Abstract
Dopamine and serotonin (5-HT) in the nucleus accumbens (ACC) and ventral tegmental area of the mesoaccumbens reward pathways have been implicated in the mechanisms underlying development of alcohol dependence. We used a C57BL/6J mouse model with increased voluntary alcohol-drinking behavior by exposing the mice to alcohol vapor for 20 consecutive days. In the alcohol-exposed mice, the expression of 5-HT2C receptor mRNA increased in the ACC, caudate nucleus and putamen, dorsal raphe nucleus (DRN), hippocampus and lateral hypothalamus, while the protein level of 5-HT2C receptor significantly increased in the ACC. The expression of 5-HT7 receptor mRNA increased in the ACC and DRN. Contents of 5-HT decreased in the ACC shell (ACCS) and DRN of the alcohol-exposed mice. The basal extracellular releases of dopamine (DA) and 5-HT in the ACCS increased more in the alcohol-exposed mice than in alcohol-naïve mice. The magnitude of the alcohol-induced ACCS DA and 5-HT release in the alcohol-exposed mice was increased compared with the control mice. Intraperitoneal (i.p.) administration or local injection into ACCS of the 5-HT2C receptor antagonist, SB-242084, suppressed voluntary alcohol-drinking behavior in the alcohol-exposed mice. But the i.p. administration of the 5-HT7 receptor antagonist, SB-258719, did not have significant effects on alcohol-drinking behavior in the alcohol-exposed mice. The effects of the 5-HT2C receptor antagonist were not observed in the air-exposed control mice. These results suggest that adaptations of the 5-HT system, especially the upregulation of 5-HT2C receptors in the ACCS, are involved in the development of enhanced voluntary alcohol-drinking behavior.
Keywords: alcohol, mesoaccumbens reward pathway, microdialysis, nucleus accumbens shell, serotonin2C receptor
Introduction
It is generally thought that alcohol is consumed for its positive reinforcing effects, and that chronic exposure to alcohol results in adaptations with abnormal drinking patterns (Clapp et al., 2008; Koob & Volkow, 2010). The dorsal raphe nucleus (DRN) supplies the ventral tegmental area (VTA) and nucleus accumbens (ACC) with serotonin (5-HT), which modulates synaptic activity in the mesoaccumbens reward pathway (Yoshimoto & McBride, 1992; Gatto et al., 1994; Koob & Le Moal, 2001). There are families of 5-HT receptors with distinct properties associated with metabotropic G-protein-coupled receptor-activated second messengers or ion channels and with amino acid sequence homologies. For example, the 5-HT2 family contains three receptor types, 5-HT2A, 5-HT2B and 5-HT2C, that are coupled to the activation of phospholipase C (Hannon & Hoyer, 2008; Hayes & Greenshaw, 2011; Kirby et al., 2011). Previously, we demonstrated that the administration of a 5-HT1A receptor-selective agonist, 8-OH-DPAT, suppressed the alcohol-induced release of dopamine (DA) and 5-HT in the ACC (Yoshimoto & McBride, 1992), and that activation of the 5-HT1A autoreceptor on the cell soma and dendrite decreases alcohol self-administration in drug-naïve rats (Silvestre et al., 1998).
Recent studies showed that the 5-HT2C receptor is linked to addictive drug effects, such as sensitization, conditioned place preference and drug self-administration (Muller & Huston, 2006; Theile et al., 2009), and is associated with side-effects of drug abuse, cocaine, including anxiety, hypolocomotion and disturbances of motor function (Barnes & Sharp, 1999; Higgins & Fletcher, 2003). While 5-HT2C receptor activation has been shown to decrease cocaine (Fletcher et al., 2004) and alcohol (Tomkins et al., 2002) self-administration, the 5-HT2A/2C receptor antagonists ritanserin and ketanserin failed to alter cocaine or amphetamine self-administration (Schenk, 2000). Generally, 5-HT2C receptors play an inhibitory role in the regulation of reward-related behavior (De Deurwaerdere et al., 2004; Hayes & Greenshaw, 2011). However, it is not clear which 5-HT receptors are involved in synaptic functions of the mesoaccumbens reward pathway that is associated with enhanced voluntary alcohol-drinking behavior.
The goal of the present study was to understand the adaptive and compensatory processes in the mesoaccumbens reward pathway, especially the serotonergic systems, that mediate the development from occasional drinking to active and compulsive drinking that leads to excessive alcohol consumption. Using an animal model with increased voluntary alcohol-drinking behavior by exposing inbred C57BL/6J mice to alcohol vapor for 20 consecutive days, we investigated the relationship between the 5-HT2C receptor in the ACC shell (ACCS) and the development of enhanced voluntary alcohol-drinking behavior.
Materials and methods
The experimental designs and procedures were approved by the Committee for Animal Research, Kyoto Prefectural University of Medicine (#21-1), Kyoto, Japan, and followed the guidelines of the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Alcohol animal models
Inbred 8-week-old male C57BL/6J mice, weighing 23–25 g (CLEA Japan, Osaka, Japan), were housed in a humidity- (60%) and temperature (23 °C)-controlled vivarium on a 12/12 h light/dark cycle (lights on 06:00 h; off 18:00 h), with ad libitum access to food (CE-2, CLEA Japan, Osaka, Japan) and water. The mice were handled briefly and placed in polycarbonate plastic cages (four or five per cage) for at least 1 week to acclimatize to our laboratory conditions. A 20-mL graduated glass tube was placed in each cage to record the consumption of tap water. To develop animal models for habitual or problem drinkers with lower sensitivity to alcohol, each standard mouse cage was kept in a sealed and clear plastic chamber into which alcohol vapor was intermittently administered, under the control of a timer, for 20 consecutive days in the vivarium.
Alcohol vapor was created by dripping 95% alcohol into a 2000-mL Erlenmeyer vacuum flask kept at 85 °C on a hot plate. Air was blown over the top of the flask at 1.5 L/min to vaporize the alcohol. The concentration of alcohol vapor was adjusted by varying the rate at which the alcohol was pumped into the flask, and ranged from approximately 22 to 27 mg/L (O’Dell et al., 2004; Walker & Koob, 2007). The animals received alcohol vapor for a maximum of 8 h per day during the dark cycle. The alcohol vapor system was turned on at 00:00 h and off at 04:00 h for the first 5 days. For the next 5 days, it was turned on at 00:00 h and off at 06:00 h. Finally, vapor was delivered from 00:00 h to 08:00 h for the last 10 days. Control C57BL/6J naïve mice were maintained under normal conditions (air-exposed groups) throughout the experimental period.
Blood alcohol concentrations were monitored at 08:00 h in two or three mice of the experimental group during the last 5 days of the alcohol vapor exposure period to confirm the maximum concentration in the preparatory experiment. Tail blood was sampled during 6 h at the end of the alcohol vapor exposure period on the last day. A sample of 20 μL of blood was obtained by the tail bleed method from 08:00 h to 14:00 h. The blood was collected into sealed 5-mL glass vials containing 5 μL of heparin (1000 USp unit/mL) as an anticoagulant and 20 μL of n-propanol as an internal standard. These vials were incubated in a water bath for 20 min at 65 °C, and 0.5 mL of vapor was then injected into a gas chromatography-flame ionization detector (GC-10A, Shimadzu, Kyoto, Japan) modified according to our previous study (Yoshimoto & Komura, 1993).
Alcohol-drinking behavior
Water was removed 12 h before performing the 4-h limited access to alcohol test. On the day of the experiment, alcohol vapor-exposed mice (n = 9) and control naïve mice (n = 8) had access to 10% (v/v) alcohol from a 20-mL graduated glass tube for a 4-h period beginning at 10:00 h. As a supplementary experiment, another group of alcohol vapor-exposed mice (n = 5) and control naïve mice (n = 5) had access to water from 20-mL graduated glass tubes for a 4-h period. The amounts consumed were measured at 0.5, 1.0, 2.0 and 4.0 h during the period of restricted access. We expressed 10% (v/v) alcohol intake as grams per kilogram per hour (the rate of alcohol consumption; g/kg/h) and total grams per kilogram in 4 hours (g/kg) and total water intake as mL per kg body weight in 4 h.
Voluntary alcohol consumption tests were performed the day after the 20-day exposure period on alcohol-exposed (n = 11) and alcohol-naïve control (n = 8) mice. Alcohol vapor-exposed mice and control mice initially had access to a 10% alcohol solution for 3 consecutive days to acclimatize them to the alcohol solution in the alcohol vapor chamber and in the normal laboratory, respectively. Then, we investigated their consumption for water or an alcohol-containing solution in the normal laboratory (Yoshimoto et al., 2000). The mice had access to 10% (v/v) alcohol and tap water in graduated glass bottles (20 mL) for 10 days. The locations of the bottles were changed daily on a random basis. The daily intakes of water (mL) and 10% alcohol (mL) were measured by means of an alcohol consumption score (g/kg body weight/day) for each mouse. Body weights were recorded every second or third day. The baseline alcohol consumption of each mouse was measured individually, and consumptions of alcohol-exposed groups and control groups were compared.
Brain monoamines and their metabolites
C57BL/6J mice of the alcohol vapor-exposed group (n = 10) and control naïve group (n = 8) were killed by decapitation at 10:00 h after alcohol inhalation for 20 consecutive days. The brain was removed, cooled on ice (this process took from 1 to 1.5 min) and then dissected using a Rodent Brain Matrix (Zivic Miller, Allison Park, PA, USA), constructed to provide coronal brain sections. Twelve brain areas, the periaqueductal gray, DRN, median raphe nucleus (MRN), VTA, substantia nigra, ACC core (ACCC), ACCS, caudate nucleus and putamen (C/P), lateral hypothalamus (LH), frontal cortex (FC), hippocampus (HP) and amygdala, were isolated using a tissue punch (1.0 mm o.d.), essentially as described by Ueda et al. (1998). The isolated brain tissues were rapidly frozen in liquid nitrogen and kept at −80 °C until the biochemical analysis. Each sample was weighed and homogenized using an ultrasonic-homogenizer in a mixture of 40 μL of 0.5 m perchloric acid, 30 μL of 0.1 m 2Na-EDTA and 30 μL of 3,4-dihydroxybenzylamine-HCl (DHBA) as internal standard. The homogenate was centrifuged (20 000 g, 20 min), and the supernatant collected and filtered using a cellulose-acetate membrane disk (Toso, Tokyo, Japan).
The tissue levels of norepinephrine (NE), DA, 3,4-dihydroxyphenylacetic acid (DOPAC), 5-HT and 5-hydroxyindoleacetic acid (5HIAA) were determined by high-performance liquid chromatography (HPLC; 3201 Nanospace, Shiseido, Tokyo, Japan) using standard curves and adjusted for handling loss with an internal standard. Separation was performed with a reversed-phase column (TSK-Gel ODS-80Ts, 5 μm particle diameter, 150 × 2.0 mm; Toso). The mobile phase consisted of 0.1 m sodium phosphate, containing 0.1 mm EDTA-2Na with 15% methanol, 5% acetonitrile and 0.3 mm sodium-1-octane sulfonic acid, adjusted to pH 2.8 with phosphoric acid. All separations were performed at a flow rate of 0.8 mL/min. The levels of DA, 5-HT and their metabolites (DOPAC and 5HIAA) were detected with electrochemical detection (ECD; 3005 Nanospace, Shiseido), using a glassy carbon electrode set at + 0.6 V vs. an Ag/AgCl reference electrode. All values are expressed as pmol/mg. Additionally, DA and 5-HT turnover indexes, changes of which indicate the increased release of DA and 5-HT at terminals, are shown as ratios of the metabolites to their respective monoamine precursors.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis: RNA preparation and real-time PCR
Another group of alcohol vapor-exposed mice and control mice (n = 5–8), as described above, were killed by decapitation at 10:00 h at the end of the first day of exposure to alcohol vapor and after 20 consecutive days of exposure. Coronal brain sections from all mice were obtained using a 2-mm punch and quickly frozen in liquid nitrogen. Total RNA from these samples was prepared using an RNAqueous Kit (Applied Biosystems, Carlsbad, CA, USA), according to the manufacturer’s instructions. cDNAs were synthesized from 1 μg of total RNA with a Transcriptor First-Strand cDNA Synthesis Kit (Roche, Basel, Switzerland), as previously described (Tanaka et al., 2009; Watanabe et al., 2009). Real-time quantitative PCR was performed in a Thermal Cycler Dice Real Time System (TaKaRa Bio, Otsu, Japan) using SYBR Premix Ex Taq II (TaKaRa Bio). PCR was carried out using 40 cycles of two-step PCR as follows: 5 s at 95 °C; and 30 s at 60 °C. Primers were designed for five 5-HT receptor subtypes: Htr1A (5-HT1A); Htr2A (5-HT2A); Htr2C (5-HT2C); Htr3 (5-HT3); and Htr7 (5-HT7); and DDR2 (DA D2 receptor) and glycerol-3-phosphate dehydrogenase (Table 1). Amplification was confirmed by a melting curve to ensure the specificity of the PCR products. On the basis of analyses and calculations integrating adequate standard curves, the relative quantification of gene expression was determined.
Table 1.
Gene names and primer sequences
| Gene name | Primer sequences |
|---|---|
| Htr1A | 5′-GGATGTTTTCCTGTCCTGGT-3′/5′-CACAAGGCCTTTCCAGAACT-3′ |
| Htr1B | 5′-TCACATGGCCATTTTTGACT-3′/5′-CAGTTTGTGGAACGCTTGTT-3′ |
| Htr2A | 5′-AGAACCCCATTCACCATAGC-3′/5′-ATCCTGTAGCCCGAAGACTG-3′ |
| Htr2C | 5′-ATGCCCCTGTCTCTGCTTGC-3′/5′-GCATAGCCGGTTCAATTCGC-3′ |
| Htr3A | 5′-CTTCCCCTTTGATGTGCAG-3′/5′-CCACTCGCCCTGATTTATG-3′ |
| Htr7 | 5′-TTCTGCAACGTCTTCATCG-3′/5′-ATTCTGCCTCACGGGGTA-3′ |
| DDR2 | 5′-GCAGGAAGCTCTCCCAGCAG-3′/5′-ATGATGGGGTTCACGGCACT-3′ |
| G3PDH | 5′-GGCATTGCTCTCAATGACAA-3′/5′-TGTGAGGGAGATGCTCAGTG-3′ |
Htr1A, 5-HT1A; Htr1B, 5-HT1B; Htr2A, 5-HT2A; Htr2C, 5-HT2C; Htr3A, 5-HT3A; Htr7, 5-HT7; DDR2, dopamine D2 receptor; G3PDH; glycerol-3-phosphate dehydrogenase.
In situ hybridization analysis of the 5-HT2C receptor
Under deep anesthesia with pentobarbital (50 mg/kg), mice were perfused via the left cardiac ventricle with 20 mL of chilled 0.1 m phosphate-buffered saline (PBS) followed by 40 mL of a fixative containing 4% paraformaldehyde in 0.1 m phosphate buffer. Brains were post-fixed in the same fixative at 4 °C overnight, and then soaked in 20% sucrose in 0.1 m PBS for 48 h at 4 °C; thereafter, frontal sections (35 μm in thickness) were cut with a cryostat and processed for in situ hybridization (Tanaka et al., 2005). Briefly, sections were deproteinized with proteinase K and acetylated in acetic anhydride and 0.1 m triethanolamine. Sections were then incubated in a hybridization buffer containing a digoxigenin (DIG)-UTP-labeled 5-HT2C riboprobe for 12 h at 60 °C. After rinsing in standard sodium citrate/50% formamide and an RNase solution, sections were transferred to a DIG coloring system (Roche Diagnostics), and reacted with 4-nitroblue tetrazolium chloride (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP) at pH 8.5 for 3 h until a blue color appeared in the cytoplasm. The antisense riboprobe for mouse 5-HT2C mRNA was synthesized from mouse 5-HT2C cDNA (1300 bp) inserted into a plasmid vector (pGEM3; Promega, Madison, WI, USA). Transcription was carried out using 120 U of T7 RNA polymerase and 1 μg of linearized template in a reaction mixture containing a DIG-labeling mixture (Roche). A sense riboprobe corresponding to the antisense probe was synthesized, but yielded no signals on in situ hybridization.
5-HT2C receptor immunohistochemistry
Coronal forebrain sections were prepared in the same manner as for the in situ hybridization experiments. Floating sections were processed for immunohistochemistry using antibodies against 5HT2C (1 : 200, sc-15081; Santa Cruz Biotechnology, Santa Cruz, CA, USA) in PBS containing 0.1% Triton X-100 for 36 h at 4 °C. The specificity of this antibody was previously examined using fluorescence immunohistochemistry (Bubar et al., 2005). For visualization using 3-3′-diaminobenzidine-4HCl (DAB; Sigma-Aldrich, St Louis, MO, USA), the sections were incubated in biotinylated anti-goat IgG (1 : 1000; Vector Lab., Burlingame, CA, USA) overnight at 4 °C and in avidin-biotin peroxidase complex solution (1 : 1000; Vector Lab) for 2 h at room temperature. Sections were exposed for 6 min at room temperature to 50 mm Tris–HCl buffer containing 0.01% DAB and 0.005% H2O2; they were then mounted on glass slides, dehydrated with a graded series of ethanol and xylene, and coverslipped with Entellan (Merk, Darmstadt, Germany). Slides were examined by light microscopy (BX50; Olympus, Tokyo, Japan).
Western blot analysis of the 5HT2C receptor in the ACC and DRN of alcohol vapor-exposed mice
Coronal sections of the ACC and DRN, obtained with a 3-mm punch, were homogenized in ice-cold PBS containing 0.5% Triton X-100 and a proteasome inhibitor cocktail (Nacalai Tesque, Kyoto, Japan). After homogenization, each sample was fractionated by centrifugation (20 000 g, 15 min, 4 °C); the supernatant was recovered and subjected to a protein assay (Watanabe et al., 2009). Protein extracts were separated by 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. After blocking, the membranes were incubated with an anti-5HT2C antibody (1 : 500) or an anti-actin antibody (1 : 1000) for 12 h at 25 °C. The membranes were then incubated with alkaline phosphatase-conjugated secondary antibodies for 1 h. Immunopositive signals were detected with the NBT and BCIP reagents.
In vivo brain microdialysis
Alcohol-naïve C57BL/6J mice (n = 20) were lightly anesthetized with chloral hydrate and placed in a Kopf stereotaxic frame. After the skull was exposed, a burr hole was drilled for a microdialysis probe guide (CMA/7 guide cannula, CMA, Solna, Sweden) that was stereotaxically implanted using the mouse brain coordinates described by Franklin & Paxinos (1997). The microdialysis guide cannula was implanted and ended 1.0 mm above the ACCS (AP, 1.2 mm anterior to the bregma; L, 0.5 mm; DV, 3.5 mm from the surface of the skull). Mice were allowed 3–5 days to recover from surgery, and were then exposed to alcohol vapor in the chamber under the same conditions as described above. After 20 consecutive days of alcohol inhalation group (n = 6) and control group (n = 6) in the normal laboratory, a mouse brain microdialysis probe (CMA/7 Microdialysis Probe, membrane length: 1.0 mm; outer diameter: 0.24 mm; steel shaft length: 7.0 mm; CMA) was inserted through the guide cannula that had been implanted over 20 days. The collections of microdialysis perfusates were then initiated approximately 3 h after inserting the probe and circulating an artificial cerebrospinal fluid (CSF) solution (in mm: NaCl, 145; KCl, 2.7; MgCl2, 1.0; CaCl2, 1.2; containing 0. mm ascorbate and pH adjusted to 7.3–7.4 with 2 mm sodium phosphate buffer) through the probe at a flow rate of 0.8 μL/min. Collections of ACCS perfusates in the control naïve mice and alcohol vapor-exposed mice were carried out only after stable pretreatment baseline values for ACCS DA and 5-HT were attained, which were usually after 60 min. Three baseline samples were collected every 20 min and analysed with the ECD-HPLC. Samples containing basal DA and 5-HT released into the ACCS were collected into a micocentrifuge tube containing 5 μL of 0.1 N HCl and 5 μL of 0.1 m 2Na-EDTA every 20 min for a 1-h period.
ACCS alcohol perfusion
Other mice with guide cannula implanted in the ACCS were maintained individually in the alcohol vapor chamber before insertion of the microdialysis probe (CMA 7 Microdialysis Probe, 1.0 mm membrane, CMA) into the ACCS through the guide cannula (CMA 7 Guide Cannula, CMA). They were allowed to move freely during the course of the experiments. The collections of microdialysis perfusates were initiated over 3 h after inserting the probe and circulating an artificial CSF. The microdialysis perfusion medium was switched from normal CSF solution to one containing 100 mm alcohol for 60 min after establishing stable baseline values of DA and 5-HT release in the ACCS dialysates, as compared with the control (n = 5) and alcohol vapor-exposed (n = 6) mice.
HPLC analysis of brain microdialysis samples
Aliquots (10 μL) of microdialysis samples were assayed for DA and 5-HT using a previously described procedure (Yoshimoto et al., 2000) modified for a small-bore ECD-HPLC to increase sensitivity. The mobile phase consisted of 20% (v/v) methanol, 0.1 m sodium dihydrogen phosphate, 0.2 mm SOS and 0.12 mm 2Na-EDTA (pH adjusted to 6.0 with 0.1 m disodium hydrogen phosphate). A C18 reversed-phase column (150 × 2.0 mm; Toso) was used at a flow rate of 0.8 μL/min (EP-700; EiCom, Kyoto, Japan). The ECD (ECD-700; EiCom, Kyoto, Japan) was usually set at a sensitivity of 0.5 nA/V with a potential of 0.65 V on the glassy carbon electrode. Detection limits were defined at approximately 0.05 nm for the DA and 5-HT dialysates. At the end of these experiments, each mouse was decapitated and the brain was removed. Coronal sections (40 μm) were cut using a cryostat microtome and stained with Cresyl violet to histologically verify placement of the microdialysis probe in the ACCS. The relative recovery rates for DA and 5-HT in these experimental conditions were about 4.4% and 3.7%, respectively.
Effects of i.p. injection of 5-HT antagonists on voluntary alcohol-drinking behavior
After exposure to alcohol vapor for 20 consecutive days, water was removed overnight before conducting the 4-h access to alcohol test. The mice were stable and exhibited reliable behavior toward alcohol and feeding. They were given doses of 0, 0.5, 1.0 and 3.0 mg/kg of 5-HT2C antagonist, SB-242084,
6-chloro-5-methyl-1-[2-(2-methylpyridyl-3-oxy)-pyrid-5-yl carbamoyl] indoline and the 5-HT7 receptor antagonist, SB-258719, 3-methyl-N-[(1R)-1-methyl-3-(4-methyl-1-piperidiny) propyl]-N-methylbenzesulfonamide hydrochloride (Sigma-Aldrich), i.p. SB-242084 and SB-258719 were first dissolved in deionized water with a drop of lactic acid to obtain a concentration of 0.1 mg/mL, and then further diluted to the required concentration, 0.1 μg/mL and 10 μm, with Ringer’s solution and the artificial CSF solution, respectively, just before administration. SB-242084 was injected into the alcohol vapor-exposed mice and control mice, and SB-258719 was injected into the alcohol vapor-exposed mice only. Control groups were given the same volume of vehicle. Thirty minutes after drug administration, the mice were given access to a 10% (v/v) alcohol solution. The consumption of alcohol (10% v/v) was measured at 0, 0.5, 1.0, 2.0 and 4.0 h in a normal laboratory.
Effects of microinjection of the 5-HT2C antagonist SB-242084 into the ACCS on voluntary alcohol-drinking behavior
Other alcohol-naïve C57BL/6J mice (n = 32) were implanted with a 24-gage microinjection guide cannula (Safelet-Cas, Nipro, Osaka, Japan) in the ACCS, as described above (Nakajima et al., 1994). Three–5 days later they were divided into two groups, alcohol vapor exposed and control. Water was removed overnight before the 4-h limited access test to show notable alcohol-drinking behavior. No significant differences in water consumption under the influence of thirst were observed between the alcohol vapor-exposed mice and control alcohol-naïve mice (Fig. 1D). On the day of the experiment, a 27-gage injection insert was attached to a microsyringe by PE-10 tubing. The 5-HT2C antagonist SB-242084 (2.5 μg/μL) or corresponding vehicle was delivered into the ACCS in a final volume of 0.2 μL at a constant flow rate of 0.1 μL/min by a 10-μL syringe and a syringe pump (CMA 400; Carnegie Medicine, Sweden; control group: saline, n = 10 and SB, n = 10; vapor group: saline, n = 6 and SB, n = 6). After the microinjection, the injection cannula was left in place for an additional 2 min before removing the cannula. At 30 min after administration of the drug or vehicle, a 10% (v/v) alcohol solution was placed in each cage. The consumption of 10% (v/v) alcohol was measured at 0, 0.5, 2.0 and 4.0 h. Results were expressed as g/kg/h and g/kg/4 h.
Fig 1.
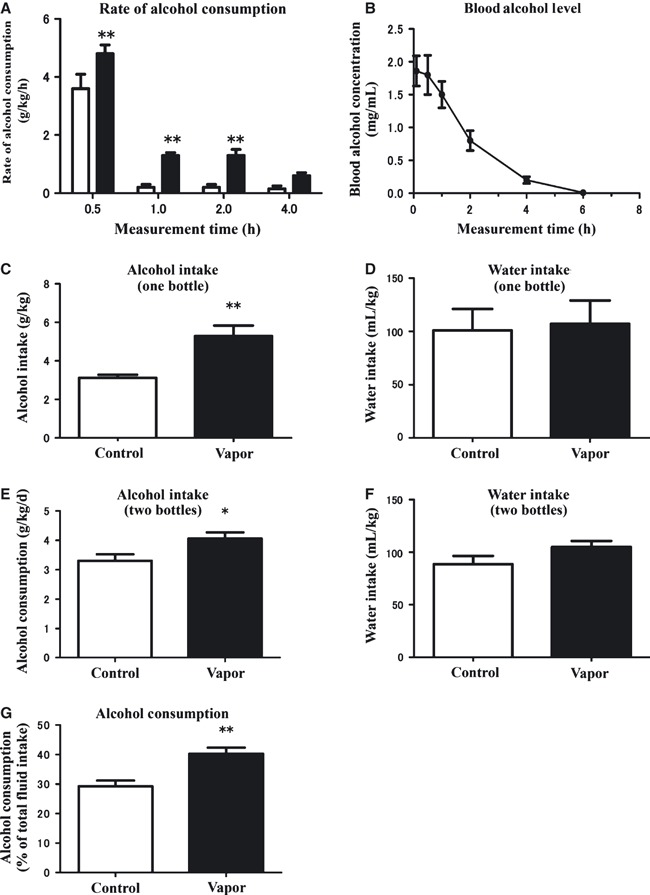
Mouse alcohol model and alcohol-drinking behavior. (A) Rate of alcohol consumption (g/kg/h) of vapor-exposed (n = 9) and alcohol-naïve (n = 8) mice during a 4-h access period. **P < 0.01 (compared with the control mice; two-way anova and then the Bonferroni post hoc t-test). (B) Changes of blood alcohol concentrations in C57BL/6J mice (n = 6) exposed to alcohol vapor for 20 consecutive days. Tail blood samples were collected at the indicated times following the end of vapor administration on the final day of alcohol inhalation. Sampling was started at 08:00 h. Values are means ± SEM. (C) Total alcohol intake (g/kg) in both groups in 4 h after a 4-h time access alcohol-drinking behavior test. **P < 0.01 (two-tailed unpaired t-test) as compared with the control mice. (D) Total water intake (mL/kg) of alcohol vapor-exposed (n = 5) and alcohol-naïve control (n = 5) mice in 4 h after a 4-h water access period. Values are means ± SEM. (E) Alcohol intake (g/kg/day) of alcohol vapor-exposed (n = 11) and alcohol-naïve control (n = 8) mice in the alcohol consumption test (two bottles). *P < 0.05 (two-tailed unpaired t-test) compared with the control mice. (F) Water intake (mL/kg/day) of alcohol vapor-exposed (n = 11) and alcohol-naïve control mice (n = 8) in the alcohol consumption test (two bottles). (G) Alcohol consumption test (% of total fluid intake) of mice exposed to alcohol vapor (n = 11) and alcohol-naïve control (n = 8) mice given the choice of water or 10% alcohol solution. **P < 0.01 (two-tailed unpaired t-test) as compared with the control mice.
Statistical analyses
Data were analysed with one- and two-way repeated-measures anovas (treatment × time) using GraphPad software. Post hoc comparisons were carried out with the Newman–Keults test, Bonferroni t-test or Tukey t-test. In all cases, the accepted level of significance was set at P < 0.05 (GraphPad Prism version 5; GraphPad Software, La Jolla, CA, USA). Body weights in the alcohol inhalation and control groups were measured at 08:00 h, the starting time of each experiment.
Results
Alcohol inhalation model and alcohol-drinking behavior
The mean maximum blood alcohol levels in alcohol vapor-exposed mice were measured at the end of 20 consecutive days of inhalation and were approximately 1.8–2.0 mg/mL at 08:00 h during the alcohol inhalation period (Fig. 1B). The blood alcohol concentration was stable during the alcohol vapor inhalation in the preparatory experiment but decreased rapidly during the primary alcohol withdrawal stage, and was approximately 0.8 mg/mL at 10:00 h when the alcohol-drinking behavior tests were carried out. There was no significant difference in body weight between the mice exposed to alcohol vapor (n = 93; 24.20 ± 1.37 g) and the control mice (n = 56; 24.15 ± 1.30 g) in the present study.
After the vapor inhalation for 20 days, water-deprived control and alcohol-exposed mice had free access to 10% alcohol for 4 h. C57BL/6J mice exposed to alcohol vapor for 20 days showed significantly higher levels of alcohol intake at 0.5, 1.0 and 2.0 h (F1,64 = 35.08, P < 0.0001, and Bonferroni post hoc test) compared with the alcohol-naïve control group (Fig. 1A), and a significantly higher total alcohol consumption (g/kg) after 4 h (Fig. 1C). There was no significant difference in water consumption between the two groups of mice (Fig. 1D). Water deprivation had no effect on the results of alcohol-drinking behavior in the 4 h access of alcohol test. It is not likely that the alcohol vapor-exposed mice took larger amounts of the 10% (v/v) alcohol solution because they were thirstier than the control mice. Mice exposed to alcohol inhalation consumed significantly more alcohol than the control mice. Mice exposed to alcohol inhalation consumed significantly higher intake than the control mice. We then measured the amounts of alcohol consumed by mice given a choice of 10% alcohol or water in the alcohol intake test. Alcohol vapor-exposed mice consumed significantly more alcohol (4.2 g/kg/day; P < 0.05, unpaired Student’s t-test) than the control group (3.2 g/kg/day; Fig. 1E). The mice exposed to alcohol vapor also showed an increase in alcohol consumption as a percentage of total fluid intake (Fig. 1G), because there was no significant difference in water intake of the two groups of mice in the alcohol consumption test (Fig. 1F). The increase in alcohol consumption among mice exposed to alcohol vapor suggests a pharmacological action of alcohol, or alcohol adaptation, in the CNS.
Monoamine levels in brain areas of mice exposed to alcohol vapor
To examine adaptive and compensatory processes in the mesoaccumbens reward pathway that mediate the development from occasional drinking to active and compulsive drinking that leads to excessive alcohol consumption, we investigated the effects of inhaling alcohol vapor for 20 consecutive days on the levels of DA and 5-HT and their metabolites, DOPAC and 5HIAA. We estimated the metabolism of DA and 5-HT by determining the levels of the monoamines and their metabolites and the ratio of [DOPAC] to [DA] and of [5HIAA] to [5-HT] in 12 brain areas of mice exposed to alcohol vapor (Table 2). Although the DA contents in the ACCC, ACCS and C/P, and the 5-HT contents in DRN and ACCS decreased significantly, the [5HIAA] : [5-HT] ratio increased in the DRN and ACCS in mice exposed to alcohol vapor (Table 2). There were no significant changes in other areas.
Table 2.
The levels of monoamines, DA and 5-HT, and their metabolites, DOPAC and 5HIAA
| pm/mg | Ratio | pm/mg | Ratio | |||||
|---|---|---|---|---|---|---|---|---|
| Area | Group | NE | DA | DOPAC | DOPAC : DA | 5-HT | 5HIAA | 5HIAA : 5-HT |
| PAG | Vapor | 0.77 ± 0.21 | 1.10 ± 0.80 | 0.80 ± 0.40 | 0.75 ± 0.41 | 1.82 ± 0.61 | 4.63 ± 0.81 | 2.91 ± 1.48 |
| Control | 0.86 ± 0.24 | 1.51 ± 1.00 | 0.80 ± 0.61 | 0.68 ± 0.38 | 2.17 ± 0.62 | 3.42 ± 1.07 | 1.90 ± 1.31 | |
| DRN | Vapor | 2.14 ± 1.45 | 1.01 ± 0.62 | 1.12 ± 0.72 | 0.92 ± 0.63 | 1.57 ± 0.40*** | 5.01 ± 1.50 | 2.96 ± 1.07*** |
| Control | 2.53 ± 1.62 | 1.12 ± 0.83 | 1.31 ± 0.84 | 1.12 ± 0.66 | 2.40 ± 0.69 | 3.28 ± 0.65 | 1.55 ± 0.99 | |
| MRN | Vapor | 1.63 ± 0.43 | 1.15 ± 0.89 | 0.81 ± 0.52 | 0.75 ± 0.27 | 1.53 ± 0.51 | 5.24 ± 1.90 | 3.50 ± 1.71* |
| Control | 2.03 ± 0.69 | 0.93 ± 1.06 | 0.44 ± 0.38 | 0.56 ± 0.25 | 2.16 ± 0.75 | 4.00 ± 1.30 | 1.88 ± 0.23 | |
| VTA | Vapor | 5.19 ± 1.19** | 1.78 ± 1.23 | 1.30 ± 0.62 | 0.98 ± 0.64 | 1.79 ± 0.77 | 3.41 ± 1.39 | 2.14 ± 1.04 |
| Control | 3.46 ± 0.86 | 1.44 ± 0.97 | 0.97 ± 0.63 | 0.74 ± 0.22 | 2.21 ± 0.29 | 3.54 ± 1.28 | 1.58 ± 0.54 | |
| SN | Vapor | 3.50 ± 0.86 | 0.91 ± 0.42 | 0.87 ± 0.30 | 1.21 ± 0.88 | 2.18 ± 0.68 | 3.83 ± 1.35 | 1.96 ± 0.99 |
| Control | 3.85 ± 1.06 | 0.93 ± 0.42 | 1.10 ± 0.90 | 0.72 ± 0.38 | 2.77 ± 0.92 | 3.20 ± 0.86 | 1.19 ± 0.20 | |
| ACCC | Vapor | 2.02 ± 1.40 | 37.84 ± 16.44*** | 16.07 ± 8.55 | 0.45 ± 0.14** | 2.11 ± 0.85 | 3.21 ± 1.07 | 1.61 ± 0.65 |
| Control | 0.80 ± 0.19 | 58.34 ± 8.46 | 12.40 ± 2.81 | 0.22 ± 0.05 | 2.16 ± 0.41 | 1.90 ± 0.38 | 0.88 ± 0.08 | |
| ACCS | Vapor | 3.30 ± 1.58 | 18.75 ± 8.50*** | 13.38 ± 4.42 | 0.85 ± 0.20** | 1.89 ± 0.54** | 3.63 ± 0.70 | 1.92 ± 0.49* |
| Control | 3.61 ± 2.20 | 26.27 ± 6.83 | 10.68 ± 7.11 | 0.41 ± 0.06 | 2.91 ± 0.69 | 2.41 ± 0.66 | 0.85 ± 0.25 | |
| C/P | Vapor | 0.33 ± 0.29 | 21.01 ± 11.67*** | 9.55 ± 4.83 | 0.56 ± 0.27 | 1.20 ± 0.15 | 3.71 ± 1.13 | 3.46 ± 1.52 |
| Control | 0.32 ± 0.26 | 29.16 ± 23.54 | 6.93 ± 3.67 | 0.35 ± 0.20 | 1.80 ± 0.80 | 2.34 ± 0.45 | 1.54 ± 0.57 | |
| LH | Vapor | 3.07 ± 1.15 | 2.25 ± 0.91 | 1.76 ± 0.53 | 0.82 ± 0.20 | 2.56 ± 0.77 | 5.57 ± 1.09 | 2.27 ± 0.45 |
| Control | 3.83 ± 0.26 | 2.05 ± 1.50 | 1.64 ± 0.49 | 1.13 ± 0.55 | 2.60 ± 0.79 | 4.72 ± 1.33 | 2.01 ± 0.96 | |
| FC | Vapor | 1.25 ± 0.31 | 0.91 ± 0.41 | 1.01 ± 0.81 | 1.22 ± 0.43 | 0.67 ± 0.20 | 0.95 ± 0.31 | 1.45 ± 0.36 |
| Control | 0.77 ± 0.08 | 1.22 ± 0.65 | 1.33 ± 0.78 | 1.42 ± 0.34 | 0.58 ± 0.18 | 0.67 ± 0.34 | 1.17 ± 0.60 | |
| HP | Vapor | 1.11 ± 0.32 | 1.31 ± 0.77 | 1.47 ± 0.77 | 1.21 ± 0.77 | 1.33 ± 0.59 | 4.02 ± 1.25** | 3.50 ± 1.50* |
| Control | 0.98 ± 0.48 | 1.81 ± 1.01 | 2.01 ± 0.67 | 1.33 ± 0.76 | 1.68 ± 0.91 | 2.33 ± 1.22 | 1.50 ± 0.57 | |
| AMY | Vapor | 1.48 ± 0.77 | 1.81 ± 0.71 | 2.01 ± 1.07 | 1.31 ± 0.71 | 2.11 ± 0.86 | 3.12 ± 0.98 | 1.41 ± 0.67 |
| Control | 2.08 ± 1.01 | 2.31 ± 1.11 | 3.04 ± 1.07 | 1.81 ± 0.92 | 3.48 ± 1.01 | 4.11 ± 1.01 | 1.22 ± 0.71 | |
5HIAA, 5-hydroxyindoleacetic acid; 5-HT, serotonin; ACCC, nucleus accumbens core; ACCS, nucleus accumbens shell; AMY, amygdala; C/P, caudate putamen; DA, dopamine; DOPAC, dihydroxyphenylacetic acid; DRN, dorsal raphe nucleus; FC, frontal cortex; HP, hippocampus; LH, lateral hypothalamus; MRN, median raphe nucleus; NE, norepinephrine; PAG, periaqueductal gray; SN, substantia nigra; VTA, ventral tegmental area. [DOPAC]/[DA] and [5HIAA]/[5-HT] ratios represent the release (turnover index) of monoamines, DA and 5-HT, respectively. Means ± SEM (vapor group, n = 10; control group, n = 8). *P < 0.05, **P < 0.01 and ***P < 0.005 (significant difference from the drug- and alcohol-naïve C57BL/6J mice, two-tailed unpaired t-test).
It is important to note that the [DOPAC] to [DA] and [5HIAA] to [5-HT] ratios in the ACCS and ACCC, and the [5HIAA] to [5-HT] ratio in the DRN significantly increased in the alcohol vapor-exposed mice. These ratios are considered an index of DA and 5-HT release (Pasinetti et al., 1992). Thus, their increases indicate increases in the release of DA and 5-HT in the ACCS, while there was no significant difference in the levels of metabolites in the DRN and ACCS after alcohol vapor inhalation (Table 2). The level of NE in the VTA, and the level of 5HIAA and ratio of [5HIAA] : [5-HT] in the MRN and HP were increased in alcohol vapor-exposed mice compared with controls (Table 2). Therefore, we propose that chronic exposure to alcohol vapor affected the DA and 5-HT systems in the ACCS of the mesoaccumbens reward pathway, which may mediate increased voluntary alcohol-drinking behavior.
mRNA expression of 5-HT and DA receptor subtypes in mice exposed to alcohol vapor
The mRNA expression levels of 5-HT1A, 5-HT2A, 5-HT2C, 5-HT3, 5-HT7 and DDR2 receptors in the ACC, C/P, DRN, HP and LH were determined in mice exposed to 1 or 20 days of alcohol vapor (Fig. 2). The complete statistical analysis of the data in Fig. 2 was shown in Table 3. The expression of 5-HT1A receptor mRNA in the C/P and LH was significantly increased after 1 day of exposure (Fig. 2A). The expression of 5-HT2A receptor mRNA was significantly increased after 1 day in the LH and after 20 days in the DRN (Fig. 2B). 5-HT2C receptor mRNA expression was significantly elevated after 1 day in C/P, HP and in all areas except LH after 20 days of exposure (Fig. 2C). No significant changes were observed for 5-HT3 RNA (Fig. 2D). The expression of new 5-HT7 mRNA was significantly increased after 1 day only in LH, and in the ACC and C/P after 20 days (Fig. 2E). Finally, the expression of DDR2 mRNA was significantly increased in the ACC, C/P and LH of mice exposed to alcohol vapor for 20 days (Fig. 2F). The particularly large increases in the ACC and C/P are consistent with a role of the DA system in the mesoaccumbens reward pathway. Expressions of 5-HT2C mRNA in the ACC, C/P and DRN, and 5-HT7 mRNA in the ACC and C/P of the mesoaccumbens reward pathway increased after 20 days alcohol inhalation (Fig. 2C and E). These serotonergic receptors may play an important role in the development of voluntary alcohol-drinking behavior or alcohol dependence. The expressions of 5-HT1A, 5-HT2A, 5-HT2C and 5-HT7 mRNA, but not 5-HT3 RNA, in the LH were increased only acutely after exposure to alcohol vapor for 1 day (Fig. 2A).
Fig 2.
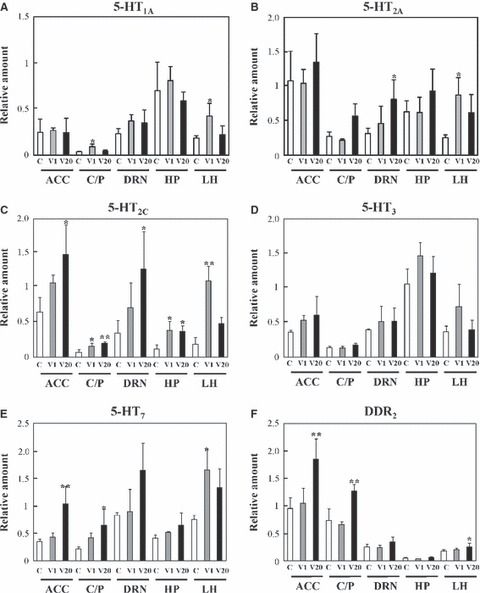
Expression of 5-HT and DA receptors subtypes. (A–F) The relative mRNA levels of (A) 5-HT1A, (B) 5-HT2A, (C) 5-HT2C, (D) 5-HT3, (E) 5-HT7 and (F) DDR2 in different brain areas of mice determined by quantitative real-time RT-PCR analysis. (C) V1, V20 denote control, alcohol vapor inhalation for 1 day, and alcohol vapor inhalation for 20 consecutive days, respectively. Values are means ± SD (n = 3–10) (*P < 0.05 and **P < 0.01; significant difference from alcohol- and drug-naïve control mice, anovapost hoc Tukey test). 5-HT, serotonin; ACC, nucleus accumbens; C/P, caudate putamen (striatum); DRN, dorsal raphe nucleus; HP, hippocampus; LH, lateral hypothalamus.
Table 3.
Statistical analyses of mRNA expressions
| ANOVA | Post hoc | ||||
|---|---|---|---|---|---|
| mRNA | Region | F-value | P-value | P-value, Tukey | |
| 5-HT1A | C/P | F2,7 = 7.32 | 0.019 | V1 (P = 0.044) | |
| LH | F2,9 = 5.90 | 0.023 | V1 (P = 0.035) | ||
| 5-HT2A | DRN | F2,9 = 4.61 | 0.042 | V20 (P = 0.048) | |
| LH | F2,7 = 4.89 | 0.047 | V1 (P = 0.039) | ||
| 5-HT2C | ACC | F2,7 = 7.32 | 0.019 | V20 (P = 0.016) | |
| C/P | F2,7 = 10.6 | 0.008 | V1 (P = 0.039) | ||
| V20 (P = 0.006) | |||||
| DRN | F2,9 = 4.87 | 0.037 | V20 (P = 0.036) | ||
| HP | F2,7 = 7.07 | 0.021 | V1 (P = 0.025) | ||
| V20 (P = 0.042) | |||||
| LH | F2,8 = 35.1 | 0.0001 | V1 (P = 0.0001) | ||
| 5-HT7 | ACC | F2,7 = 14.2 | 0.0035 | V20 (P = 0.0049) | |
| C/P | F2,8 = 4.45 | 0.050 | V20 (P = 0.043) | ||
| LH | F2,9 = 6.91 | 0.015 | V1 (P = 0.012) | ||
| DDR2 | ACC | F2,14 = 16.6 | 0.0002 | V20 (P = 0.0003) | |
| C/P | F2,17 = 38.5 | < 0.0001 | V20 (P < 0.0001) | ||
| LH | F2,16 = 3.68 | 0.049 | V20 (P = 0.042) | ||
ACC, nucleus accumbens; C/P, caudate nucleus and putamen; DRN, dorsal raphe nucleus; HP, hippocampus; LH, lateral hypothalamus.
Histochemical analysis and Western blotting of 5-HT2c in the ACC
We examined the distribution of 5-HT2C in the ACC of control mice by in situ hybridization and receptor immunohistochemistry, and analysed its expression in the ACC and DRN of alcohol vapor-exposed or control mice by Western blotting. The abundant expression of 5-HT2C was observed in the ACC using in situ hybridization (Fig. 3A) and immunohistochemistry (Fig. 3B). 5-HT2C-expressing cells were distributed in the ACCC and the ACCS.
Fig 3.
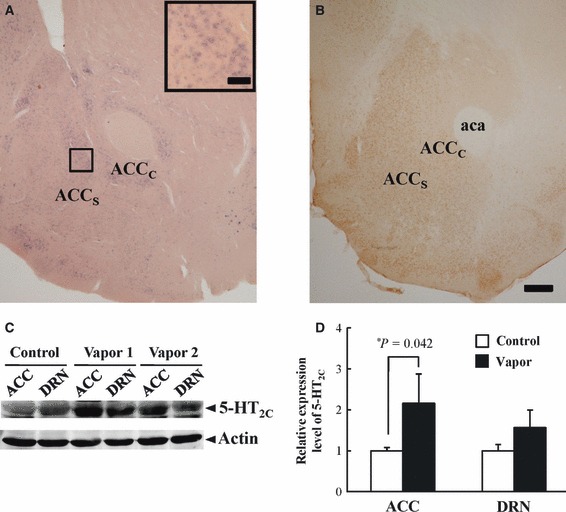
Histochemical expression and Western blotting of 5-HT2C. (A) In situ hybridization of 5-HT2C mRNA in the ACC by histochemistry. The square in the upper right is a magnified picture of the transitional zone between the two regions. (B) Immunohistochemistry of 5-HT2C in the ACC. The distribution of immunoreactive cells was similar to that of cells expressing 5-HT2C mRNA. Scale bars: 20 μm (A); 100 μm (B). (C) Expression of 5-HT2C was quantified with Western blotting analysis in different brain areas of mice exposed to alcohol vapor inhalation for 20 consecutive days, compared with the control groups. Protein extracts from alcohol vapor (lanes 3–6) or control (lanes 1 and 2) mice were subjected to Western blot analysis, followed by the immunodetection of 5-HT2C (upper) and actin (lower). (D) Band densities were quantified using Image-J software. The 5-HT2C band densities were normalized by the corresponding actin band densities. Values are means ± SD (n = 3 in each group). *P = 0.042 against control, Student’s t-test. 5-HT, serotonin; aca, anterior commissure anterior part; ACC, nucleus accumbens; ACCC, nucleus accumbens core; ACCS, nucleus accumbens shell; DRN, dorsal raphe nucleus.
Figure 3C shows Western blotting analysis of 5-HT2C receptor and actin in ACC and DRN under control and alcohol vapor conditions. The alcohol vapor inhalation for 20 consecutive days resulted in significant increase of the 5-HT2C receptor in the ACC compared with control mice (Fig. 3D).
Brain microdialysis in the ACCS of mice exposed to alcohol vapor
To more closely examine the 5-HT and DA systems, we measured the release of these monoamines in the ACCS via a brain microdialysis guide cannula and probe (placements are shown in Fig. 4A and B). We focused on the ACCS as the target area because it is linked to alcohol-drinking behavior (Koob & Volkow, 2010) and various members of the 5-HT receptor family are expressed there (Hoyer et al., 2002).
Fig 4.
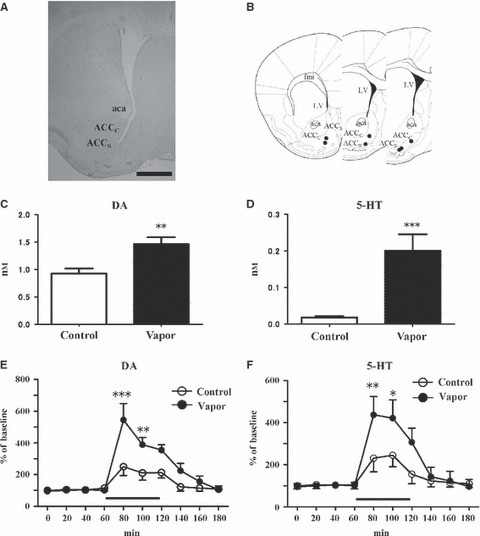
Releases of DA and 5-HT and effects of alcohol perfusion into the ACCS. (A) Placement of brain microdialysis guide cannulae or probes. All guide cannulae or probes were aimed at the right nucleus ACCS. Scale bar: 1 mm. (B) Black circles represent the guide cannula or probe tip as visualized by the manual inspection of Nissl-stained brain sections after collecting the dialysate from the ACC through the dialysis probe membrane. (C) Basal physical concentration of DA in the dialysate monitored by microdialysis in the ACCS after alcohol vapor exposure for 20 consecutive days (n = 6). **P < 0.01 compared with the alcohol-naïve control groups (n = 6; two-tailed unpaired t-test). (D) Basal concentration of 5-HT in the dialysate monitored by microdialysis in the ACCS after alcohol vapor exposure for 20 consecutive days (n = 5). Basal 5-HT release in the control group was at the limit of detection using our ECD-HPLC assay (n = 5). ***P < 0.005 comparison with the alcohol-naïve control groups (two-tailed unpaired t-test). (E, F) Time courses of changes in the extracellular levels of DA (E) and 5-HT (F) in the ACCS of the control (open circles; n = 5) and alcohol vapor-exposed mice (filled circles; n = 6) after the perfusion of 100 mm ethanol for 60 min. After establishing a stable baseline, 100 mm alcohol was perfused with the artificial CSF for 60 min through the microdialysis probe membrane (bar). *P < 0.05, **P < 0.01 and ***P < 0.005 (compared with the control mice; one-way anova and Bonferroni’s post hoc t-test). Values are means ± SEM. 5-HT, serotonin; aca, anterior commissure anterior part; ACCC, nucleus accumbens core; ACCS, nucleus accumbens shell; DA, dopamine; fmi, forceps minor corpus callosum; LV, lateral ventricle.
The basal extracellular concentration of DA in the dialysates of mice exposed to alcohol vapor (1.56 ± 0.25 nm) was significantly higher than that in the control mice (0.97 ± 0.15 nm; Fig. 4C). The extracellular concentration of 5-HT was also significantly higher in mice exposed to alcohol vapor (0.22 ± 0.10 nm; Fig. 4D) compared with the control group (the detectable limit of 5-HT with the ECD was approximately 1.0 pm).
Alcohol perfusion in the ACCS of mice exposed to alcohol vapor
To examine the direct effects of alcohol on monoamine release, we directly perfused the ACC with alcohol. Administration of alcohol (100 mm) through the microdialysis membrane increased the release of DA in both groups. However, the magnitude of the release was significantly greater in the alcohol vapor-exposed group (Fig. 4E; treatment: F1,90 = 20.54, P < 0.0001; time: F9,90 = 17.65, P < 0.0001). Alcohol-induced 5-HT release was also higher in the vapor-exposed mice than in the control group (Fig. 4F; treatment: F1,90 = 8.11, P < 0.005; time: F9,90 = 10.57, P < 0.0001). These results suggest that the varicosities and terminals of 5-HT neurons in the ACCS of alcohol vapor-exposed mice had developed greater sensitivity to alcohol.
Effects of i.p. injection of the systemic 5-HT antagonists on voluntary alcohol-drinking behavior
To examine the connection between 5-HT receptors (specifically 5-HT2C and 5-HT7 receptors) and drinking behavior, we systemically administered specific 5-HT receptor subtype antagonists and investigated alcohol-drinking behavior over a 4-h period in alcohol vapor-exposed and control mice. There was no significant effect of SB-242084 in the control mice (time × treatment: F9,160 = 1.312 n.s.; time: F3,160 = 101.8, P < 0.0001; treatment: F3,160 = 1.876 n.s.; Fig. 5A). On the other hand, in the alcohol-exposed mice, alcohol consumption decreased in an SB-242084 dose-dependent manner (F3,116 = 29.68, P < 0.0001) and altered in the time course (F3,116 = 106.7, P < 0.0001).Treatment with SB-242084 (0.5–3.0 mg/kg, i.p.) significantly reduced voluntary alcohol-drinking behavior in the alcohol-exposed mice (time × treatment: F9,116 = 98.62, P < 0.0001; Fig. 5B). We observed no significant differences in voluntary alcohol consumption in the alcohol-exposed groups following the administration of the 5-HT7 receptor antagonist, SB-258719 (Fig. 5C).
Fig 5.
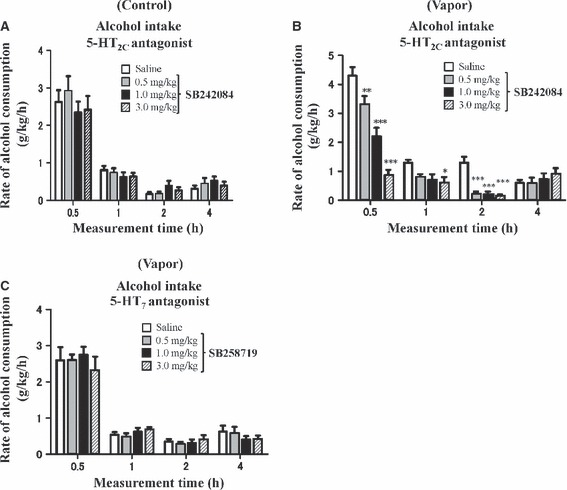
Effects of systemic 5-HT receptor antagonists on drinking behavior. (A, B) Rate of alcohol consumption (g/kg/h) over 4 h following administration of the 5HT2C antagonist SB-242084 (0, 0.5, 1.0 and 3.0 mg/kg i.p.) in control mice (A) (total n = 44) and mice exposed to alcohol for 20 consecutive days (B) (total n = 32). *P < 0.05, **P < 0.01 and ***P < 0.005 compared with saline vehicle (Bonferroni post hoc t-test). (C) Rate of alcohol consumption (g/kg/h) over 4 h following administration of the 5-HT7 receptor antagonist SB-258719 (0, 0.5, 1.0 and 3.0 mg/kg i.p., total n = 32) in mice exposed to alcohol for 20 consecutive days. All drug treatments were administered 30 min prior to the initiation of the drinking test. Values are means ± SEM. 5-HT, serotonin.
Effects of microinjection of SB-242084 into the ACCS on voluntary alcohol-drinking behavior
We investigated the effect of microinjecting SB-242084 into the ACCS on alcohol-drinking behavior over a 4-h period in the alcohol vapor-exposed and control mice. Treatment with SB-242084 (0.5 μg) significantly decreased voluntary alcohol-drinking behavior at 0.5 h (treatment: F1,80 = 6.233, P < 0.05; time: F3,80 = 46.27, P < 0.0001; and treatment × time: F3,80 = 2.206, n.s.) in the control groups, and at 0.5 and 1 h (treatment: F1,48 = 15.00, P < 0.005; time: F3,48 = 73.81, P < 0.0001; treatment × time: F3,48 = 2.932, P < 0.005) in the alcohol vapor-exposed mice, respectively (Fig. 6A and C). The microinjection of SB-242084 into the ACCS of mice exposed to alcohol vapor inhalation caused a significant decrease in total 4-h alcohol consumption (P < 0.05, unpaired Student’s t test) compared with saline (Fig. 6D). In contrast, there was no significant difference in the control naïve mice (Fig. 6B).
Fig 6.
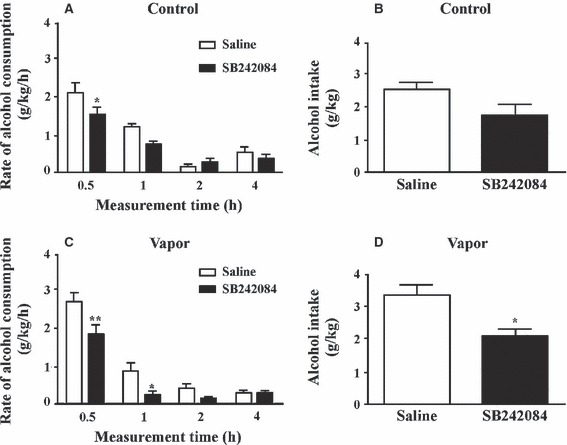
Effects of microinjection of SB-242084 into the ACCS on drinking behavior. (A, C) Effects of the microinjection of 5-HT2C antagonist SB-242084 into ACCS of the control mice and mice exposed to alcohol vapor inhalation on the rate of alcohol consumption (g/kg/h). SB-242084 (0.5 μg/0.2 μL) or the same volume of vehicle were administered in the control (A: saline, n = 10; SB, n = 10) and alcohol vapor inhalation groups (C: saline, n = 6; SB, n = 6). Each mouse received all treatments at 30 min prior to the initiation of the test. Two-way anova and Bonferroni post hoc test (*P < 0.05 and **P < 0.01). (B, D) Effects of the microinjection of 5-HT2C antagonist SB-242084 into the ACCS on the total alcohol intake (g/kg) in the control (B: n = 16) and mice exposed to alcohol vapor inhalation at the end of 20 consecutive days (D: n = 16). *P < 0.05 (two-tailed unpaired t-test) as compared with the control mice. Values are means ± SEM.
Discussion
An adequate animal model exhibiting the symptoms of alcoholism is of critical importance to understanding brain mechanisms in alcoholics. However, alcoholism is a complex behavioral disorder involving the excessive intake of alcohol, and the development of tolerance and dependence. The inhalation procedure has advantages, because the alcohol dose and duration of exposure can be precisely controlled, and the level of intoxication can be maintained stable (Becker, 2000). Chronic intermittent alcohol vapor inhalation in male C57BL/6J mice was reported to result in an increased alcohol-drinking behavior when blood alcohol concentration reached above 1.75 mg/mL (Griffin et al., 2009). In the present study, C57BL/6J mice showed a significantly higher level of alcohol intake after alcohol vapor inhalation for 20 consecutive days (Fig. 1G) than after normal air inhalation (control) without any physiologically serious withdrawal syndromes or changes in body weight. C57BL/6J mice given intermittent access of 20% (v/v) alcohol on alternate days showed more than 20 g/kg/day of ethanol intake for 3 weeks, which was greater than mice given continuous alcohol access (Hwa et al., 2011). In the present study the time of alcohol vapor exposure was restricted to 8 h/day for 20 days to simulate an intermittent access to alcohol solutions. An intermittent access method may best model human alcohol dependence with withdrawal symptoms. After the exposure of alcohol inhalation for 20 days, alcohol-exposed mice showed alcohol intakes of approximately 5.3 g/kg/4 h and 4.2 g/kg/day on the time access alcohol test and alcohol consumption test, respectively (Fig. 1C and E). On the other hand, C57BL/6J mice given continuous access to 10% and 20% alcohol showed about 9.3 g/kg/day and 11.8 g/kg/day of alcohol intake, respectively (Hwa et al., 2011). The intermittent access to 20% alcohol drinking protocol can produce alcohol intakes of up to two to three times higher in the inbred strains of mice. Different values of alcohol consumption in the same inbred strains of C57BL/6J mice may result from different drinking metrics in the two-bottle alcohol test. The use of intermittent or restricted access to alcohol may be advantageous in the development of alcohol animal models.
The role of the 5-HT system in voluntary alcohol-drinking behavior has been extensively investigated (Lesch, 2005; Kirby et al., 2011). Voluntary alcohol intakes are negatively correlated with levels of 5-HT in the mesoaccumbens reward pathway (McBride et al., 1993), and low levels of 5-HT neurotransmission are associated with increased alcohol-drinking behavior (Strother et al., 2005). In the selectively bred, alcohol-preferring and non-preferring lines of rats, levels of 5-HT in the FC, ACC and C/P were lower in alcohol-preferring rats (Zhou et al., 1994). In the present study, lower levels of 5-HT in the DRN and an increase in the [5HIAA] to [5-HT] ratio in the DRN and ACCS were found in the alcohol vapor-exposed mice that exhibited increased alcohol-drinking behavior (Table 2). An increase in the [5HIAA] to [5-HT] ratio indicates an increase in 5-HT release, as supported by its upregulation in the synaptic terminals of the ACCS (Fig. 4D).
Alcohol activates the release of DA and 5-HT in the ACC, which is a key area in the mesoaccumbens reward pathway (Yoshimoto & McBride, 1992; Clapp et al., 2008). Recent results indicate that the ACCS, but not ACCC, is a site that mediates the reinforcing action of alcohol, and that the ACCS of alcohol-preferring rats is more sensitive to the reinforcing effects of alcohol (Engleman et al., 2009). This suggests the possibility that mice with increased alcohol-drinking behavior may require alcohol to stimulate the release of sufficient amounts of DA and 5-HT in the ACCS (Fig. 4C and D) as a reward. In other words, these mice consume alcohol to maintain a moderately high blood alcohol level (Fig. 1B) to adapt to or compensate for the increased activity of 5-HT in the DRN-ACCS 5-HT system.
Our regimen of alcohol vapor inhalation resulted in increased 5-HT release in the ACCS and decreased 5-HT content in the DRN and ACCS (Fig. 4D; Table 2), and induced an elevation of 5-HT availabilities in the synaptic clefts of the ACCS. Furthermore, a higher release of 5-HT of the ACCS with alcohol perfusion via a microdialysis membrane in mice exposed to alcohol vapor inhalation than in control mice (Fig. 4F) would estimate more activated serotonergic neurons in the ACCS. These results may be related to inhibition of clearance independent of the serotonin transporter (SERT), because physiologically relevant doses of alcohol inhibited the clearance of 5-HT from extracellular fluid in the HP of a knockout mouse with the genetic inactivation of SERT (Boyce-Rustay et al., 2006; Daws et al., 2006). Increased voluntary alcohol drinking to sustain the biological availability of 5-HT in the ACCS is associated with the adaptation of 5-HT2C receptor functions as evidenced by increased 5-HT2C expression in the ACC (Figs 1G, 2C and 3C). Together, these observations suggest that alterations in 5-HT2C receptor functions and 5-HT transmission in the ACCS contribute to the drive to consume alcohol.
In the development of enhanced voluntary alcohol-drinking behavior in mice exposed to alcohol vapor we found the increased expression of specific 5-HT and DA receptor subtypes in the reward pathway (Fig. 2). The expression of 5-HT2C receptor in mice exposed to alcohol vapor was significantly elevated in the ACC of the mesoaccumbens reward pathway (Fig. 3D), suggesting that enhanced alcohol drinking in the alcohol models was strongly attributed to adapted 5-HT2C receptor functions in the ACC. 5-HT2A mRNA in the DRN was also increased following alcohol inhalation (Fig. 2B). Stimulation of 5-HT2A and 5-HT2C receptors coupled to the Gq protein activates phospholipase C (Muller & Huston, 2006); thus, activation of the phosphatidylinositide-signaling pathway may be involved with the development of increased alcohol drinking. And it follows then, that increased 5-HT2C receptor density in the alcohol models might have caused the upregulation of serotonergic function in the ACCS participating in the development of voluntary alcohol-drinking behavior. Furthermore, the expression of 5-HT7 receptor mRNA, coupled with Gs proteins (Barnes & Sharp, 1999), in mice exposed to alcohol vapor was increased in the ACC and C/P (Fig. 2E). In the present study, no changes in 5-HT3 receptors were observed in the development of enhanced alcohol-drinking behavior (Fig. 2D).
Both the i.p. administration and microinjection into the ACCS of SB-242084 suppressed the consumption of alcohol in a dose-dependent manner (Figs 5B and 6C). When the 5-HT2C antagonist was administered during the primary stage of alcohol withdrawal, alcohol drinking was suppressed (Figs 5B and 6C). It was suggested that the alcohol-exposed mice showed the alteration of 5-HT2C receptor sensitivity to alcohol with the increase of density in the ACC (Fig. 3D). In accordance with this, the 5-HT2C receptor antagonist mianserin blocked the development of alcohol sensitization when given in the acute or primary phase of daily alcohol injection (Ferraz & Boerngen-Lacerda, 2008). Similarly, pretreatment with 1 mg/kg SB-242084 subcutaneously completely prevented cocaine-seeking behavior (Burbassi & Cervo, 2008). We explain this as follows: the 5-HT2C antagonist SB-242084 antagonized 5-HT2C receptor function and may alter the clearance of 5-HT in the ACCS of alcohol vapor-exposed mice, thereby suppressing alcohol-drinking behavior (Figs 5B and 6D). Regarding the relationship between the activation of 5-HT clearance and alcohol-drinking behavior, it was reported that pharmacological agents, fluoxetine, imipramine and amitriptyline, which inhibit 5-HT reuptake from the synapses, reduce alcohol-drinking behavior (Nestler & Aghajanian, 1997). Furthermore, SERT knockout mice exhibit decreased alcohol preference and consumption (Boyce-Rustay et al., 2006). We observed increases in 5-HT2C receptor density after chronic intermittent alcohol vapor inhalation that contribute to the increase of 5-HT release in the ACCS (Figs 2C and 4D). During chronic alcohol vapor exposure, there are adaptive changes related to tolerance to alcohol, or increased alcohol-drinking behavior, that result in increases in 5-HT2C receptor density that contribute to the excitatory effects and the increase of 5-HT release in the ACCS (Figs 2C and 4D). The potentiation of selective serotonin reuptake inhibitor (SSRI) fluoxetine-induced elevation in hippocampal 5-HT levels was reproduced by the 5-HT2C receptor-selective antagonist SB-242084. 5-HT2C receptor antagonist augmented the action of SSRI as the availability of synaptic 5-HT (Cremers et al., 2004). It was suggested that the compound SB-242084 shows the possibility of dual properties, working as an antagonist of 5-HT2C receptor as well as an indirect 5-HT uptake blocker, thereby increasing the availability of synaptic 5-HT in the ACCS of mice exposed to alcohol vapor.
It was reported that 5-HT2C receptor-mediated phosphoinositide hydrolysis associated with phospholipase C was elevated more in alcohol-preferring P rats (alcohol-drinking models) than in non-preferring rats (Pandey et al., 1996; Qu et al., 2005). This suggests that the local injection of SB-242084 antagonizes the 5-HT2C-receptor-mediated phosphoinositide hydrolysis in the ACCS. Thus, both an elevation of Ca2+ with the increase of inositol 2,4,5-triphosphate and the activation of 5-HT2C receptor-mediated phosphoinositide hydrolysis associated with phospholipase C in the ACCS appear to be involved in Ca2+-triggered serotonergic neural firing mechanism (Sudhof, 2004) and the enhanced alcohol-drinking behavior in mice exposed to alcohol vapor.
In addition to the 5-HT2C receptor, other 5-HT receptors may also be involved in increased alcohol consumption. Although the expression of 5-HT7 mRNA also increased in mice exposed to alcohol vapor (Fig. 2E), the 5-HT7 receptor antagonist had no significant effect on alcohol-drinking behavior in mice exposed to alcohol vapor (Fig. 5C). There is accumulating evidence that the 5-HT7 receptor plays a role in psychiatric disorders (Bonaventure et al., 2002; Mnie-Filali et al., 2007; Sarkisyan et al., 2010). Interestingly, the administration of alcohol vapor for just 1 day resulted in an acute, but transient, increase in the expression of 5-HT1A, 5-HT2A and 5-HT2C, and 5-HT7 receptor mRNAs in the LH (Fig. 2).
In conclusion, we found that 8-week-old male C57BL/6J mice exhibited enhanced alcohol-drinking behavior after being exposed to alcohol vapor for 20 consecutive days. There was no significant difference in water intake and body weight between the alcohol vapor-exposed mice and control mice. This occurred in association with an increased expression of the 5-HT2C receptors in the ACCS. The administration of a 5-HT2C antagonist, not only through systemic infusion but also through local injection into the ACCS, suppressed the increase in alcohol-drinking behavior and alcohol preference in alcohol vapor-exposed mice. These effects were not observed after the administration of a 5-HT7 antagonist. Therefore, our findings support the hypothesis that the activation of 5-HT2C receptors in the ACCS of the mesoaccumbens reward pathway plays a pivotal role in increased alcohol-drinking behavior.
Acknowledgments
This work was supported by Grants-in-Ais for Scientific Research to K.Y. and M.T. and ‘Development of biomarker candidates for social behavior’ carried out under the Strategic Research Program for Brain Sciences to M.K. from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Glossary
- 5HIAA
5-hydroxyindoleacetic acid
- 5-HT
serotonin
- ACC
nucleus accumbens
- ACCC
nucleus accumbens core
- ACCS
nucleus accumbens shell
- BCIP
5-bromo-4-chloro-3-indolyl phosphate
- C/P
caudate nucleus and putamen
- CSF
cerebrospinal fluid
- DA
dopamine
- DAB
3-3′-diaminobenzidine-4HCl
- DIG
digoxigenin
- DOPAC
3,4-dihydroxyphenylacetic acid
- DRN
dorsal raphe nucleus
- ECD
electrochemical detection
- FC
frontal cortex
- HP
hippocampus
- HPLC
high-performance liquid chromatography
- LH
lateral hypothalamus
- MRN
median raphe nucleus
- NBT
4-nitroblue tetrazolium chloride
- NE
norepinephrine
- PBS
phosphate-buffered saline
- RT-PCR
reverse transcriptase-polymerase chain reaction
- SERT
serotonin transporter
- SSRI
selective serotonin reuptake inhibitor
- VTA
ventral tegmental area
References
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Becker HC. Animal models of alcohol withdrawal. Alcohol Res. Health. 2000;24:105–113. [PMC free article] [PubMed] [Google Scholar]
- Bonaventure P, Nepomuceno D, Kwok A, Chai W, Langlois X, Hen R, Stark K, Carruthers N, Lovenberg TW. Reconsideration of 5-hydroxytryptamine (5-HT)(7) receptor distribution using [(3)H]5-carboxamidotryptamine and [(3)H]8-hydroxy-2-(di-n-propylamino)tetraline: analysis in brain of 5-HT(1A) knockout and 5-HT(1A/1B) double-knockout mice. J. Pharmacol. Exp. Ther. 2002;302:240–248. doi: 10.1124/jpet.302.1.240. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daws LC, Holmes A. Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol. Clin. Exp. Res. 2006;30:1957–1965. doi: 10.1111/j.1530-0277.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Seitz PK, Thomas ML, Cunningham KA. Validation of a selective serotonin 5-HT(2C) receptor antibody for utilization in fluorescence immunohistochemistry studies. Brain Res. 2005;1063:105–113. doi: 10.1016/j.brainres.2005.09.050. [DOI] [PubMed] [Google Scholar]
- Burbassi S, Cervo L. Stimulation of serotonin2C receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology. 2008;196:15–27. doi: 10.1007/s00213-007-0916-7. [DOI] [PubMed] [Google Scholar]
- Clapp P, Bhave SV, Hoffman PL. How adaptation of the brain to alcohol leads to dependence. A pharmacological perspective. Alcohol Res. and Health. 2008;31:310–339. [PMC free article] [PubMed] [Google Scholar]
- Cremers TIFH, Giorgette M, Bosker FJ, Hogg S, Arnt J, Mørk A, Honig G, Bøgesø KP, Westerink BH, Boer HD, Wirksotrom HV, Tecott LH. Inactivation of 5-HT2C receptors potentiates consequences of serotonin reuptake blockade. Neuropsychopharmacol. 2004;29:1782–1789. doi: 10.1038/sj.npp.1300474. [DOI] [PubMed] [Google Scholar]
- Daws LC, Montanez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, Millstein RA, Wiedholz LM, Murphy DL, Holmes A. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J. Neurosci. 2006;26:6431–6438. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerdere P, Navailles S, Berg KA, Clarke WP, Spampinato U. Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J. Neurosci. 2004;24:3235–3241. doi: 10.1523/JNEUROSCI.0112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, Ding ZM, Oster SM, Toalston JE, Bell RL, Murphy JM, McBride WJ, Rodd ZA. Ethanol is self-administered into the nucleus accumbens shell, but not the core: evidence of genetic sensitivity. Alcohol. Clin. Exp. Res. 2009;33:2162–2171. doi: 10.1111/j.1530-0277.2009.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraz IC, Boerngen-Lacerda R. Serotonin 5-HT2 receptor antagonist does not reverse established ethanol-induced sensitization but blocks its development and expression. Pharmacol. Biochem. Behav. 2008;88:456–464. doi: 10.1016/j.pbb.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Chintoh AF, Sinyard J, Higgins GA. Injection of the 5-HT2C receptor agonist Ro60-0175 into the ventral tegmental area reduces cocaine-induced locomotor activity and cocaine self-administration. Neuropsychopharmacol. 2004;29:308–318. doi: 10.1038/sj.npp.1300319. [DOI] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Tokyo: Academic Press; 1997. [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Griffin WC, III, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Cli. Exp. Res. 2009;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav. Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hayes DJ, Greenshaw AJ. 5-HT receptors and reward-related behaviour: a review. Neurosci. Biobehav. Rev. 2011;35:1419–1449. doi: 10.1016/j.neubiorev.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Fletcher PJ. Serotonin and drug reward: focus on 5-HT2C receptors. Eur. J. Pharmacol. 2003;480:151–162. doi: 10.1016/j.ejphar.2003.08.102. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBodd JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol. Clin. Exp. Res. 2011;35:1–10. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Zeeb FD, Winstanley CA. Contributions of serotonin in addiction vulnerability. Neuropharmacol. 2011;61:421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacol. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacol. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP. Alcohol dependence and gene x environment interaction in emotion regulation: is serotonin the link? Eur. J. Pharmacol. 2005;526:113–124. doi: 10.1016/j.ejphar.2005.09.027. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Gatto GJ, Levy AD, Yoshimoto K, Lumeng L, Li TK. CNS mechanisms of alcohol self-administration. Alcohol Alcohol. Suppl. 1993;2:463–467. [PubMed] [Google Scholar]
- Mnie-Filali O, Lambas-Senas L, Zimmer L, Haddjeri N. 5-HT7 receptor antagonists as a new class of antidepressants. Drug News Perspect. 2007;20:613–618. doi: 10.1358/dnp.2007.20.10.1181354. [DOI] [PubMed] [Google Scholar]
- Muller CP, Huston JP. Determining the region-specific contributions of 5-HT receptors to the psychostimulant effects of cocaine. Trends Pharmacol. Sci. 2006;27:105–112. doi: 10.1016/j.tips.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Inui A, Teranishi A, Miura M, Hirosue Y, Okita M, Himori N, Baba S, Kasuga M. Effects of pancreatic polypeptide family peptides on feeding and learning behavior in mice. J. Pharmacol. Exp. Ther. 1994;268:1010–1014. [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol. Clin. Exp. Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Lumeng L, Li TK. Serotonin2C receptors and serotonin2C receptor-mediated phosphoinositide hydrolysis in the brain of alcohol-preferring and alcohol-nonpreferring rats. Alcohol. Clin. Exp. Res. 1996;20:1038–1042. doi: 10.1111/j.1530-0277.1996.tb01944.x. [DOI] [PubMed] [Google Scholar]
- Pasinetti GM, Osterburg HH, Kelly AB, Kohama S, Morgan DG, Reinhard JF, Jr, Stellwagen RH, Finch CE. Slow changes of tyrosine hydroxylase gene expression in dopaminergic brain neurons after neurotoxin lesioning: a model for neuron aging. Brain Res. Mol. Brain Res. 1992;13:63–73. doi: 10.1016/0169-328x(92)90045-d. [DOI] [PubMed] [Google Scholar]
- Qu Y, Villacreses N, Murphy DL, Rapoport SI. 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology. 2005;180:12–20. doi: 10.1007/s00213-005-2231-5. [DOI] [PubMed] [Google Scholar]
- Sarkisyan G, Roberts AJ, Hedlund PB. The 5-HT(7) receptor as a mediator and modulator of antidepressant-like behavior. Behav. Brain Res. 2010;209:99–108. doi: 10.1016/j.bbr.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S. Effects of the serotonin 5-HT(2) antagonist, ritanserin, and the serotonin 5-HT(1A) antagonist, WAY 100635, on cocaine-seeking in rats. Pharmacol. Biochem. Behav. 2000;67:363–369. doi: 10.1016/s0091-3057(00)00377-4. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Palacios JM, Fernandez AG, O’Neill MF. Comparison of effects of a range of 5-HT receptor modulators on consumption and preference for a sweetened ethanol solution in rats. J. Psychopharmacol. 1998;12:168–176. doi: 10.1177/026988119801200209. [DOI] [PubMed] [Google Scholar]
- Strother WN, Lumeng L, Li TK, McBride WJ. Dopamine and serotonin content in select brain regions of weanling and adult alcohol drinking rat lines. Pharmacol. Biochem. Behav. 2005;80:229–237. doi: 10.1016/j.pbb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Iijima N, Miyamoto Y, Fukusumi S, Itoh Y, Ozawa H, Ibata Y. Neurons expressing relaxin 3/INSL 7 in the nucleus incertus respond to stress. Eur. J. Neurosci. 2005;21:1659–1670. doi: 10.1111/j.1460-9568.2005.03980.x. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Watanabe Y, Yoshimoto K. Regulation of relaxin 3 gene expression via cAMP-PKA in a neuroblastoma cell line. J. Neurosci. Res. 2009;87:820–829. doi: 10.1002/jnr.21895. [DOI] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Role of 5-hydroxytryptamine2C receptors in Ca2+-dependent ethanol potentiation of GABA release onto ventral tegmental area dopamine neurons. J. Pharmacol. Exp. Ther. 2009;329:625–633. doi: 10.1124/jpet.108.147793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins DM, Joharchi N, Tampakeras M, Martin JR, Wichmann J, Higgins GA. An investigation of the role of 5-HT(2C) receptors in modifying ethanol self-administration behaviour. Pharmacol. Biochem. Behav. 2002;71:735–744. doi: 10.1016/s0091-3057(01)00710-9. [DOI] [PubMed] [Google Scholar]
- Ueda S, Aikawa M, Ishizuya-Oka A, Koibuchi N, Yamaoka S, Yoshimoto K. Age-related degeneration of the serotoninergic fibers in the zitter rat brain. Synapse. 1998;30:62–70. doi: 10.1002/(SICI)1098-2396(199809)30:1<62::AID-SYN8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol. Clin. Exp. Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Ikegawa M, Naruse Y, Tanaka M. A novel splicing variant form suppresses the activity of full-length signal transducer and activator of transcription 5A. FEBS J. 2009;276:6312–6323. doi: 10.1111/j.1742-4658.2009.07339.x. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Komura S. Monitoring of ethanol levels in the rat nucleus accumbens by brain microdialysis. Alcohol Alcohol. 1993;28:171–174. [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ. Regulation of nucleus accumbens dopamine release by the dorsal raphe nucleus in the rat. Neurochem. Res. 1992;17:401–407. doi: 10.1007/BF00969884. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Ueda S, Nishi M, Yang Y, Matsushita H, Takeuchi Y, Kato B, Kawai Y, Noritake K, Kaneda S, Sorimachi Y, Yasuhara M. Changes in dopamine transporter and c-Fos expression in the nucleus accumbens of alcohol-tolerant rats. Alcohol. Clin. Exp. Res. 2000;24:361–365. [PubMed] [Google Scholar]
- Zhou FC, Bledsoe S, Lumeng L, Li TK. Reduced serotonergic immunoreactive fibers in the forebrain of alcohol-preferring rats. Alcohol. Clin. Exp. Res. 1994;18:571–579. doi: 10.1111/j.1530-0277.1994.tb00912.x. [DOI] [PubMed] [Google Scholar]


