Abstract
The therapeutic benefit of nitrosoureas or temozolomide for glioblastoma is limited mainly by O6-methylguanine-DNA methyltransferase (MGMT) expression. The aim of this study was to evaluate the effectiveness of various anticancer drugs for MGMT-positive glioblastoma. Seventy-four glioblastoma patients were administered various anticancer drugs according to drug sensitivity testing. For the individualization, drug-induced apoptosis was quantified by flow cytometry in the primary culture of surgically resected tumor cells. The MGMT protein expression was analyzed by immunohistochemistry. The median survival of the patients receiving the individualized chemotherapy was 19.4 months (95% CI, 15.9–22.1). The patients with negative MGMT immunostaining had significantly longer survival than those with positive MGMT immunostaining [median survival, 22.3 months (95% CI, 17.6–27.0) vs. 15.1 months (95% CI, 13.4–16.8); p=0.0188]. For MGMT-positive tumors, the platinum agents and the taxanes were more frequently selected for administration than the other categories of anticancer agents. The patient survival period of MGMT-positive glioblastomas treated with the platinum agents or the taxanes [median survival, 20.1 months (95% CI, 18.0–22.7)] was significantly longer than that of MGMT-positive tumors treated with nitrosoureas (p=0.0026), and was equivalent to that of MGMT-negative glioblastomas (p=0.3047). These results suggest that the platinum agents and the taxanes offer the best probability to be effective against immunohistochemically MGMT-positive glioblastomas.
Keywords: O6-methylguanine-DNA methyltransferase, immunohistochemistry, taxane, platinum, glioma
Introduction
The currently available optimum treatment for glioblastoma consists of cytoreductive surgery followed by radiotherapy and chemotherapy (1). This conventional therapeutic strategy results in a median survival of 12–15 months in consecutive, non-selected glioblastoma patients (2–4). Most large-scale clinical studies on chemotherapy for malignant gliomas have utilized nitrosoureas (2,3), and have usually produced negative results regarding the survival gain for glioblastoma patients. Although temozolomide chemotherapy contributes to a significant improvement in patient survival, its benefit is usually restricted to tumors without O6-methylguanine-DNA methyltransferase (MGMT) expression (4,5). Since the level of MGMT expression strongly influences the efficacy of nitrosoureas or temozolomide (5–9), the establishment of novel therapeutic strategies for MGMT-positive glioblastoma is one of the main issues in contemporary neurooncology.
Current cancer treatments for categories of patients generally require the selection of therapy made on the basis of clinical trials conducted on large populations. However, the heterogeneity in drug sensitivity partly reduces the clinical success gained with these empiric chemotherapeutic regimens used for the general patient population (1). A therapeutic strategy with a protocol modified case by case according to drug sensitivity is termed ‘individualized’ or ‘tailor-made’ chemotherapy (10). Published clinical studies using in vitro drug sensitivity tests (DST) have shown improved patient response rates as compared with empiric regimens (11–15). The lessons learned from individualized chemotherapy may be valuable in planning chemotherapy regimens for glioblastoma as various anticancer drugs are actually administered in clinics.
We treated glioblastoma patients with various anticancer agents according to individualized protocols selected by DST. However, individualization of chemotherapy cannot easily be adopted in every institution, since it is both time-consuming and non-economical. In this report, the efficacy of each anticancer agent for glioblastoma was retrospectively examined in relation to the MGMT expression status by immunohistochemistry. This information provides a clue for the selection of anticancer drugs against glioblastoma expressing a high level of MGMT or those harboring unmethylated promoter of the MGMT gene.
Materials and methods
Patients
Seventy-four consecutive patients newly diagnosed with glioblastoma according to WHO classification were treated with individualized chemotherapy at Chiba University Hospital or Chiba Cancer Center Hospital from 1995 to 2004. All of the patients treated during this period were evaluated and included in the study without exclusion. The study protocol was approved by the institutional review board, and a written informed consent was obtained from all of the patients or a guardian. The patient characteristics are summarized in Table I. Magnetic resonance imaging (MRI), with and without gadolinium enhancement, was performed preoperatively and postoperatively before the initiation of radio-chemotherapy. Regarding extent of resection, the total/subtotal resection was defined as 90% or more reduction of the tumor volume in the postoperative MRI. The biopsy meant the CT-guided stereotactic needle biopsy, and partial removal covered all other situations. Toxicity was graded according to the National Cancer Institute’s Common Toxicity Criteria version 3.0.
Table I.
Patient characteristics (n=74).
| Age (years) | |
| Mean | 51.5 |
| Range | 15–77 |
| Gender | |
| Male | 49 (66%) |
| Female | 25 (34%) |
| Karnofsky performance score | |
| ≥70 | 42 (57%) |
| <70 | 32 (43%) |
| Tumor location | |
| Left | 37 (50%) |
| Right | 28 (38%) |
| Midline | 9 (12%) |
| Extent of surgery | |
| Total/Subtotal | 46 (62%) |
| Partial/Biopsy | 28 (38%) |
Drug sensitivity test (DST)
Direct quantification of apoptosis by means of flow cytometric DNA analysis is widely used in basic research and has been successfully utilized for clinical DST (15–17). Cell suspensions prepared from surgically resected tumor tissues were incubated with each of 25 different anticancer drugs already being used in clinical practice (cyclophosphamide, ifosphamide, nimustine, ranimustine, cisplatin, carboplatin, adriamycin, daunomycin, pirarubicin, epirubicin, aclarubicin, mitoxantrone, etoposide, camptothecin, methotraxate, 5-fluorouracil, thioinosine, cytosine arabinoside, mitomycin C, bleomycin, vincristine, vinblastine, vindesine, paclitaxel and docetaxel). The in vitro drug concentrations were set both at the peak plasma concentration when the clinically recommended doses were provided and at 1/10 of that level (18). Drug-induced apoptosis was quantified with a flow cytometer (FACScan; Becton Dickinson, Mountain View, CA, USA) as the sub-G1 population. To confirm the presence of drug-induced apoptosis, morphological examinations of the nuclei were also performed on the same samples. DNA integrity assessed by the FCM analysis correlated well with the morphological changes in the nuclei.
Treatment protocols
For individualization of chemotherapy, the most effective drug in vitro was routinely selected as the key drug for each individual patient. In addition, one or two drugs were selected for combination with the key drug according to their degree of effectiveness and their mechanism of pharmaceutical action. The doses and schedules of chemotherapy regimens were determined on the basis of clinically recommended doses. When no agent was positive in vitro, the patients were treated with a modified PCV chemotherapy with substitution of lomustine with nimustine (nimustine 75 mg/m2, vincristine 1 mg/m2 and procarbazine 100 mg/day) (19). For all patients, the conventional 60-Gy radiotherapy with a megavoltage machine was started within 2 weeks of surgical removal in conjunction with the chemotherapy.
MGMT immunohistochemistry
For immunohistochemical analysis, paraffin-embedded samples were sliced and mounted on glass slides. Mouse monoclonal anti-MGMT antibody MT3.1 (1:200 dilution; Chemicon, Inc., Temecula, CA, USA) was used as the primary antibody. A heat-induced epitope was formed using microwaves in 10 mM citric acid buffer at pH 7.2. The samples were incubated with the antibody overnight in the same buffer followed by incubation with the biotinylated secondary antibody (1:500 dilution; Dako, Tokyo, Japan). The bound antibodies were visualized by the avidin biotinylated peroxidase complex method and diaminobenzidine tetrachloride (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Human liver was used as the positive control, and the negative control was achieved by omitting the primary antibody from the procedure. Tissue specimens that showed staining of >10% of the malignant cells were considered positive for MGMT.
Statistical analysis
The primary end-point of this study was overall survival and the secondary end-points were progression-free survival and safety. Survival curves were generated using the Kaplan-Meier method, and the survival rates were compared with the log-rank test. The patient survival duration was calculated from the date of surgery until the date of last follow-up or death, and progression-free survival until the date of recurrence detection or until the last follow-up. The Fisher’s exact probability test and χ2 test were used to evaluate the statistical significance of the differences between patient characteristics of the two groups. Cox’s proportional hazard model was used to analyze the prognostic variables. The hazard ratios for death were calculated considering adjustment for age, Karnofsky performance status score and the extent of resection.
Results
Efficacy of individualized chemotherapy
All specimens from the 74 patients were examined for their in vitro susceptibility to the 25 anticancer drugs. In this series of newly diagnosed glioblastoma patients, the success rate of the DST was 100%. There was remarkable heterogeneity in the most effective drug. The median survival time of all of the 74 glioblastoma patients treated with the individualized chemotherapy was 19.4 months (95% CI, 15.9–22.1), and the 2-year survival rate was 36.5% (95% CI, 24.3–48.7). The median progression-free survival was 9.2 months (95% CI, 7.6–12.3) (Fig. 1). The survival periods could be favorably compared with those treated with temozolomide, the present-day standard regimen for glioblastoma.
Figure 1.
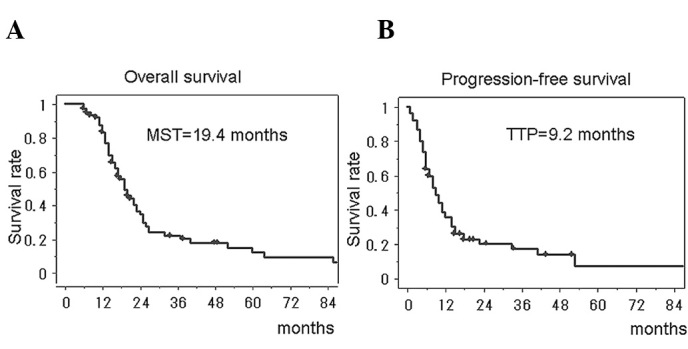
Kaplan-Meier analyses of overall survival (A) and progression-free survival (B) in the patients with glioblastoma treated with individualized chemotherapy. MST, median survival time; TTP, time to tumor progression.
The univariate analysis showed that the clinical factors previously known to affect the survival of patients with glioblastoma were correlated with favorable prognosis in this study; a Karnofsky performance status score of ≥70%, tumor resection of ≥90% and <50 years of age. The multivariate analysis showed that <50 years of age and tumor resection of ≥90% were significantly associated with favorable prognosis (Table II, p=0.0002 and p=0.0211, respectively).
Table II.
Multivariate analyses for favorable prognostic factors.
| Hazard ratio (95% CI) | P-value | |
|---|---|---|
| Age (<50 vs. ≥50) | 0.41 (0.26–0.66) | 0.0002 |
| Karnofsky performance score (≥70 vs. <70) | 0.69 (0.47–1.00) | 0.0607 |
| MGMT expression (negative vs. positive) | 0.59 (0.41–0.88) | 0.0081 |
| Extent of resection (≥90 vs. <90) | 0.60 (0.39–0.93) | 0.0211 |
MGMT expression and chemosensitivity
The MGMT-positive rate was 53.7% for the 74 glioblastomas (Fig. 2). According to the DST, 58 tumors (78%) had at least one effective drug, and the other 16 tumors (22%) were negative for all of the 25 anticancer drugs examined (all-drug-resistant tumors). The relationship between the MGMT expression status and chemosensitivity was analyzed (Fig. 3). For the MGMT-positive tumors, the alkylating agents were selected only in two cases, and the topoisomerase inhibitors were never selected for administration as a key drug. The platinum agents were more frequently effective against the MGMT-positive tumors than against the MGMT-negative tumors. The taxanes were equally selected either in the MGMT-negative or the MGMT-positive group. Most of the all-drug-resistant tumors (14 out of 16 cases, 87.5%) were included in the MGMT-positive group (p=0.0019). Thus, the MGMT expression status significantly influenced the in vitro chemosensitivity of almost all categories of anticancer agents except for the taxanes.
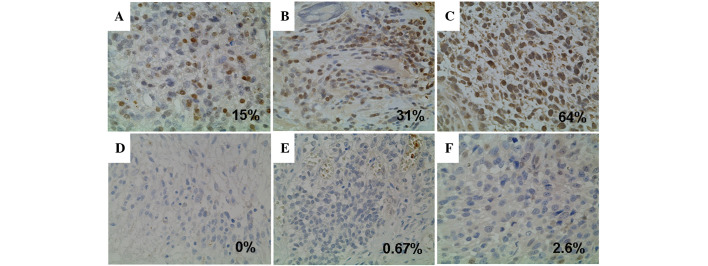
Figure 2.
Immunohistochemical analyses for MGMT expression. The representative cases for positive (A, B and C) and negative (D, E and F) immunostaining. The cut-off point for positive MGMT expression was set at 10%.
Figure 3.
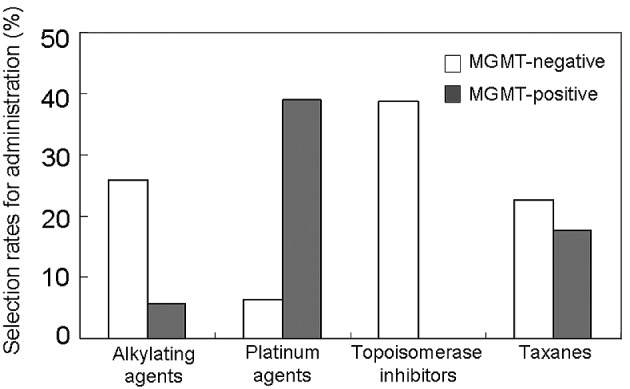
Selection rates of each anticancer drug as a key drug for administration; comparisons between the MGMT-negative and -positive glioblastomas.
MGMT expression and survival
The patients with negative MGMT immunostaining had significantly longer survival than those with positive MGMT [median survival, 22.3 months (95% CI, 17.6–27.0) vs. 15.1 months (95% CI, 13.4–16.8); p=0.0188] (Fig. 4). Immunohistochemical MGMT expression status had a significant impact on the survival period in the multivariate analysis (Table II). The survival period of the patients with MGMT-positive tumors treated with the platinum agents or the taxanes [median survival, 20.1 months (95% CI, 18.0–22.7)] was equivalent to that of the MGMT-negative tumors (p=0.3047) (Fig. 5). In contrast, the survival time of the patients with all-drug-resistant MGMT-positive tumors (n=14) who were treated with the nitrosourea-based chemotherapy (the modified PCV therapy) [median survival, 13.0 months (95% CI, 11.4–14.6)] was significantly shorter than both that of the patients with MGMT-negative tumors (p=0.0007) and that of MGMT-positive tumors treated with the platinum agents or the taxanes (p=0.0026).
Figure 4.
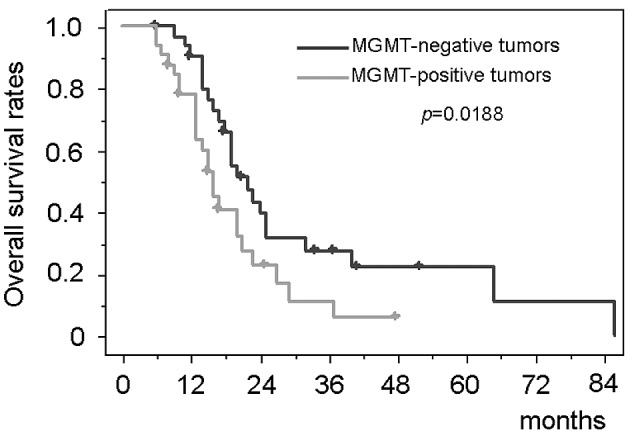
Kaplan-Meier analyses of the overall survival compared between MGMT-negative and MGMT-positive glioblastoma patients treated with individualized chemotherapy.
Figure 5.
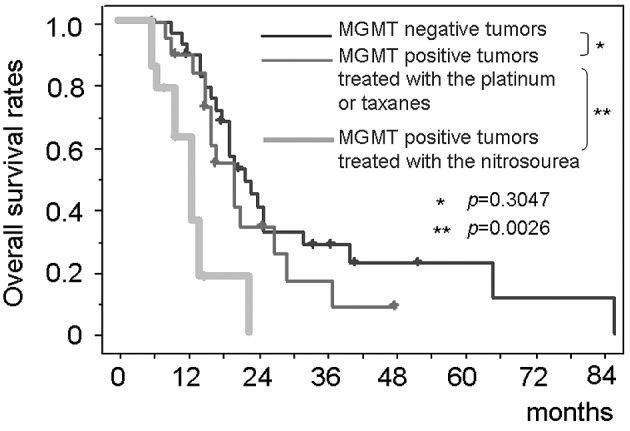
Kaplan-Meier analyses of the overall patient survival compared among MGMT-negative glioblastoma, MGMT-positive tumors treated with nitrosoureas and those treated with the platinum agents or the taxanes.
Safety evaluation
We monitored the adverse events, with special focus on the hematological toxic effects. They were graded according to the National Cancer Institute’s Common Toxicity Criteria version 3.0. Grade 3 or 4 neutropenia was observed in 9 patients (12.2%), and severe pneumonia occurred in 3 patients (4.1%). However, there was no treatment-related death in the present series.
Discussion
Our results suggest that individualized chemotherapy with anticancer drugs prospectively selected based on in vitro chemosensitivity tests for each glioblastoma patient provides a median survival of 19.4 months which compares favorably with most of the previously reported studies (2–4). The potentially poor prognosis groups with age greater than 50 years, Karnofsky performance status score less than 70% and surgical resection less than 90% particularly benefited from the individualized chemotherapy. Therefore, this study suggests an important new direction in the chemotherapy for glioblastoma.
An important implication of this study is that a large number of MGMT-positive glioblastomas were effectively treated with individualized chemotherapy. Many studies have indicated that MGMT is a significant prognostic factor for shorter survival rates in glioblastoma patients (5–7), whereas its prognostic value remains controversial (8,9). Efficacy of temozolomide also depends significantly on the level of MGMT expression (5). Therefore, one of the most important issues in contemporary neurooncology is how to treat glioblastoma with high MGMT expression. Therapeutic strategies for MGMT-positive glioblastoma currently under consideration have been designed to deplete MGMT and to combine other agents which are not affected by MGMT (21). The present results demonstrate that currently available anticancer drugs are much more effective when administered to those most likely to respond. An individualized or tailored strategy based on multiple biological information of the tumor would be one of the effective approaches to treat MGMT-positive glioblastoma.
However, an individualization strategy cannot easily be adopted in every institution. The lessons learned from individualized chemotherapy may be valuable in planning chemotherapy regimens for MGMT-positive glioblastoma. The present study suggests that the platinum agents and the taxanes can potentially prolong the survival of patients with MGMT-positive glioblastoma. The platinum agents as well as temozolomide and O6-benzylguanine can abrogate MGMT activity (22). Several studies have also shown that the antitumor activity of platinum agents is not affected by MGMT activity (23–25). This knowledge has led to Phase II clinical trials with promising results (26–28). Although MGMT affected the efficacies of diverse anticancer drugs, only the taxanes were independent from the MGMT status. Taxanes were clinically used in some trials without marked improvement in the efficacy for gliobastoma (29–31). However, multiple molecular mechanisms were reported to affect their efficacies for glioma cells (32). It is preferable to treat MGMT-positive glioblastoma with multi-modality regimens including platinum agents or the taxanes.
Currently, MGMT expression is estimated mainly by methylation-specific PCR or immunohistochemistry (5–9). Methylation-specific PCR is highly sensitive but is unable to assess intratumoral heterogeneity and contaminating normal cells such as endothelial cells (8,9). A methylated band is observed even when cells that carry MGMT promoter hypermethylation represent only a minor portion of the tumor. Regulation of MGMT expression is a more complex phenomenon in which abnormal promoter methylation is not the sole determining factor. We employed immunohistochemistry to directly evaluate the final functional molecule and the intratumoral heterogeneity, although an objective threshold for evaluation may not be easily set. We used a cut-off value of 10%, whereas the reported values vary from 5 to 35% (8,9,33,34). The results showed a tendency of polarization of the MGMT-positive rate in the tumors with rates of less than 5% and those with more than 35%. Consequently, the overall positive rate in our study is consistent with published reports employing immunohistochemistry.
This is the first report to show that individualization of chemotherapy can potentially prolong the survival of non-selected consecutive glioblastoma patients with high MGMT expression without any additional toxicity. When the stratification based on the MGMT expression is available, platinum agents or the taxanes offer the highest probability for effectiveness against MGMT-positive glioblastomas.
References
- 1.Shapiro WR. Current therapy for brain tumors: back to the future. Arch Neurol. 1999;56:429–432. doi: 10.1001/archneur.56.4.429. [DOI] [PubMed] [Google Scholar]
- 2.Medical Research Council Brain Tumor Working Party Randomized trial of procarbazine, lomustine and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trial. J Clin Oncol. 2001;19:509–518. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- 3.Glioma Meta-analysis Trialist (GMT) Group Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 6.Jaeckle KA, Eyre HJ, Townsend JJ, et al. Correlation of tumor O6-alkylguanine-DNA methyltransferase levels with survival of malignant astrocytoma patients treated with bis-chloroethylnitrosourea: a Southwest Oncology Group study. J Clin Oncol. 1998;16:3310–3315. doi: 10.1200/JCO.1998.16.10.3310. [DOI] [PubMed] [Google Scholar]
- 7.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 8.Brell M, Tortosa A, Verger E, et al. Prognostic significance of O6-alkylguanine-DNA methyltransferase determined by promoter hypermethylation and immunohistochemical expression in anaplatic gliomas. Clin Cancer Res. 2005;11:5167–5174. doi: 10.1158/1078-0432.CCR-05-0230. [DOI] [PubMed] [Google Scholar]
- 9.Chinot OL, Barrie M, Fuentes S, et al. Correlation between O6-alkylguanine-DNA methyltransferase and survival in inoperable newly diagnosed glioblastoma patients treated with neoadjuvant temozolomide. J Clin Oncol. 2007;25:1470–1475. doi: 10.1200/JCO.2006.07.4807. [DOI] [PubMed] [Google Scholar]
- 10.Cortazar P, Johnson BE. Review of the efficacy of individualized chemotherapy selected by in vitro drug sensitivity testing for patients with cancer. J Clin Oncol. 1999;17:1625–1631. doi: 10.1200/JCO.1999.17.5.1625. [DOI] [PubMed] [Google Scholar]
- 11.Alonso K. Human tumor stem cell assay. A prospective clinical trial. Cancer. 1984;54:2475–2479. doi: 10.1002/1097-0142(19841201)54:11<2475::aid-cncr2820541127>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Von Hoff DD, Sandbach JF, Clark GM, et al. Selection of cancer chemotherapy for a patient by an in vitro assay versus a clinician. J Natl Cancer Inst. 1990;82:110–116. doi: 10.1093/jnci/82.2.110. [DOI] [PubMed] [Google Scholar]
- 13.Gazdar AF, Steinberg SM, Russell EK, et al. Correlation of in vitro drug-sensitivity testing with response to chemotherapy and survival in extensive-stage small cell lung cancer: a prospective clinical trial. J Natl Cancer Inst. 1990;82:117–124. doi: 10.1093/jnci/82.2.117. [DOI] [PubMed] [Google Scholar]
- 14.Cortazar P, Gazdar AF, Woods E, et al. Survival of patients with limited-stage small cell lung cancer treated with individualized chemotherapy selected by in vitro drug sensitivity testing. Clin Cancer Res. 1997;3:741–747. [PubMed] [Google Scholar]
- 15.Iwadate Y, Fujimoto S, Namba H, Yamaura A. Promising survival for patients with glioblastoma multiforme treated with individualised chemotherapy based on in vitro drug sensitivity testing. Br J Cancer. 2003;89:1896–1900. doi: 10.1038/sj.bjc.6601376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwadate Y, Fujimoto S, Sueyoshi K, et al. Prediction of drug cytotoxicity in 9L rat brain tumor by using flow cytometry with a deoxyribonucleic acid-binding dye. Neurosurgery. 1997;40:782–788. doi: 10.1097/00006123-199704000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Iwadate Y, Fujimoto S, Yamaura A. Differential chemosensitivity in human intracerebral gliomas measured by flow cytometric DNA analysis. Int J Mol Med. 2002;10:187–192. [PubMed] [Google Scholar]
- 18.Alberts DS, Chen HSG. Tabular Summary of Pharmacokinetic Parameters Relevant to In Vitro Drug Assay. Alan R. Liss; New York: 1980. pp. 351–359. [PubMed] [Google Scholar]
- 19.Higuchi Y, Iwadate Y, Yamaura A. Treatment of low-grade oligodendroglial tumors without radiotherapy. Neurology. 2004;63:2384–2386. doi: 10.1212/01.wnl.0000147243.02317.28. [DOI] [PubMed] [Google Scholar]
- 20.Iwadate Y, Namba H, Sueyoshi K. Intra-arterial ACNU and cisplatin chemotherapy for the treatment of glioblastoma multiforme. Neurol Med Chir. 1995;35:598–603. doi: 10.2176/nmc.35.598. [DOI] [PubMed] [Google Scholar]
- 21.Mason WP, Cairncross JG. Drug insight: temozolomide as a treatment for malignant glioma – impact of a recent trial. Nat Clin Pract Neurol. 2005;1:88–95. doi: 10.1038/ncpneuro0045. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka S, Kobayashi I, Utsuki S, Oka H, Yasui Y, Fujii K. Down-regulation of O6-methylguanine-DNA methyltransferase gene expression in gliomas by platinum compounds. Oncol Rep. 2005;14:1275–1280. [PubMed] [Google Scholar]
- 23.Beith J, Hartley J, Darling J, Souhami R. DNA interstrand cross-linking and cytotoxicity induced by chloroethylnitrosoureas and cisplatin in human glioblastoma cell lines which vary in cellular concentration of O6-alkylguanine-DNA methyltransferase. Br J Cancer. 1997;75:500–505. doi: 10.1038/bjc.1997.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preuss I, Thust R, Kaina B. Protective effect of O6-methylguanine-DNA methyltransferase (MGMT) on the cytotoxic and recombinigenic activity of different antineoplastic drugs. Int J Cancer. 1996;65:506–512. doi: 10.1002/(SICI)1097-0215(19960208)65:4<506::AID-IJC19>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Fruehauf JP, Brem H, Parker R, et al. In vitro drug response and molecular markers associated with drug resistance in malignant gliomas. Clin Cancer Res. 2006;12:4523–4532. doi: 10.1158/1078-0432.CCR-05-1830. [DOI] [PubMed] [Google Scholar]
- 26.Balana C, Ramirez JL, Rosell R, et al. O6-methylguanine-DNA methyltransferase methylation in serum and tumor DNA predicts response to 1,3-bis(2-chloroethyl)-1-nitrosourea but not to temozolomide plus cisplatin in glioblastoma multiforme. Clin Cancer Res. 2003;9:1461–1468. [PubMed] [Google Scholar]
- 27.Watanabe T, Katayama Y, Ogino A, et al. Preliminary individualized chemotherapy for malignant astrocytomas based on O6-methylguanine-deoxyribonucleic acid methyltransferase methylation analysis. Neurol Med Chir. 2006;46:387–394. doi: 10.2176/nmc.46.387. [DOI] [PubMed] [Google Scholar]
- 28.Silvani A, Eoli M, Salmaggi A, et al. Phase II trial of cisplatin plus temozolomide, in recurrent and progressive malignant glioma patients. J Neurooncol. 2004;66:203–208. doi: 10.1023/b:neon.0000013479.64348.69. [DOI] [PubMed] [Google Scholar]
- 29.Rosenthal MA, Gruber ML, Glass J, et al. Phase II study of combination taxol and estramustine phosphate in the treatment of recurrent glioblastoma multiforme. J Neurooncol. 2000;47:59–63. doi: 10.1023/a:1006426215005. [DOI] [PubMed] [Google Scholar]
- 30.Langer CJ, Ruffer J, Rhodes H, et al. Phase II Radiation Therapy Oncology Group trial of weekly paclitaxel and conventional external beam radiation therapy for supratentorial glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;51:113–119. doi: 10.1016/s0360-3016(01)01597-8. [DOI] [PubMed] [Google Scholar]
- 31.Ashamalla H, Zaki B, Mokhtar B, et al. Fractionated stereotactic radiotherapy boost and weekly paclitaxel in malignant gliomas clinical and pharmacokinetics results. Technol Cancer Res Treat. 2007;6:169–176. doi: 10.1177/153303460700600303. [DOI] [PubMed] [Google Scholar]
- 32.Karmakar S, Banik NL, Ray SK. Combination of all-trans retinoic acid and paclitaxel-induced differentiation and apoptosis in human gliobastoma U87MG xenografts in nude mice. Cancer. 2008;112:596–607. doi: 10.1002/cncr.23223. [DOI] [PubMed] [Google Scholar]
- 33.Friedman HS, McLendon RE, Kerby T, et al. DNA mismatch repair and O6-alkylguanine-DNA alkyltransferase analysis and response to Temodal in newly diagnosed malignant glioma. J Clin Oncol. 1998;16:3851–3857. doi: 10.1200/JCO.1998.16.12.3851. [DOI] [PubMed] [Google Scholar]
- 34.Mollemann M, Wolter M, Feisberg J, et al. Frequent promoter hypermethylation and low expression of the MGMT gene in oligodendroglial tumors. Int J Cancer. 2005;113:379–385. doi: 10.1002/ijc.20575. [DOI] [PubMed] [Google Scholar]