Abstract
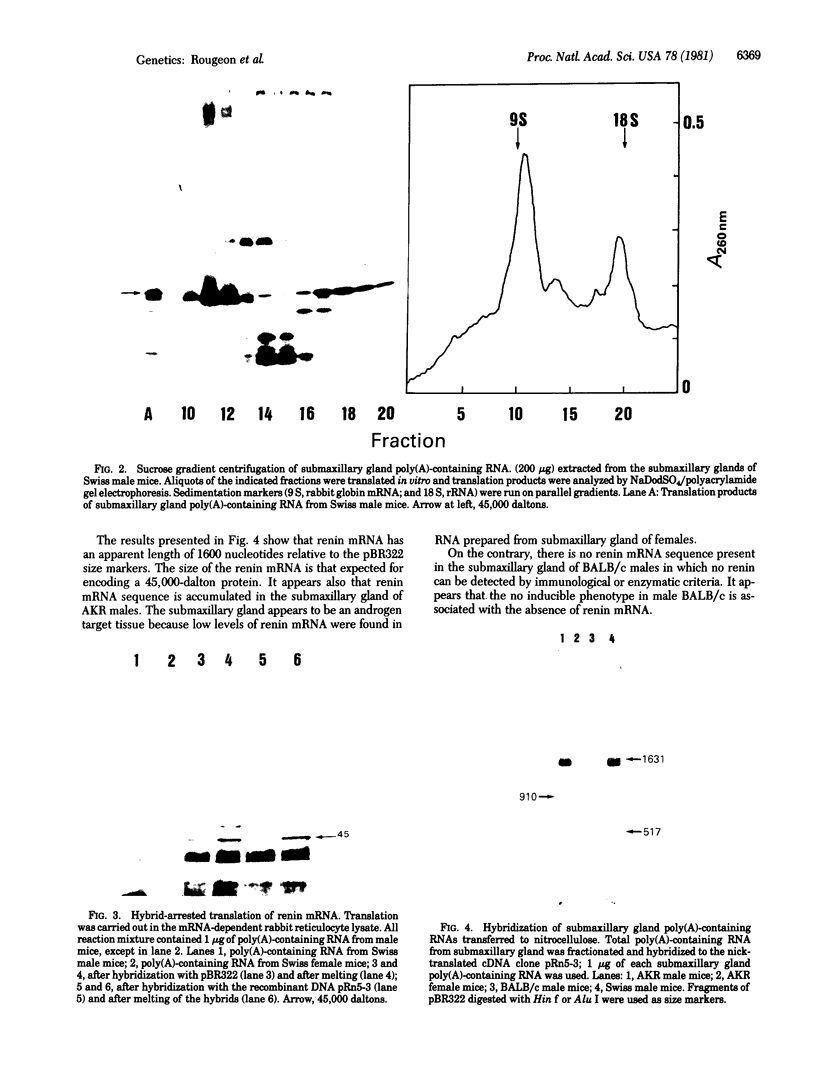
The mRNA encoding mouse renin has been partially purified from total poly(A)-containing RNA of submaxillary glands of male Swiss mice. Corresponding cDNAs were cloned in the Pst I site of pBR322. Recombinants have been characterized by differential screening and hybrid-arrested translation. The DNA of clone pRn3-5 has been used to study the expression of renin mRNA in the submaxillary gland and in the kidney of different mouse strains. The renin mRNA from submaxillary gland and kidney have the same length (1600 nucleotides) and appear to be the products of the same gene. In vitro translation of mRNAs and RNA blotting experiments have shown that renin mRNA sequences are accumulated in the submaxillary gland of males of AKR and Swiss strains but not in the gland of male BALB/c.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Nageotte R., Chambraud B., Rougeon F. Mouse immunoglobulin genes: a bacterial plasmid containing the entire coding sequence for a pre-gamma 2a heavy chain. Nucleic Acids Res. 1980 Mar 25;8(6):1231–1241. doi: 10.1093/nar/8.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Nucleotide sequence of a cloned cDNA corresponding to secreted mu chain of mouse immunoglobulin. Gene. 1980 Dec;12(1-2):77–86. doi: 10.1016/0378-1119(80)90017-7. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bhoola K. D., Dorey G., Jones C. W. The influence of androgens on enzymes (chymotrypsin-and trypsin-like proteases, renin, kallikrein and amylase) and on cellular structure of the mouse submaxillary gland. J Physiol. 1973 Dec;235(2):503–522. doi: 10.1113/jphysiol.1973.sp010400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Byyny R. L., Orth D. N., Cohen S., Doyne E. S. Epidermal growth factor: effects of androgens and adrenergic agents. Endocrinology. 1974 Sep;95(3):776–782. doi: 10.1210/endo-95-3-776. [DOI] [PubMed] [Google Scholar]
- Dobberstein B., Garoff H., Warren G., Robinson P. J. Cell-free synthesis and membrane insertion of mouse H-2Dd histocompatibility antigen and beta 2-microglobulin. Cell. 1979 Aug;17(4):759–769. doi: 10.1016/0092-8674(79)90316-7. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii D. N., Shooter E. M. Regulation of nerve growth factor synthesis in mouse submaxillary glands by testosterone. J Neurochem. 1975 Dec;25(6):843–851. doi: 10.1111/j.1471-4159.1975.tb04416.x. [DOI] [PubMed] [Google Scholar]
- Lee D. C., McKnight G. S., Palmiter R. D. The action of estrogen and progesterone on the expression of the transferrin gene. A comparison of the response in chick liver and oviduct. J Biol Chem. 1978 May 25;253(10):3494–3503. [PubMed] [Google Scholar]
- Malling C., Poulsen K. Direct measurement of high molecular weight forms of renin in plasma. Biochim Biophys Acta. 1977 Apr 25;491(2):542–550. doi: 10.1016/0005-2795(77)90299-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelakis A. M., Yoshida H., Menzie J., Murakami K., Inagami T. A radioimmunoassay for the direct measurement of renin in mice and its application to submaxillary gland and kidney studies. Endocrinology. 1974 Apr;94(4):1101–1105. doi: 10.1210/endo-94-4-1101. [DOI] [PubMed] [Google Scholar]
- Misono K. S., Inagami T. Characterization of the active site of mouse submaxillary gland renin. Biochemistry. 1980 Jun 10;19(12):2616–2622. doi: 10.1021/bi00553a013. [DOI] [PubMed] [Google Scholar]
- Oliver W. J., Gross F. Effect of testosterone and duct ligation on submaxillary renin-like principle. Am J Physiol. 1967 Aug;213(2):341–346. doi: 10.1152/ajplegacy.1967.213.2.341. [DOI] [PubMed] [Google Scholar]
- Paigen K. Acid hydrolases as models of genetic control. Annu Rev Genet. 1979;13:417–466. doi: 10.1146/annurev.ge.13.120179.002221. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Quantitation of parameters that determine the rate of ovalbumin synthesis. Cell. 1975 Mar;4(3):189–189. doi: 10.1016/0092-8674(75)90167-1. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Poulsen K., Nielsen A. H. Renin in the mouse kidney has a molecular weight of 40 000. Clin Sci (Lond) 1981 Jan;60(1):41–46. doi: 10.1042/cs0600041. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roskam W. G., Rougeon F. Molecular cloning and nucleotide sequence of the human growth hormone structural gene. Nucleic Acids Res. 1979 Sep 25;7(2):305–320. doi: 10.1093/nar/7.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Tosi M., Pittet A. C., Fabiani L., Wellauer P. K. Tissue-specific expression of mouse alpha-amylase genes. J Mol Biol. 1980 Sep 5;142(1):93–116. doi: 10.1016/0022-2836(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS J. A comparison of conalbumin and transferrin in the domestic fowl. Biochem J. 1962 May;83:355–364. doi: 10.1042/bj0830355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M., Erdös E. G., Dunn J. F., Wilson J. D. Genetic control of renin activity in the submaxillary gland of the mouse. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1185–1189. doi: 10.1073/pnas.74.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M., Erdös E. G., Wilson J. D., Taylor B. A. Location on chromosome 1 of Rnr, a gene that regulates renin in the submaxillary gland of the mouse. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5623–5626. doi: 10.1073/pnas.75.11.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]








