Abstract
Study Objectives:
Several studies have assessed the association between midday nap and cardiovascular outcomes and reported heterogenous results. Concern exists that confounding might have distorted these results and contributed to discrepancies among them. This study prospectively examines the association between midday nap habits and the occurrence of coronary artery disease in a non-Mediterranean population.
Participants:
The baseline examination of 4,123 participants aged 45–75 years.
Measurements:
Measurements included interviews, physical examinations, laboratory tests, and electron beam computed tomography. We studied the influence of midday nap habits on risk of coronary artery disease. We adjusted for several potential confounders including measures of subclinical atherosclerosis—such as coronary calcium score and ankle brachial index—at baseline. Cardiac events during a median follow-up of 8.1 years were defined as nonfatal myocardial infarction and sudden cardiac death.
Results:
Overall, 135 of 4,123 subjects (3.3%) either suffered from acute myocardial infarction (81 subjects) or died due to a sudden cardiac death (54 subjects) during follow-up. After adjustment for several confounders including measures of subclinical atherosclerosis, regular long (> 60 min) midday nap was associated with an increased hazard ratio of cardiac events (hazard ratio 2.12, 95% confidence interval 1.11–4.05).
Conclusions:
As our detailed confounder analyses showed, confounding is not the sole explanation for this finding. Future research on midday naps should focus on biological mechanisms that may be responsible for increasing the risk of coronary artery disease among subjects taking regular long midday naps.
Citation:
Stang A; Dragano N; Moebus S; Möhlenkamp S; Schmermund A; Kälsch H; Erbel R; Jöckel KH. Midday naps and the risk of coronary artery disease: results of the Heinz Nixdorf Recall Study. SLEEP 2012;35(12):1705–1712.
Keywords: Cohort studies, myocardial infarction, sudden cardiac death, sleep initiation and maintenance disorders, risk factors, coronary arteriosclerosis, tomography, x-ray computed
INTRODUCTION
Several epidemiologic studies have been conducted on the association between taking midday nap (in short: nap) and the incidence or mortality of coronary artery disease (CAD). Nap characteristics were associated with both an increased and decreased risk of CAD incidence or mortality including average nap duration,1,2 and frequency of naps per week.3–6 The association between naps and occurrence of CAD may be modified by gender.5
In our recent cross-sectional study, we showed that sleep characteristics are associated with classical risk factors of coronary artery disease and subclinical measures of atherosclerosis in Germany.7 Until now, studies on the relation between naps and risk of CAD have only provided relative risk estimates adjusted for classical risk factors. The lack of adjustment of subclinical measures of atherosclerosis at baseline might have produced residual confounding in those studies, that is, subclinical CAD might have prompted people to take naps and also triggered acute cardiac events. We present results of the Heinz Nixdorf Recall Study, a population-based prospective cohort study of the comparative predictive value of modern risk stratification techniques for coronary events.8,9 Our aim is to describe the association between naps and coronary artery disease (defined as nonfatal myocardial infarction or sudden cardiac death), taking into account subclinical atherosclerosis and other potential confounders.
METHODS
Design
The rationale, design, and methods of the Heinz Nixdorf Recall Study have been described in detail.8,9 Briefly, from December 2000 through August 2003, we recruited 4,814 participants aged 45–75 years residing in the industrial cities Essen, Bochum, and Mülheim, in the Ruhr area of Germany from mandatory residence lists, with a response proportion of 56%.10
Follow-Up
Annual postal questionnaires assessed the morbidity status (i.e., medication, hospital admissions, outpatient diagnoses of cardiovascular disease) during follow-up. Self-reported incident cardiovascular morbidity was validated by review of hospital records and records of the attending physicians (see below). All death certificates of the 3 cities under study were regularly screened. Deceased participants were contemporarily tracked back to obtain as much information as possible to verify causes of death.
Study Endpoints and Verification of Study Endpoints
Primary end points for this study were based on unequivocally documented coronary events that met predefined study criteria.8,9 We considered a myocardial infarction event based on symptoms, electrocardiographic signs, and enzymes (levels of creatine kinase [CK-MB]) as well as troponin T or I, and necropsy as: (1) nonfatal acute myocardial infarction; and (2) sudden cardiac death that occurred between the baseline examination and last follow-up.11 Medical records were obtained in 100% of all reported endpoints. An external criteria and endpoint committee blinded for conventional risk factor status and measures of subclinical atherosclerosis (coronary calcium score [CAC] score and ankle-brachial index [ABI]) reviewed all documents and classified potential endpoints as primary endpoints thereafter and assigned event dates.
Midday Nap Characteristics
The interview-based frequency categories for naps were never, < 1 per week, 1–4 per week, 5–6 per week, and daily. We defined naps taken ≥ 5 days per week as regular naps and naps taken less often as irregular.12 The latter group was combined with those reporting no naps, as the cardiovascular risk factor prevalence was virtually identical in these groups.7 Regular nap was categorized as short (≤ 1 h) or long nap (> 1 h). We did not ask for current night shift work. However, all participants who reported naps reported sleeping at night.
Covariates
The interview on nocturnal sleep habits included questions on sleep duration and nocturnal sleep disturbances during the 4 weeks before interview: difficulty falling asleep (DFA), difficulty maintaining sleep (DMS), and early morning arousal (EMA). Self-reported frequency of nocturnal sleep disturbances was assessed as never, sometimes (≤ 1 per week), frequently (≥ 2 times per week), and nearly every night. We defined persons having regular nocturnal sleep disturbances if they occurred nearly every night.13
The baseline examinations included interviews for cardiovascular risk factors; anthropometric measurements such as height, weight, waist-to-hip ratio, blood pressure, and ABI; comprehensive laboratory tests of blood and urine; resting and exercise electrocardiograms; and electron beam computed tomography (EBT) to assess coronary calcium quantities (CAC score) according to the method of Agatston et al.14 We assessed depressed mood with the Center for Epidemiologic Studies Depression (CES-D) scale.15,16 Participants with a self-reported history of CAD (myocardial infarction, revascularization of coronary arteries including balloon dilatation, and coronary bypass surgery) at baseline were defined as having manifest CAD and were excluded from the analysis (327 of 4,814). The study organization was certified according to DIN EN ISO9001:2000.17
Statistical Methods
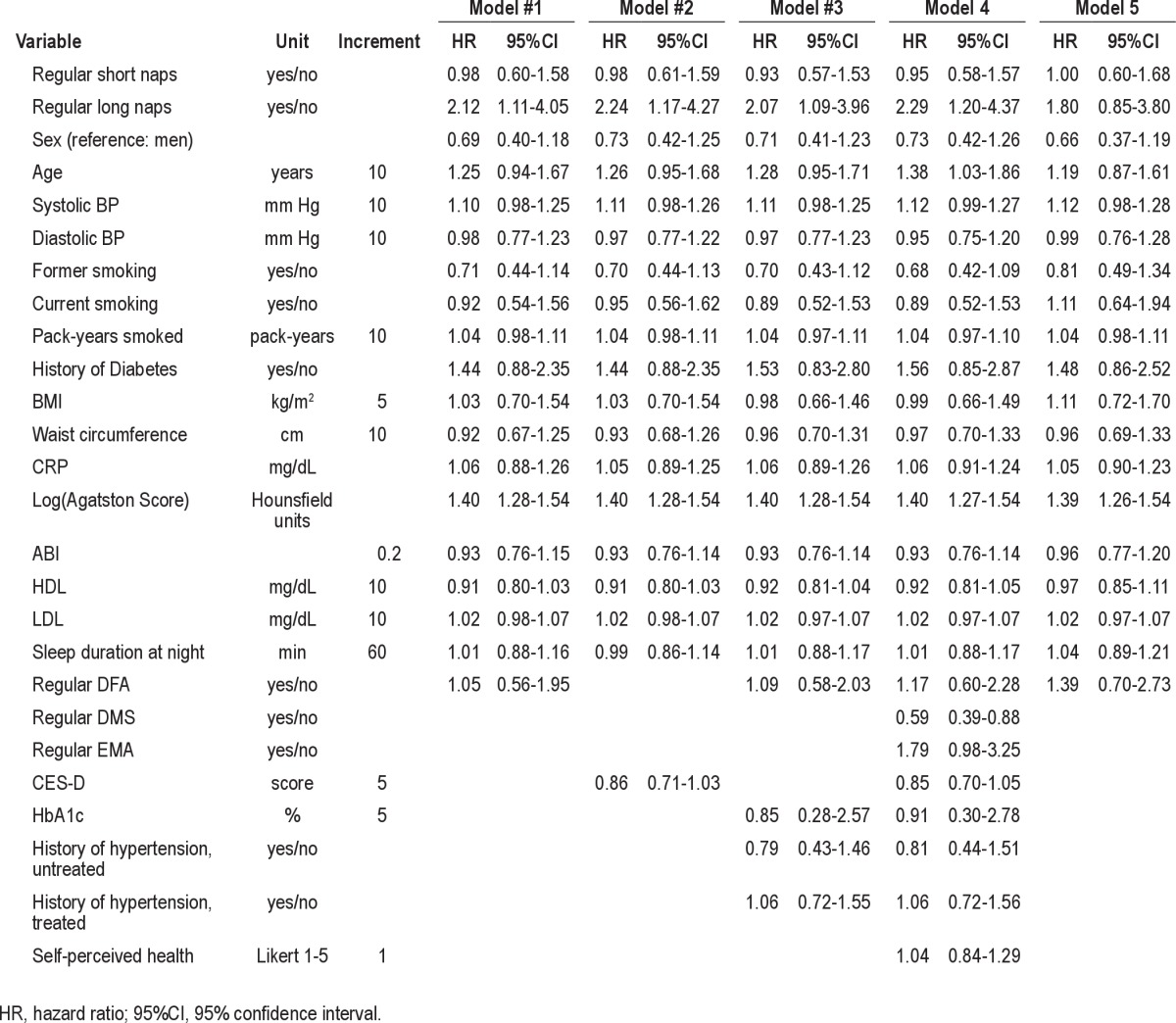
We excluded 327 subjects with manifest CAD at baseline (history of myocardial infarction, revascularization of coronary arteries). We excluded an additional 364 subjects with missing data on the exposure or any adjustment variable, resulting in a final study size of 4,123 subjects. The closing date of follow-up was June 19, 2011. The median follow-up time was 8.1 years (maximum 10.3 years). We identified minimally sufficient adjustment sets using a diagram that represents the relations among the exposure, outcome, and other variables.18 (See Figure S1 in supplemental material.) Potential adjustment variables were age and gender, CAD risk factors including blood pressure, smoking (never, former, current), diabetes, BMI, waist circumference, hs-CRP, CAC score, ABI and lipids, modified CES-D, difficulties falling asleep (DFA), and sleep duration at night. Two minimally sufficient adjustment sets included age, gender, CAD risk factors, and sleep duration at night. One also included difficulties falling asleep, the other one included CES-D.
We used crude and multivariable Cox proportional hazards regression to estimate unadjusted and adjusted hazard ratios and corresponding 95% confidence intervals (95%CIs) with respect to nap categories. We checked the assumption of proportional hazards by use of Schoenfeld residual plots. We used the Cox model to estimate adjusted probabilities of primary events by nap group based on mean values of the covariates in the model.
We performed several types of sensitivity analyses. One type addressed the grouping of naps based on the frequency per week and mean duration per day by estimating hazard ratios for more nap subgroups than in the main analysis (no naps, irregular naps, regular naps < 60 min, regular naps 60 min, regular naps > 60 min). Another type addressed confounding by adjusting for the second minimally sufficient adjustment set, by adjusting for more detailed confounders of the main analysis (HbA1c, self-reported history of hypertension and treatment of hypertension, pack-years of smoked cigarettes), and by additionally adjusting for self-perceived health in the recent 12 months, any sleep disturbance at night, and others. A third type addressed more specifically the issue of residual confounding due to undetected prodromal CAD in “healthy” nap takers despite adjustment for subclinical measures of atherosclerosis. For example, we excluded all subjects with potential symptoms of angina pectoris, with poor self-perceived health status, or with a history of stroke. As 123 of 4,123 (3.0%) subjects had missing data for depressive mood, we used multiple imputation to generate CES-D values for missing data.
RESULTS
Overall, 671 (16.3%) subjects reported regular naps (Figure 1). Whereas among men, the frequency of nap taking was not associated with the mean self-reported duration of naps, frequency of naps was positively associated with mean self-reported nap duration among women. Self-reported sleep durations of regular nap takers showed peaks at 30 min, 60 min, and 90 min. Only 105 of 671 (15.6%) reported regular naps that took more than 60 min (Figure 2).
Figure 1.
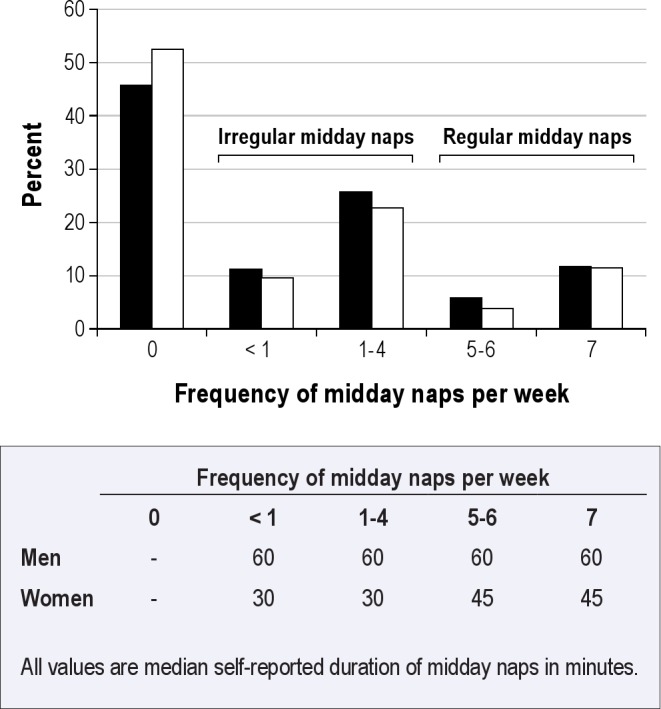
Frequency of midday naps and median self-reported midday naps durations among 4,123 subjects of the Heinz Nixdorf Recall Study, Germany, 2000–2003. Black bars, men; white bars, women.
Figure 2.
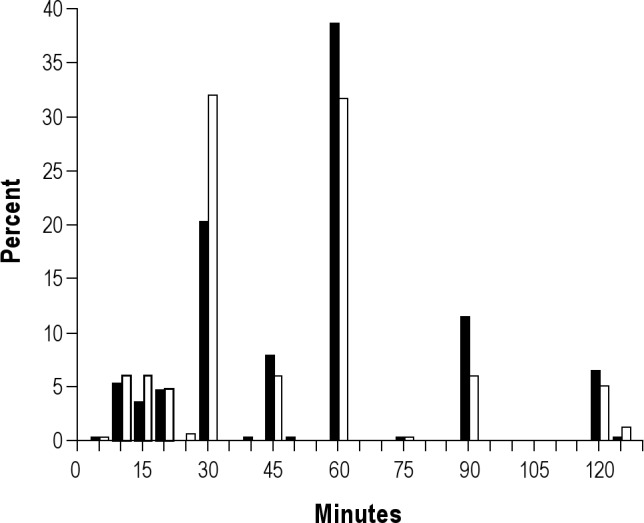
Relative frequency of self-reported durations of midday naps among 671 subjects taking at least five midday naps per week, Heinz Nixdorf Recall Study, Germany, 2000–2003. Black bars, men; white bars, women. Subjects with sleep durations > 120 min were collapsed to group 125 min (1.5% of observation).
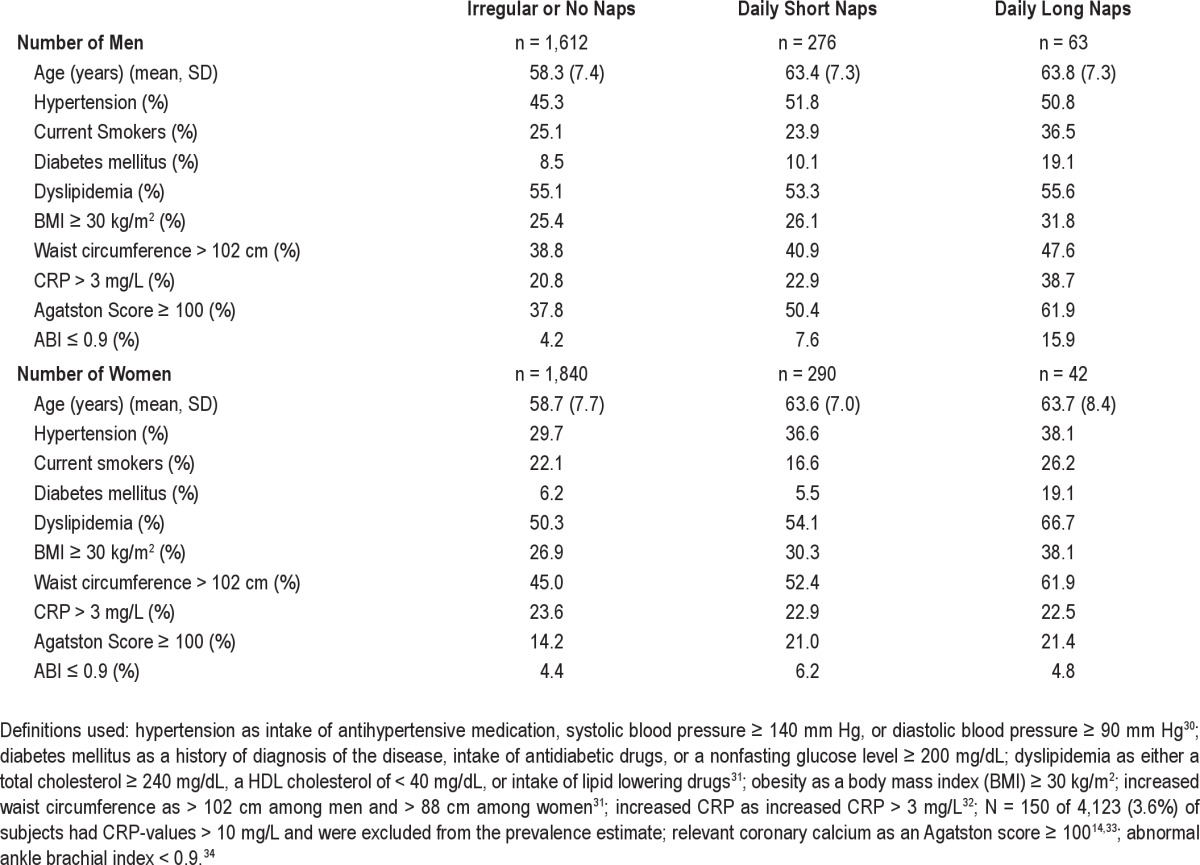
Napping was not associated with the self-reported median sleep duration at night. Regular long nap takers more frequently reported a poor self-perceived health status and more frequently were depressive according to CES-D (Table 1). Regular nap takers were older among both men and women. Regular long nap takers differed on several variables that are also potentially related to coronary artery disease including smoking, diabetes mellitus, obesity and increased waist circumference, CAC score, decreased ABI, increased CRP (men only), and dyslipidemia (women only) (Table 2).
Table 1.
Sleep characteristics, self-perceived poor health status, and depressive mood by type of midday nap taker among 4,123 subjects without manifest coronary artery disease of the Heinz Nixdorf Recall Study, Germany, 2000–2003
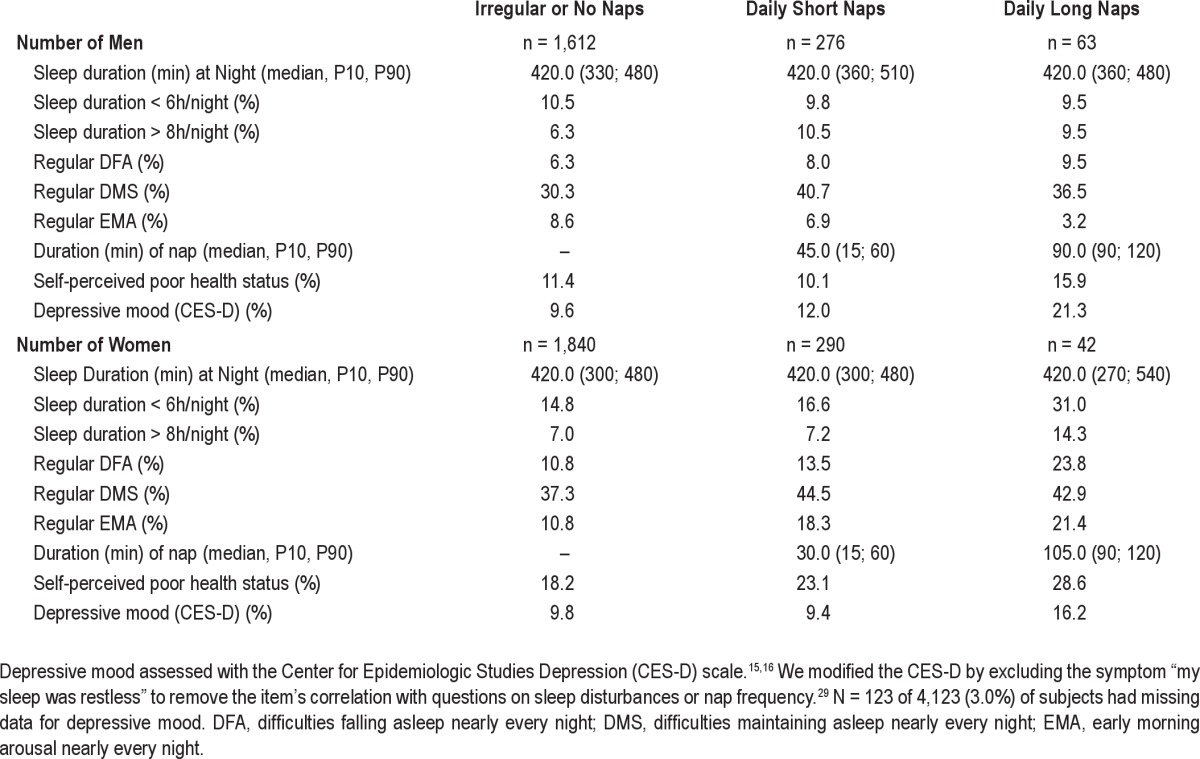
Table 2.
Cardiovascular risk factors by type of nap taker among 4,123 subjects without manifest coronary artery disease of the Heinz Nixdorf Recall Study, Germany 2000–2003
Overall, 135 of 4,123 subjects (3.3%) either suffered from acute myocardial infarction (81 subjects) or died due to a sudden cardiac death (54 subjects) during follow-up. Regular long nap takers showed considerably higher event rates than the other nap groups. The crude estimated hazard ratios comparing long nap takers with no and irregular nap takers were 3.22 (95%CI: 1.55–6.69) and 4.81 (95%CI: 1.47–15.74) among men and women, respectively (Table 3, Figure 3). Adjustment for several potential confounders including subclinical signs of atherosclerosis at baseline (ABI and CAC) only partially explained this observation. Adjusted hazard ratios were > 2 among both men and women. The estimated hazard ratio for regular long nap takers was higher for women than men (Table 3).
Table 3.
Event rates (per 1,000 person-years) and estimated hazard ratios of cardiac events according to midday napping habits
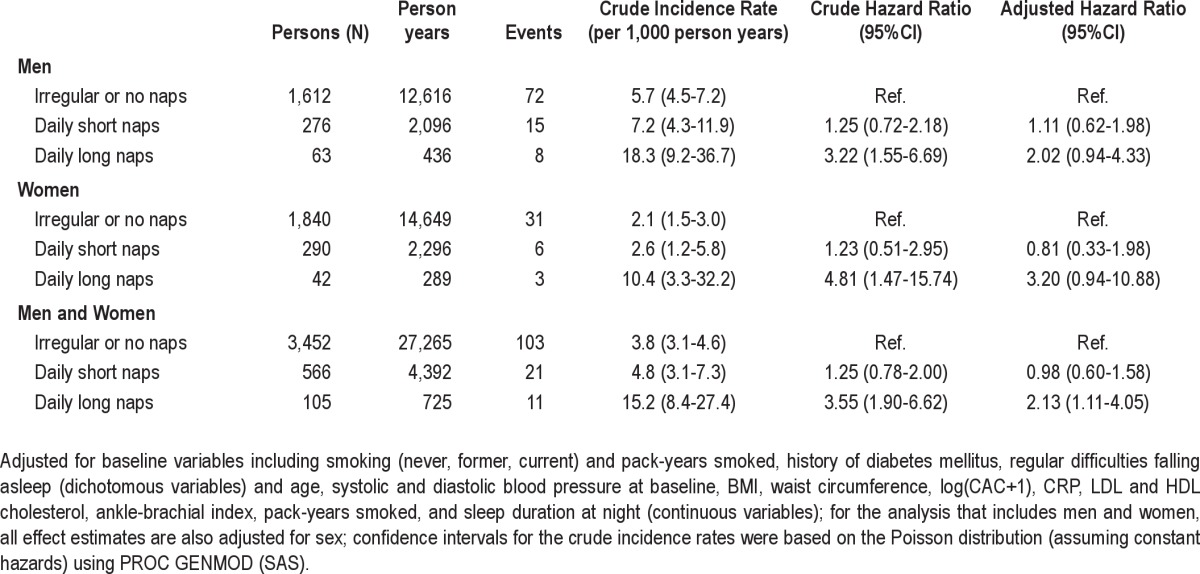
Figure 3.
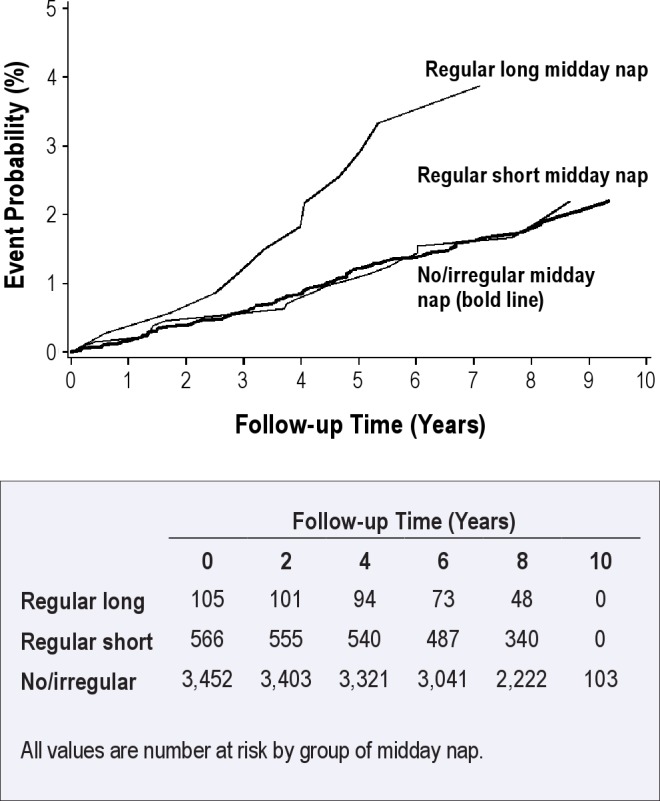
Estimated probabilities of cardiac events by categories of midday naps adjusted for potential confounders among 4,123 subjects of the Heinz Nixdorf Recall Study, Germany. Midday naps taken ≥ 5 days per week are regular midday naps; regular short midday naps (≤ 1 hour); regular long midday naps (> 1 hour).
A more detailed analysis of nap habits (no naps, irregular naps, regular naps < 60 min, regular naps 60 min, regular naps > 60 min) showed that irregular naps, regular naps < 60 min, and regular naps of 60 min were not associated with the risk of CAD. However, subjects with regular naps > 60 min had an increased risk of CAD (Table S1 in supplemental material). Sensitivity analyses that accounted for alternative confounder adjustments and a more refined adjustment of potential confounders did not markedly alter our observations. Furthermore, exclusion of subjects with angina pectoris history at baseline (Rose questionnaire) did not markedly change the results.
Only the exclusion of subjects with poor self-perceived health in the recent 12 months (at baseline) resulted in some change of the effect estimates, although the estimated hazard ratios for long nap takers remained 1.80 (95%CI: 0.85–3.80) (Table 4). The stratification of subjects by gender- and age-specific CAC score percentiles showed that long nap taking was associated with an increased risk of CAD within the group of subjects with CAC scores ≥ 50th percentile for age and gender (data not shown).
Table 4.
Sensitivity analyses of the association between type of midday naps and cardiac events among 4,123 subjects without manifest coronary artery disease of the Heinz Nixdorf Recall Study, Germany 2000–2003
DISCUSSION
Taking daily long naps was associated with cardiac events among both men and women. Adjustment for classical CAD risk factors and subclinical measures of atherosclerosis including ankle brachial index and coronary calcium did not substantially change our finding. We undertook several sensitivity analyses. However, our adjusted relative risk estimates only slightly changed.
Our study is the first that has examined the association between midday naps and CAD risk in a non-Mediterranean population in Europe. In contrast to previous studies, we were able to adjust not only for classical risk factors of CAD but also for subclinical measures of atherosclerosis at baseline, further reducing the potential of residual confounding. As prodromal undetected CAD might have prompted subjects to take naps and therefore could produce confounding, we furthermore tried to minimize this bias by exclusion of subjects with self-reported angina pectoris symptoms and poor self-perceived health status at baseline. In addition, all primary endpoints were reviewed by a blinded external endpoint committee to minimize misclassification of outcomes, and we included both nonfatal myocardial infarction and sudden cardiac deaths.
The comparison of our studies with previous studies is complicated as studies differed considerably by methodology and populations. For example, we prospectively assessed the occurrence of nonfatal myocardial infarctions and sudden cardiac deaths among subjects free of CAD at baseline; endpoints were reviewed by an external criteria and endpoint committee; and adjustment of confounding also included measures of subclinical atherosclerosis. The Athens EPIC cohort study measured CAD outcomes based on death certificates and did not adjust for measures of subclinical atherosclerosis.6 Burazeri et al. assessed cardiovascular mortality including a broad range of cardiac diseases in a population of Jerusalem residents that also included patients with CAD at baseline.5 Campos et al. studied myocardial infarction survivors in their case-control study in Costa Rica and did not adjust for measures of subclinical atherosclerosis.3 Perhaps the most important difference between our study and other studies is related to the population studied: we studied a non-Mediterranean population of Western Europe, whereas the other studies included either populations of Mediterranean regions or populations living considerably closer to the equator. Bursztyn recently stated that “in more northern or more southern countries, people may take a midday nap for different reasons: fatigue or somnolence in the former and a lifelong cultural habit in the latter.”19 In line with this view, napping in Western European non-Mediterranean populations may have different health consequences than Mediterranean populations.
Tanabe et al. recently discussed two biologic mechanisms that could explain increased risk of cardiovascular diseases among nap takers.20 First, data from 24-h ambulatory monitoring suggest that blood pressure during a daytime nap decreases to a level similar to that at night.21,22 A recent study on acute changes in cardiovascular function during the onset period of daytime sleep showed that the period between lights out and sleep onset was associated with the largest acute reduction in blood pressure during one afternoon nap.23 After awakening in the morning, blood pressure rises rapidly by activation of the sympathetic nervous system. The morning has the highest risk of the day for cardiovascular events. After nap, blood pressure also rapidly rises and might explain the association with sympathetic nervous activation and CAD risk. Second, acute changes in posture in the morning have prothrombotic effects that could also occur after daytime napping and may trigger thrombotic cardiovascular events.24,25 However, our findings imply that these effects (blood pressure rise and prothrombotic effects) are modified by nap duration, that is, these effects occur only after long naps.
In their experimental study, Mulcahy et al. analyzed changes of cardiovascular parameters (heart rate, blood pressure, and heart rate blood pressure product [called double product]) during and after a 2-h midday nap (i.e., a long midday nap) and compared these changes with changes during and after night sleep among 10 healthy subjects. The period immediately after a 2-h midday nap provided the second largest surge of the double product (98% increase) after the morning surge (102% increase).26 We could not find any sleep study that investigated the association between duration and sleep stages of midday naps and changes of cardiovascular parameters. A sleep study focusing on duration and stages of sleep at night among 14 volunteers showed that the decline of heart rate during sleep depends on the duration of sleep regardless of stage of sleep.27 A larger decline of the heart rate, blood pressure, and double product during sleep induces a larger surge of these parameters after awakening. We speculate that long midday naps are associated with larger declines of cardiovascular parameters followed by larger surges of these parameters than is seen in short midday naps.The double product is a major determinant of cardiac oxygen consumption and vascular stress impact.26,28 In addition, according to our analyses, these mechanisms would play a particularly important role among subjects with high coronary calcium burden.
There are several factors that limit our results. First, the information on sleep habits came from self-reports and was not validated. Second, the interview questions did not draw a distinction between voluntary and involuntary afternoon naps (i.e., falling asleep while intending to remain awake), which may have different health effects. We also did not ask for the context in which napping is most common, such as the typical time during the day for napping, nap environment (e.g., light and noise level), mode of awakening, sleepiness after awakening, and sleep satisfaction. Third, obesity, snoring, and daytime sleepiness have been reported to be key markers of sleep apnea,29–31 which is plausibly associated with napping. We were unable to assess the association between sleep apnea and napping because we did not collect information on snoring and sleep apnea. We therefore additionally adjusted for any sleep disturbances at night as surrogate information for sleep apnea. The weak association between napping and obesity may reflect at least some of the consequences of sleep apnea. However, additional adjustment for these variables results did not change our results. Despite several ways to address confounding including confounders such as subclinical measures of atherosclerosis at baseline and running subgroup analyses, residual confounding may be still present if the adjustment variables did not capture these confounders.
In conclusion, we found an increased risk for acute myocardial infarctions and sudden cardiac death among men and women who take regular long midday naps in a non-Mediterranean Western European population of people aged 45–75 years. As our detailed confounder analyses showed, confounding is not the sole explanation for this finding. Future research on midday napping should focus on the association between duration and sleep stages of midday nap and corresponding changes of cardiovascular and humoral factors (e.g., catecholamines, renin activity, plasma plasminogen activator inhibitor, platelet aggregability) during midday nap and thereafter.
ACKNOWLEDGMENTS
The authors thank the Heinz Nixdorf Foundation, Germany, for their generous support of this study. The authors are indebted to all study participants and to the dedicated personnel of both the study centre of the Heinz Nixdorf Recall Study and the EBT-scanner facilities as well as to the investigative group, in particular to R. Seibel, D. Grönemeyer, U. Roggenbuck, S. Slomiany, M. Bauer, R. Peter, and H. Hirche. We thank Dr. O. Kuβ for his statistical advice.
Author Contributions
R. Erbel and K.-H. Jöckel, the principal investigators of the study, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: R. Erbel, K.-H. Jöckel, A. Stang, S. Moebus, A. Schmermund, S. Möhlenkamp, N. Dragano. Acquisition of data: R. Erbel, K.-H. Jöckel, A. Stang, S. Moebus, A. Schmermund, S. Möhlenkamp, H. Kälsch, N. Dragano. Analysis and interpretation of data: A. Stang, N. Dragano. Drafting of the manuscript: A. Stang, N. Dragano, R. Erbel, K.-H. Jöckel, S. Moebus, A. Schmermund, S. Möhlenkamp, H. Kälsch, N. Dargano. Obtained funding: R. Erbel, K.H. Jöckel, A. Schmermund, N. Dragano, S. Moebus, A. Stang. Study supervision: R. Erbel, K.-H. Jöckel.
Funding/Support
This work was supported by the German Ministry of Education and Science (BMBF), and the German AeroCenter (Deutsches Zentrum für Luftund Raumfahrt [DLR], Bonn, Germany). Assessment of psychosocial factors and neighborhood level information is funded by the German Research Council (DFG; Project SI 236/8-1 and SI 236/9-1). The authors acknowledge the support of the Sarstedt AG – Co. (Nümbrecht, Germany) concerning laboratory equipment.
DISCLOSURE STATEMENT
This was not an industry supported study, but Sarstedt AG – Co. provided laboratory equipment for the study. Dr. Möhlenkamp has received speaker honoraria from AstraZeneca and Novartis Pharmaceuticals. Dr. Jöckel has received research and/or grant support from Medice Arzneimittel Pütter, Neuro Consil, Sanofi Aventis, Mundipharma, MDS Nordion, Phoenux, KKS Marburg, and ZKS Köln. He has also served as a consultant for Weinberg Group. Dr. Stang has received remuneration for a speaking engagement for Bristol-Myers Squibb. The other authors have indicated no financial conflicts of interest.
SUPPLEMENTAL MATERIAL
Causal diagram of the association between midday naps and risk of cardiac events. CAD RF, risk factors of coronary heart disease (including blood pressure, smoking, diabetes mellitus, body mass index, waist circumference, CRP, coronary calcium score according to Agatston, ankle-brachial index, serum lipids); DFA, difficulty falling asleep; Minimally sufficient adjustment set (A, R, K, U) and (A, R, F, U).
Estimated hazard ratios of cardiac events by four different categories of napping habits
REFERENCES
- 1.Trichopoulos D, Tzonou A, Christopoulos C, Havatzoglou S. Siesta and risk of coronary heart disease. Stress Med. 1988;4:143–8. doi: 10.1016/s0140-6736(87)90848-8. [DOI] [PubMed] [Google Scholar]
- 2.Kalandidi A, Tzonou A, Toupadaki N, et al. A case-control study of coronary heart disease in Athens, Greece. Int J Epidemiol. 1992;21:1074–80. doi: 10.1093/ije/21.6.1074. [DOI] [PubMed] [Google Scholar]
- 3.Campos H, Siles X. Siesta and the risk of coronary heart disease: results from a population-based, case-control study in Costa Rica. Int J Epidemiol. 2000;29:429–37. [PubMed] [Google Scholar]
- 4.Bursztyn M, Ginsberg G, Hammerman-Rozenberg R, Stessman J. The siesta in the elderly: risk factor for mortality? Arch Intern Med. 1999;159:1582–6. doi: 10.1001/archinte.159.14.1582. [DOI] [PubMed] [Google Scholar]
- 5.Burazeri G, Gofin J, Kark JD. Siesta and mortality in a Mediterranean population: a community study in Jerusalem. Sleep. 2003;26:578–84. doi: 10.1093/sleep/26.5.578. [DOI] [PubMed] [Google Scholar]
- 6.Naska A, Oikonomou E, Trichopoulou A, Psaltopoulou T, Trichopoulos D. Siesta in healthy adults and coronary mortality in the general population. Arch Intern Med. 2007;167:296–301. doi: 10.1001/archinte.167.3.296. [DOI] [PubMed] [Google Scholar]
- 7.Stang A, Dragano N, Poole C, et al. Daily siesta, cardiovascular risk factors, and measures of subclinical atherosclerosis: results of the Heinz Nixdorf Recall Study. Sleep. 2007;30:1111–9. doi: 10.1093/sleep/30.9.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmermund A, Möhlenkamp S, Stang A, et al. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL Study. Risk Factors, Evaluation of Coronary Calcium and Lifestyle. Am Heart J. 2002;144:212–8. doi: 10.1067/mhj.2002.123579. [DOI] [PubMed] [Google Scholar]
- 9.Erbel R, Mohlenkamp S, Moebus S, et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56:1397–406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Stang A, Moebus S, Dragano N, et al. Baseline recruitment and analyses of nonresponse of the Heinz Nixdorf Recall Study: identifiability of phone numbers as the major determinant of response. Eur J Epidemiol. 2005;20:489–96. doi: 10.1007/s10654-005-5529-z. [DOI] [PubMed] [Google Scholar]
- 11.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–53. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 12.Bursztyn M, Ginsberg G, Stessman J. The siesta and mortality in the elderly: effect of rest without sleep and daytime sleep duration. Sleep. 2002;25:187–91. doi: 10.1093/sleep/25.2.187. [DOI] [PubMed] [Google Scholar]
- 13.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.Radloff LS. A self-reported depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 16.Denollet J, Sys SU, Stroobant N, Rombouts H, Gillebert TC, Brutsaert DL. Personality as independent predictor of long-term mortality in patients with coronary heart disease. Lancet. 1996;347:417–21. doi: 10.1016/s0140-6736(96)90007-0. [DOI] [PubMed] [Google Scholar]
- 17.International Organization for Standardization. ISO 9001:2000. Internet. 2011 10-1-2001. [Google Scholar]
- 18.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 19.Bursztyn M. Mad dogs and Englishmen go out in the midday sun. Sleep. 2010;33:285–6. doi: 10.1093/sleep/33.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanabe N, Iso H, Seki N, et al. Daytime napping and mortality, with a special reference to cardiovascular disease: the JACC study. Int J Epidemiol. 2010;39:233–43. doi: 10.1093/ije/dyp327. [DOI] [PubMed] [Google Scholar]
- 21.Stergiou GS, Malakos JS, Zourbaki AS, Achimastos AD, Mountokalakis TD. Blood pressure during siesta: effect on 24-h ambulatory blood pressure profiles analysis. J Hum Hypertens. 1997;11:125–31. doi: 10.1038/sj.jhh.1000383. [DOI] [PubMed] [Google Scholar]
- 22.Bursztyn M, Mekler J, Wachtel N, Ben Ishay D. Siesta and ambulatory blood pressure monitoring. Comparability of the afternoon nap and night sleep. Am J Hypertens. 1994;7:217–21. doi: 10.1093/ajh/7.3.217. [DOI] [PubMed] [Google Scholar]
- 23.Zaregarizi M, Edwards B, George K, Harrison Y, Jones H, Atkinson G. Acute changes in cardiovascular function during the onset period of daytime sleep: comparison to lying awake and standing. J Appl Physiol. 2007;103:1332–8. doi: 10.1152/japplphysiol.00474.2007. [DOI] [PubMed] [Google Scholar]
- 24.Tofler GH, Brezinski D, Schafer AI, et al. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987;316:1514–8. doi: 10.1056/NEJM198706113162405. [DOI] [PubMed] [Google Scholar]
- 25.Thrall G, Lane D, Carroll D, Lip GY. A systematic review of the prothrombotic effects of an acute change in posture: a possible mechanism underlying the morning excess in cardiovascular events? Chest. 2007;132:1337–47. doi: 10.1378/chest.06-2978. [DOI] [PubMed] [Google Scholar]
- 26.Mulcahy D, Wright C, Sparrow J, et al. Heart rate and blood pressure consequences of an afternoon SIESTA (Snooze-Induced Excitation of Sympathetic Triggered Activity) Am J Cardiol. 1993;71:611–4. doi: 10.1016/0002-9149(93)90524-g. [DOI] [PubMed] [Google Scholar]
- 27.Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10:253–64. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- 28.Bursztyn M, Mekler J, Ben Ishay D. The siesta and ambulatory blood pressure: is waking up the same in the morning and afternoon? J Hum Hypertens. 1996;10:287–92. [PubMed] [Google Scholar]
- 29.Kripke DF, Ancoli-Israel S, Mason WJ. Sleep apnea: association with deviant sleep durations and increased mortality. In: Guilleminault C, Partinen M, editors. Obstructive sleep apnea syndrome: clinical research and treatment. New York: Raven Press Ltd; 1990. pp. 9–14. [Google Scholar]
- 30.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 31.Stradling JR, Davies RJ. Sleep 1: Obstructive sleep apnoea/hypopnoea syndrome: definitions, epidemiology, and natural history. Thorax. 2004;59:73–8. doi: 10.1136/thx.2003.007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 33.Rumberger JA. Clinical use of coronary calcium scanning with computed tomography. Cardiol Clin. 2003;21:535–47. doi: 10.1016/s0733-8651(03)00089-4. [DOI] [PubMed] [Google Scholar]
- 34.Kröger K, Stang A, Kondratieva J, et al. Prevalence of peripheral arterial disease-results of the Heinz Nixdorf recall study. Eur J Epidemiol. 2006;21:279–85. doi: 10.1007/s10654-006-0015-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Causal diagram of the association between midday naps and risk of cardiac events. CAD RF, risk factors of coronary heart disease (including blood pressure, smoking, diabetes mellitus, body mass index, waist circumference, CRP, coronary calcium score according to Agatston, ankle-brachial index, serum lipids); DFA, difficulty falling asleep; Minimally sufficient adjustment set (A, R, K, U) and (A, R, F, U).
Estimated hazard ratios of cardiac events by four different categories of napping habits


