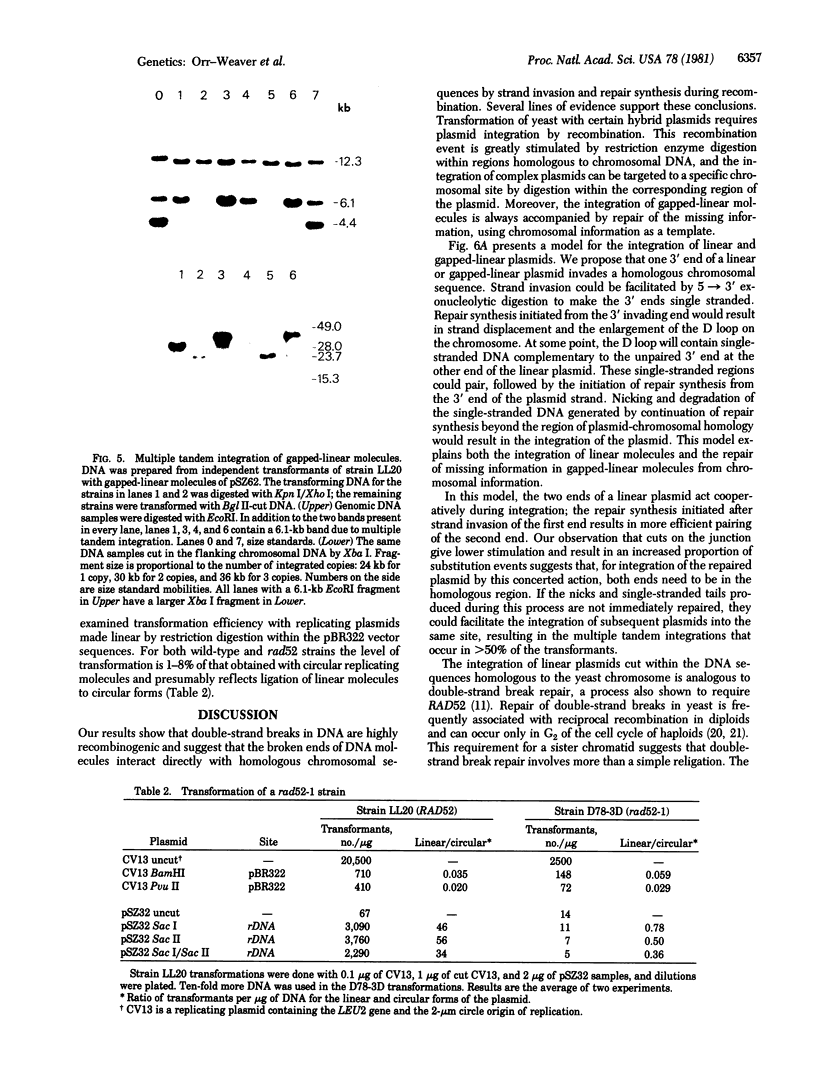
Abstract
DNA molecules that integrate into yeast chromosomes during yeast transformation do so by homologous recombination. We have studied the way in which circular and linear molecules recombine with homologous chromosomal sequences. We show that DNA ends are highly recombinogenic and interact directly with homologous sequences. Circular hybrid plasmids can integrate by a single reciprocal crossover, but only at a low frequency. Restriction enzyme digestion within a region homologous to yeast chromosomal DNA greatly enhances the efficiency of integration. Furthermore, if two restriction cuts are made within the same homologous sequence, thereby removing an internal segment of DNA, the resulting deleted-linear molecules are still able to transform at a high frequency. Surprisingly, the integration of these gapped-linear molecules results in replacement of the missing segment using chromosomal information. The final structure is identical to that obtained from integration of a circular molecule. The integration of linear and gapped-linear molecules, but not of circular molecules, is blocked by the rad52-1 mutation. Consideration of models for plasmid integration and gene conversion suggests that RAD52 may be involved in the DNA repair synthesis required for these processes. Implications of this work for the isolation of integrative transformants, fine-structure mapping, and the cloning of mutations are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunborg G., Resnick M. A., Williamson D. H. Cell-cycle-specific repair of DNA double strand breaks in Saccharomyces cerevisiae. Radiat Res. 1980 Jun;82(3):547–558. [PubMed] [Google Scholar]
- Chlebowicz E., Jachymczyk W. J. Repair of MMS-induced DNA double-strand breaks in haploid cells of Saccharomyces cerevisiae, which requires the presence of a duplicate genome. Mol Gen Genet. 1979 Jan 2;167(3):279–286. doi: 10.1007/BF00267420. [DOI] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Fogel S., Mortimer R., Lusnak K., Tavares F. Meiotic gene conversion: a signal of the basic recombination event in yeast. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1325–1341. doi: 10.1101/sqb.1979.043.01.152. [DOI] [PubMed] [Google Scholar]
- Haber J. E., Rogers D. T., McCusker J. H. Homothallic conversions of yeast mating-type genes occur by intrachromosomal recombination. Cell. 1980 Nov;22(1 Pt 1):277–289. doi: 10.1016/0092-8674(80)90175-0. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., McIndoo J., Strathern J. N., Hicks J. B. Evidence for a physical interaction between the transposed and the substituted sequences during mating type gene transposition in yeast. Cell. 1980 Nov;22(1 Pt 1):291–298. doi: 10.1016/0092-8674(80)90176-2. [DOI] [PubMed] [Google Scholar]
- Kupersztoch Y. M., Helinski D. R. A catenated DNA molecule as an intermediate in the replication of the resistance transfer factor R6K in Escherichia coli. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1451–1459. doi: 10.1016/0006-291x(73)91149-2. [DOI] [PubMed] [Google Scholar]
- Leblon G., Rossignol J. L. Mechanism of gene conversion in Ascobolus immersus. 3. The interaction of heteroallelas in the conversion process. Mol Gen Genet. 1973 Apr 12;122(2):165–182. doi: 10.1007/BF00435189. [DOI] [PubMed] [Google Scholar]
- Malone R. E., Esposito R. E. The RAD52 gene is required for homothallic interconversion of mating types and spontaneous mitotic recombination in yeast. Proc Natl Acad Sci U S A. 1980 Jan;77(1):503–507. doi: 10.1073/pnas.77.1.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation in Escherichia coli: cryogenic preservation of competent cells. J Bacteriol. 1977 Oct;132(1):349–351. doi: 10.1128/jb.132.1.349-351.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S., Prakash L., Burke W., Montelone B. A. Effects of the RAD52 Gene on Recombination in SACCHAROMYCES CEREVISIAE. Genetics. 1980 Jan;94(1):31–50. doi: 10.1093/genetics/94.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzkin B., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Feb;74(2):487–491. doi: 10.1073/pnas.74.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Martin P. The repair of double-strand breaks in the nuclear DNA of Saccharomyces cerevisiae and its genetic control. Mol Gen Genet. 1976 Jan 16;143(2):119–129. doi: 10.1007/BF00266917. [DOI] [PubMed] [Google Scholar]
- Resnick M. A. The repair of double-strand breaks in DNA; a model involving recombination. J Theor Biol. 1976 Jun;59(1):97–106. doi: 10.1016/s0022-5193(76)80025-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stadler D. R., Towe A. M. Evidence for meiotic recombination in Ascobolus involving only one member of a tetrad. Genetics. 1971 Jul;68(3):401–413. doi: 10.1093/genetics/68.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Wu R. Insertion of a genetic marker into the ribosomal DNA of yeast. Plasmid. 1979 Oct;2(4):536–554. doi: 10.1016/0147-619x(79)90053-2. [DOI] [PubMed] [Google Scholar]
- Weiffenbach B., Haber J. E. Homothallic mating type switching generates lethal chromosome breaks in rad52 strains of Saccharomyces cerevisiae. Mol Cell Biol. 1981 Jun;1(6):522–534. doi: 10.1128/mcb.1.6.522. [DOI] [PMC free article] [PubMed] [Google Scholar]