00)00147-1. [DOI] [PubMed] [Google Scholar]
- 39.Krakow B, Melendrez D, Lee SA, Warner TD, Clark JO, Sklar D. Refractory insomnia and sleep-disordered breathing: a pilot study. Sleep Breath. 2004;8:15–29. doi: 10.1007/s11325-004-0015-5. [DOI] [PubMed] [Google Scholar]
- 40.Krakow B, Melendrez D, Sisley B, Warner TD, Krakow J. Nasal dilator strip therapy for chronic sleep maintenance insomnia: a case series. Sleep Breath. 2004;8:133–40. doi: 10.1007/s11325-004-0133-0. [DOI] [PubMed] [Google Scholar]
- 41.Krakow B, Melendrez D, Sisley B, et al. Nasal dilator strip therapy for chronic sleep-maintenance insomnia and symptoms of sleep-disordered breathing: a randomized controlled trial. Sleep Breath. 2006;10:16–28. doi: 10.1007/s11325-005-0037-7. [DOI] [PubMed] [Google Scholar]
- 42.Guilleminault C, Palombini L, Poyares D, Chowdhuri S. Chronic insomnia, premenopausal women and sleep disordered breathing: part 2.Comparison of nondrug treatment trials in normal breathing and UARS post menopausal women complaining of chronic insomnia. J Psychosom Res. 2002;53:617–23. doi: 10.1016/s0022-3999(02)00463-4. [DOI] [PubMed] [Google Scholar]
- 43.Guilleminault C, Davis K, Huynh NT. Prospective randomized study of patients with insomnia and mild sleep disordered breathing. Sleep. 2008;31:1527–33. doi: 10.1093/sleep/31.11.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohayon MM. Relationship between chronic painful physical condition and insomnia. J Psychiatr Res. 2005;39:151–9. doi: 10.1016/j.jpsychires.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Silva GE, Vana KD, Goodwin JL, Sherrill DL, Quan SF. Identification of patients with sleep disordered breathing: comparing the four-variable screening tool, STOP, STOP-Bang, and Epworth Sleepiness Scales. J Clin Sleep Med. 2011;7:467–72. doi: 10.5664/JCSM.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wetzler RG, Winslow DH. New solutions for treating chronic insomnia: an introduction to behavioral sleep medicine. J Ky Med Assoc. 2006;104:502–12. [PubMed] [Google Scholar]
- 47.Ancoli-Israel S, Richardson GS, Mangano RM, Jenkins L, Hall P, Jones WS. Long-term use of sedative hypnotics in older patients with insomnia. Sleep Med. 2005;6:107–13. doi: 10.1016/j.sleep.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 48.Morin CM. Combined therapeutics for insomnia: should our first approach be behavioral or pharmacological? Sleep Med. 2006;7:S15–19. doi: 10.1016/j.sleep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Roehrs T, Roth T. ‘Hypnotic’ prescription patterns in a large managed-care population. Sleep Med. 2004;5:463–6. doi: 10.1016/j.sleep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Roth T. Introduction: advances in our understanding of insomnia and its management. Sleep Med. 2007;8:25–6. doi: 10.1016/j.sleep.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Rosenberg R, Roach JM, Scharf M, Amato DA. A pilot study evaluating acute use of eszopiclone in patients with mild to moderate obstructive sleep apnea syndrome. Sleep Med. 2007;8:464–70. doi: 10.1016/j.sleep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Luyster FS, Buysse DJ, Strollo PJ., Jr. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med. 2010;6:196–204. [PMC free article] [PubMed] [Google Scholar]
- 53.Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150:481–5. doi: 10.1164/ajrccm.150.2.8049833. [DOI] [PubMed] [Google Scholar]
- 54.Series F. Can improving sleep influence sleep-disordered breathing? Drugs. 2009;69:77–91. doi: 10.2165/11532000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 55.Ohayon MM, Krystal A, Roehrs TA, Roth T, Vitiello MV. Using difficulty resuming sleep to define nocturnal awakenings. Sleep Med. 2010;11:236–41. doi: 10.1016/j.sleep.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]