Abstract
This review examines vestibular compensation and vestibular rehabilitation from a unified translational research perspective. Laboratory studies illustrate neurobiological principles of vestibular compensation at the molecular, cellular and systems levels in animal models that inform vestibular rehabilitation practice. However, basic research has been hampered by an emphasis on ‘naturalistic’ recovery, with time after insult and drug interventions as primary dependent variables. The vestibular rehabilitation literature, on the other hand, provides information on how the degree of compensation can be shaped by specific activity regimens. The milestones of the early spontaneous static compensation mark the re-establishment of static gaze stability, which provides a common coordinate frame for the brain to interpret residual vestibular information in the context of visual, somatosensory and visceral signals that convey gravitoinertial information. Stabilization of the head orientation and the eye orientation (suppression of spontaneous nystagmus) appear to be necessary by not sufficient conditions for successful rehabilitation, and define a baseline for initiating retraining. The lessons from vestibular rehabilitation in animal models offer the possibility of shaping the recovery trajectory to identify molecular and genetic factors that can improve vestibular compensation.
Keywords: Vestibular compensation, vestibular rehabilitation therapy, gaze control, head control, locomotion
1. Introduction
Recent decades of basic research into cellular and molecular mechanisms of vestibular compensation have produced important insights into functional plasticity of the central nervous system. For example, a considerable body of evidence indicates that vestibulo-ocular reflex plasticity is mediated by protein kinase C (PKC) -dependent mechanisms in modular vestibulocerebellar cortico-nuclear microcircuits termed microcomplexes, which are small networks involving the inferior olive, vestibular nuclei, nucleus prepositus hypoglossi and the flocculonodular lobe (Ito, 2001). Inhibition of flocculonodular lobe Purkinje cell PKC blocks adaptive modification of vestibulo-ocular (De Zeeuw et al., 1998) and optokinetic (Shutoh et al., 2003) responses. In this regard, it is significant that Purkinje cells in different flocculonodular lobe microcomplexes show transient molecular changes in specific PKC isoform during compensation for unilateral ablation of vestibular endorgans (Balaban and Romero, 1998; Balaban et al., 1999; Goto et al., 1997) and that PKC inhibition retards the resolution of spontaneous nystagmus in these animals (Balaban et al., 1999). Inhibition of flocculus PKC also prevented a compensatory increase in the intrinsic excitability of medial vestibular nucleus neurons during early vestibular compensation (Johnston et al., 2002). Although cerebellar long term depression (LTD) is induced in Purkinje cells by processes that require simultaneous parallel and climbing fiber activity and PKC activation (De Zeeuw et al., 1998; Ito, 2001; Leitges et al., 2004; Linden and Connor, 1995), the PKC-mediated effects on both cerebellar motor learning and oculomotor aspects of vestibular compensation likely include mechanisms in addition to LTD (Faulstich et al., 2006; Schonewille et al., 2011). A number of comprehensive reviews provide additional examples of the value of vestibular compensation as a model system for investigating dynamic neural reorganization of sensorimotor processes (Cullen et al., 2009; Dutia, 2010; Gliddon et al., 2005; Lacour and Tighilet, 2010).
Unfortunately, these advances in understanding of basic processes of vestibular compensation have found very little translational application into therapies that improve compensation outcomes in patients. By contrast, vestibular rehabilitation therapy has been a remarkable success story. This communication explores implications of Lacour’s (Lacour, 2006) observation that vestibular compensation includes a rapid, ‘vestibulo-centric’ static process and a longer term, dynamic, distributed learning process. We suggest that the focus of the basic scientific approach on spontaneous compensation after peripheral vestibular ablation elucidates only mechanisms that bring a patient to the functional point of entry into rehabilitation therapy. Vestibular rehabilitation therapy improves dynamic performance by replacing systematically some previously compensatory strategies with new, learned strategies that enhance functional recovery. The therapy is guided by three implicit assumptions. Firstly, spontaneous compensation is not optimal. Secondly, compensation is viewed as a top-down, ordered process, proceeding from eye-head stabilization to dynamic gait stabilization. Thirdly, effective rehabilitation involves stepwise guidance of adaptive learning to achieve interim goals of increasing complexity. For example, a first goal in vestibular rehabilitation is to overcome the voluntary (‘adaptive’) limitations on head movements that patients often adopt to minimize precipitating disequilibrium and nausea (Herdman and Whitney, 2007); other interim goals may only be intermediate steps to enable further improvement. The implication is obvious: scientific paradigms for studying vestibular compensation should be expanded to elucidate mechanisms that are likely to be clinically meaningful for improving rehabilitation outcomes.
There is a common fallacy that translational research is strictly a ‘bottom-up’ process from the laboratory bench, via animal model trials, to the clinic. To the contrary, the fundamental importance of the clinical to basic research path in experimental medicine was emphasized by Claude Bernard (Bernard, 1875, c1989) more than 130 years ago in the statement that experimental medicine “ …claims knowledge of the laws of healthy and diseased organisms, not only as to foresee phenomena, but also so as to be able to regulate and alter them within certain limits. Accordingly, we easily perceive that medicine tends to become experimental, and that every physician who gives his patients active medicine cooperates in building up experimental medicine.” Stated simply, basic investigators have the task of providing therapeutically relevant explanations for the outcomes of the patient-oriented and disease-oriented clinical research. Hence, this review focuses on lessons from procedures and outcomes of vestibular rehabilitation therapy that can inform the experimental analysis of vestibular compensation. Conversely, we discuss how principles from laboratory studies of vestibular compensation inform and are validated by clinical rehabilitation practice.
2. Vestibular Rehabilitation as an Experimental Approach to Clinically Relevant Vestibular Compensation
2.1 History and relationship with ‘stages of compensation’
Vestibular rehabilitation is a therapeutic approach to enhance functional vestibular compensation. Sir Terence Cawthorne and F.S. Cooksey (Cawthorne, 1945; Cawthorne and Cooksey, 1946; Cawthorne, 1949) are credited with formalizing the concept of vestibular rehabilitation by developing a balance rehabilitation strategy for British soldiers injured in the Second World War. Despite the success of their work, vestibular rehabilitation was largely ignored until half a century later, when a number of investigators began to develop new outcome- based strategies for treating balance disorders (Herdman, 1990; Norré and Beckers, 1988; Odkvist and Odkvist, 1988; Shumway-Cook and Horak, 1990; Smith-Wheelock et al., 1991). The empirical successes of the treatment resulted in an expansion of the discipline, with gradual introduction into mainstream physical therapy educational programs. Finally, a Cochran review concluded in 2007 that “there is moderate to strong evidence that vestibular rehabilitation is a safe, effective management for unilateral peripheral vestibular dysfunction, based on a number of high quality randomized controlled trials” (Hillier and McDonnell, 2007). This conclusion was reaffirmed in 2011 with a second Cochrane review (Hillier and McDonnell, 2011).
Vestibular rehabilitation therapy commences in the second of three classically described stages of experimental and clinical compensation to unilateral vestibular dysfunction (Llinas and Walton, 1979; Smith and Curthoys, 1989; Spiegel and Sommer, 1944). The acute or critical phase is characterized by sensations of vertigo, spontaneous nystagmus in the light, postural symptoms both at rest and during gait, and neck torsion. The second phase has been termed symptom resolution, which marks a high impact window for rehabilitation therapy. It ends with final phase when there is no further improvement, which can be termed ‘terminal compensation’. However, this characterization belies, as stated by Curthoys and Halmagyi (Curthoys and Halmagyi, 1999), that vestibular compensation is “composed of a number of processes which are intertwined seamlessly to provide the basis for a continuum of recovery.” These compensatory processes affect responses that include tonic (horizontal and torsional) eye position, sensation of body position or motion (e.g., vertigo), dynamic performance and axis of rotation of vestibulo-ocular reflexes, eye-head coordination, static postural maintenance and gait.
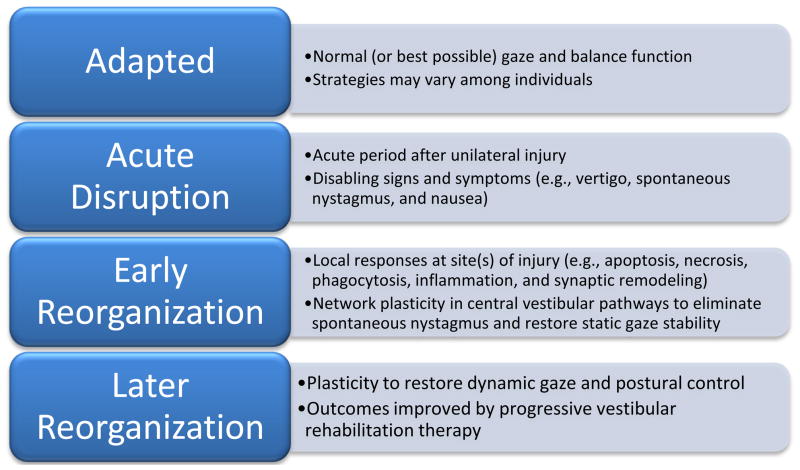
An alternative conceptualization is to consider the scenario of a unilateral vestibular insult as the sudden replacement of stable, adapted gaze and balance with a chaotic orientation state (Figure 1). The early biological response has two components, (1) repair and remodeling at sites of injury and (2) adaptive functional responses at multiple intact sites within balance control pathways. The acute responses fall within the purview of ‘vestibulo-centric’ static adaptations (Lacour, 2006), while the latter processes correspond to longer term dynamic adaptations. The acute, naturalistic responses have been the focus of basic research. The later functional reorganization, on the other hand, is targeted by vestibular rehabilitation.
Figure 1.
Schematic overview of vestibular compensation and rehabilitation
2.2 Therapeutic goals and strategies for vestibular rehabilitation
The primary goal of rehabilitation therapy is to improve balance function, reduce the risk of falling, and improve the patient’s quality of life. Stated from a basic science perspective, the therapist tries to achieve the best possible functional vestibular compensation (Herdman and Whitney, 2007). The approaches are designed to improve functional balance performance through retraining. The tools for retraining are defined operationally as adaption, substitution, and desensitization. In adaptation, a patient learns to adapt to the component of balance that is working improperly either by ignoring it or repairing it. Hence, it is a modality-specific strategy to reach the best restoration of residual function. Substitution refers to a process of learning to substitute other sensory information or activities to improve balance performance. For example, visual and haptic cues can be used to compensate for residual deficits in vestibular transduction; in the absence of visual input (eyes closed), patients with bilateral vestibular dysfunction rely on proprioceptive input for roll plane sway stabilization during static stance (Horlings et al., 2008). Desensitization is the process of learning to tolerate an abnormal response. It is very similar to cognitive and behavioral therapies for anxiety disorders (Beidel and Horak, 2001).
These recovery strategies are recruited by specific exercises that are designed to decrease dizziness (a percept), improve static and dynamic balance function (motor performance), and increase general activity levels (restoration of daily activities) (Herdman and Whitney, 2007; Whitney and Sparto, 2011). Because they involve self-generated activity, the exercises provide stimulation to foster integration (compensation) of the somatosensory, visual, and vestibular information processing for graded levels of skilled spatial orientation. Exercises to decrease dizziness may focus on exposure to specific stimulus profiles for habituation or attenuation of the perceptual response. Balance retraining exercises are designed to improve organization of sensory information for balance control and coordination of muscle responses. General activity exercise involves a daily aerobic exercise program of progressive walking, cycling, or swimming. One states the obvious by asserting that the success of these strategies in improving functional balance performance is a direct reflection of their ability to engage adaptive processes to achieve compensation. Because compensation is a dynamic process, one expects that the best empirical results will be achieved when each modality of intervention is delivered at the appropriate time in the process.
3. Top-down strategies in clinical assessment and vestibular rehabilitation
3.1 Therapeutic progression proceeds from head control to locomotion
Empirical principles for vestibular rehabilitation practice include an implicit top-down strategy for progression of exercises. The therapy progression can be summarized from the literature (Herdman and Whitney, 2007; Whitney and Sparto, 2011) as follows. If a patient is incapable of standing safely, they remain seated and perform active eye and head movements while fixating on a point in a lighted room. If the patient is capable of standing safely, static postural control exercises are initiated simultaneously. The difficulty of the exercises is then increased progressively, within tolerance limits of the patient. For example, the speed and frequency of head movements are increased and the head movements can be performed with eyes closed. For postural exercises, the base of support is altered and head movement exercises of progressive difficulty are performed simultaneously. A similar progression of therapy strategy is followed for dynamic gait rehabilitation. A natural adaptive progression from head control on seated body, to head control while standing to head control while walking (i.e., from eye-head coordination to static postural coordination to dynamic postural coordination) has contributed to the empirical success of vestibular rehabilitation.
The term optimization is inappropriate for systems such as balance control, where performance simply needs to be ‘good enough’ for daily activities. Experience teaches that vestibular, visual and somatosensory modalities have different patterns of influence on postural control among normal subjects (Collins and De Luca, 1995; Lacour et al., 1997). Therefore, it is not surprising that there are multiple strategies for sensory weighting after balance rehabilitation (e.g.,, (Horak et al., 1990; Lacour et al., 1997)). Hence, the top-down trajectory to terminal compensation and adaptation should be conceptualized as an sequential process of satisficing (Simon, 1955; Simon, 1956) during more challenging activities.
The vestibular rehabilitation approach to compensation is based upon the concept that functional restoration requires a systematic reintegration of the multiple sensory modalities involved in balance in an appropriate order (and time frame) to improve outcomes. The therapeutic goal is achieved by re-establishing relationships between balance-related sensory signals that are referenced to the head, the retina or postcranial body regions (trunk and limbs). Vestibular sensory information is head-referenced; semicircular canal and otolith organs provide linear and angular acceleration signals relative to their fixed orientation in the skull. Because eye movements alter the orientation of the retinae (eccentricity and vergence angle) relative to the head, visual information for spatial orientation must placed in head-referenced (vestibular) coordinates. Proprioceptive and visceral information are body referenced (Balaban and Yates, 2004).
3.2 Rehabilitation exercises target functional subsystems
A vestibular physical therapy program integrates exercise procedures that are designed to improve performance of the vestibulo-ocular reflex (VOR), cervico-ocular reflex (COR), depth perception (DP), somatosensory retraining (SS), dynamic gait, and aerobic function (Herdman and Whitney, 2007; Whitney and Sparto, 2011). The VOR and COR exercises engage plasticity of these reflex pathways. The DP exercises are designed to recalibrate depth and spatial perception of the current sensory information. These VOR, COR, and DP exercises are graded in difficulty, based on velocity of head and object motion. They are also performed, in a progression of difficulty, while sitting, standing and walking to foster top-down integration of motor control.
The SS exercises are more integrative and require compensation to the extent that a patient can stand. They are performed while standing and are introduced in order of increasing difficulty. The functional difficulty is augmented by narrowing the base of support, making the surface uneven, or changing the compliance of the substrate. Large amplitude head and trunk movements are also employed to increase the range of somatosensory input to span the normal functional range. These exercises include the Proprioceptive Neuromuscular Facilitation (PNF) techniques of slow reversal head and neck patterns, modified chopping and lifting for head and trunk in progression from supine, to sitting, and standing postures and total body mass rolling activities. Dynamic gait exercises extend activities to walking. These activities are graded in difficulty by changing direction, performing with the eyes closed, increasing speed of ambulation, walking on soft surfaces, or navigating stairs. An aerobic exercise home program increases progressively the time, speed, or distance that the patient can tolerate.
Clinical experience indicates that a top-down rehabilitation strategy is tolerated well by patients and produces impressive outcomes in terms of both subjective patient and objective functional metrics. The status of VOR function and eye tracking function at the initiation of therapy are an important factor in selecting initial therapeutic procedures and interim target outcomes. Patients simply cannot master somatosensory exercise and PNF skills without at first gaining control of eye motion and eye-head coordination (Herdman and Whitney, 2007; Whitney and Sparto, 2011). As a result, a great deal of time and attention is directed, both initially and throughout therapy, at challenging the VOR and gaze control. The integration of the head motion with eye motion during gaze shifts is an important precursor for performance of somatosensory exercises, PNF tasks, and mastery of basic cardiovascular and strength exercises. Poor static and dynamic head positioning also creates a strain on the neck which may result in negative feedback on balance function through altered COR or impaired interpretation of torso and limb somatosensory cues.
4. Progression of exercise difficulty in vestibular rehabilitation: Compensation as a stepwise learning process
4.1 Spontaneous compensation without rehabilitation therapy
4.1.1 Sensorimotor performance
Experience with vestibular rehabilitation indicates that outcomes are improved by stepwise achievement of interim goals, which may require replacing previously compensatory strategies with new, learned strategies that enhance functional recovery. The first benchmark, resolution of nausea, severe vertigo and spontaneous nystagmus, is typically achieved prior to initiating rehabilitation (Herdman and Whitney, 2007). It has long been recognized that this benchmark is a necessary but insufficient condition for vestibular recovery. Although spontaneous nystagmus in the light resolves quickly (Curthoys and Halmagyi, 1999; Halmagyi et al., 2010; Smith and Curthoys, 1989), this functional milestone alone is not prognostic for immediate improvement in balance function or successful rehabilitation. In rats, tonic head deviation improves markedly within one hour of unilateral vestibular injury (Llinas and Walton, 1979), but spontaneous nystagmus in the light resolves over approximately 48 hours (Balaban et al., 1999; Llinas and Walton, 1979). Patients with sudden unilateral vestibular dysfunction show a similar sequence of early compensation within the 3–5 days prior to earliest initiation of therapeutic vestibulo-ocular exercises (Enticott et al., 2005; Herdman et al., 1995; Vereeck et al., 2008). Tonic yaw head deviation often improves within 2–3 days, with only slight head-on-trunk yaw or roll deviation when standing seven days after injury (Borel et al., 2002). Spontaneous nystagmus in patients often resolves during the same time frame in the light, but nystagmus reappears when visual fixation is abolished by Frenzel lenses or in darkness for a more prolonged period of time. These early static (or tonic) adaptations stabilize (1) the orientation of head re: trunk and (2) the orientation of the retinas re: head, providing a common coordinate frame for interpreting residual vestibular information with visual, somatosensory and visceral signals that convey gravitoinertial information. We suggest that these changes mark a first milestone of functional compensation, which provides a reference frame for reestablishing balance function but includes self-limiting features that are overcome by rehabilitation. A static head tilt aligns the head to the perceived orientation from the intact saccule and utricle, in the absence of input from the damaged side. In addition, patients often adopt voluntary limitations on head movements to minimize disequilibrium and nausea (Herdman and Whitney, 2007), which is ‘unlearned’ by VOR and COR rehabilitation exercises.
From a functional perspective, it is equally important to recognize that the influence of vestibular, visual and somatosensory (including proprioceptive) information on postural control varies among normal subjects (Collins and De Luca, 1995; Lacour et al., 1997; Peterka et al., 2011), presumably because there is no unique optimal strategy, A corollary to this principle is that the first milestone of compensation reflects trade-offs between multiple demands for reorganizing balance control. The tradeoffs can also produce collateral consequences. One cautionary case was raised by Peterka and Benolken (Peterka and Benolken, 1995) in studies of visually induced postural sway in normal subjects and patients who had compensated for bilateral vestibular loss. They found no differences in somatosensory cue sensitivity and argued that apparent visual sensitivity changes were a secondary effect of the loss of vestibular suppression of postural sway in the patients.
The high prevalence of the clinically significant head tilt and static head position deviation after recovery from spontaneous nystagmus has been noted repeatedly (Halmagyi et al., 2010). Although the magnitude of spontaneous sway normalizes over the first month after damage (Fetter et al., 1991; Lacour et al., 1997), static postural control is strongly dependent upon the availability of visuospatial information during the first three months after unilateral vestibular injury. Tonic trunk yaw and roll deviation both resolve in the light within 30 days of injury, but trunk yaw deviation and a combined head-trunk roll deviation persist in the dark for at least 3 months (Borel et al., 2002). One is faced often with patients who no longer have acute spontaneous nystagmus but still have imbalance and visual abnormalities (head tilt, impaired smooth pursuit, and limits on head motion). In fact, the persistent static head tilt, visual dependence and limited spontaneous head motion are components of the motor strategies utilized to suppress the acute spontaneous nystagmus in the light and re-establish a common reference frame for vestibular, visual and somatic balance-related information. Hence, self-imposed limits on head movement are a simple (but effective) strategy to stabilize head-referenced senses.
The dynamic performance of vestibulo-ocular reflexes (VORs) improves more gradually and recovery is often incomplete. The horizontal VOR performance is attenuated and highly asymmetric during the first week after unilateral damage, with a gradual increase in gain and increased symmetry within one year (Allum et al., 1988; Allum and Ledin, 1999). However, considerable asymmetry can still persist chronically (1–5 years) during transient head movements (Crane and Demer, 1998). Similarly, the horizontal VOR time constant is shortened bidirectionally for the first month after insult, recovering gradually within 1 year to normal performance only for rotation toward the intact side (Allum et al., 1988). By contrast, dynamic trunk sway during bipedal stance (eyes closed) is almost normal after 3 months of compensation. However, trunk sway resolves more slowly (eyes open or closed) for more difficult tasks, such as one-legged stance and gait (Allum and Adkin, 2003). A skilled therapist uses a patient’s perceived gradient in task difficulty to define the sequence from therapeutic progression of retraining in vestibular rehabilitation.
4.1.2 Cognitive and information processing impairment and vestibular compensation
Quantitative analysis of empirical case histories and detailed interview data indicate that both vertiginous patients and individuals undergoing vestibular rehabilitation perceive cognitive limitations after developing the balance disorder (Jacobson and Newman, 1990; Morris et al., 2008; Yardley et al., 1992; Yardley and Putman, 1992). This perception is sufficiently prevalent and robust that impaired ability for concentration, attention and/or memory meets statistical criteria for inclusion in the Dizziness Handicap Inventory (DHI) and related questionnaire instruments for subjective impairment (Hazlett et al., 1996; Jacobson and Newman, 1990; Morris et al., 2008; Morris et al., 2009; Yardley et al., 1992). However, this item is far from a specific or sensitive detector of cognitive function. The formulators of the DHI noted originally that subjective difficulty concentrating is correlated with items indicating both functional and emotional effects (Jacobson and Newman, 1990). Later multivariate analyses of DHI results (both factor analysis and latent variable modeling) confirm that this item is related to a sense of impaired quality of life, including activities daily living and social interactions (Asmundson et al., 1999; Perez et al., 2001; Tamber et al., 2009; Vereeck et al., 2007).
Studies of interactions between postural control and cognitive task performance have yielded explicit evidence of slower and less accurate auditory choice reaction time task performance in patients who have compensated from unilateral vestibular dysfunction (Talkowski et al., 2005; Yardley et al., 2001). Seated subjects with a documented pre-existing vestibular disorder showed significantly prolonged reaction times from control subjects for tone-related tasks of varying complexity, including a go/no-go task (Talkowski et al., 2005; Yardley et al., 2001). The patient group also made more errors on a more complex task using spoken numbers as stimuli (Yardley et al., 2001). However, simple auditory reaction time performance was unchanged relative to the control subjects (Talkowski et al., 2005). These studies provide objective support that more sluggish information processing may contribute to the perception of difficulties with concentration and memory by these patients. Longitudinal tracking of cognitive task performance is needed to assess how information processing capabilities are impacted by processes of vestibular compensation.
These changes in choice reaction time performance may provide a metric of dysfunction and reorganization of vestibular influences on regions such as the amygdala, prefrontal cortex and hippocampus. Functional imaging studies have shown that caloric irrigation or galvanic vestibular stimulation produce increased blood flow in cognition-related regions that include the posterior and anterior cingulate gyri, orbitofrontal cortex, and prefrontal cortex (Bottini et al., 2001; Bucher et al., 1998; Dieterich et al., 2003; Dieterich and Brandt, 2008; Eikhoff et al., 2006; Fasold et al., 2002; Lobel et al., 1998; Suzuki et al., 2001). Caloric stimulation produces similar effects on blood flow in the hippocampus (Bottini et al., 2001; Dieterich et al., 2003; Suzuki et al., 2001) and hippocampal atrophy has been associated with bilateral vestibular lesions (Brandt et al., 2005). It is, therefore, of particular interest that cognitive function can be impaired by dendritic atrophy in the prefrontal cortex (Arnstein, 2009) and the hippocampus (Ulrich-Lai and Herman, 2009) that accompanies acute and/or chronic effects of anxiety and stress. The vulnerability of these regions increases with aging (Lupien et al., 2009) and the dendritic atrophy (and altered spine turnover) at these sites can also be elicited by pharmacological manipulation of glucocorticoid receptors (Liston and Gan, 2011; McEwen, 2010). These stress responses are also mediated by structures such as the dorsal raphe nucleus, locus coeruleus and the central amygdaloid nucleus (Joëls and Baram, 2009), which are linked to vestibular information processing (Balaban et al., 2011; Halberstadt and Balaban, 2006a; Halberstadt and Balaban, 2006b; Halberstadt and Balaban, 2007; Schuerger and Balaban, 1999). Hence, studies of the cognitive aspects of vestibular compensation will need to be sensitive to effects of incidental chronic and acute stress responses, in addition to standard measures of sensorimotor and cognitive performance.
4.2 Vestibular rehabilitation therapy interventions: improving dynamic recovery
Empirical outcomes from vestibular rehabilitation practice suggest that dynamic VOR recovery is limited, to a large extent, by the restricted range of spontaneous head motion that is adopted during static head and trunk compensation (Herdman and Whitney, 2007). This limited movement range of the head in space is an adaptation (a form of natural vestibular compensation) to minimize visual-vestibular mismatch consequences created by an abnormal dynamic VOR. However, limited head motion greatly limits engagement of the profound plasticity of human vestibulo-ocular reflexes (Gauthier and Robinson, 1975; Gonshor and Melvill Jones, 1976a; Gonshor and Melvill Jones, 1976b; Kramer et al., 1998; Schubert et al., 2008; Shelhamer et al., 1992; Shelhamer et al., 2002) for the recovery of dynamic VOR function. The therapist utilizes active head-based exercises to drive active recovery of gaze (and hence, VOR) control. When treatment is initiated, the exercises are most commonly performed while seated, which eliminates poor postural control as a confounding factor and creates a stable base for eye-head-trunk coordination training. Graded integrated recovery is achieved by introducing active head movement exercises while the patient is standing, followed by introduction while walking.
The time course for dynamic VOR recovery is influenced profoundly by exercise therapy. Significant recovery usually requires approximately four weeks of therapy and there is no evidence of improvement at four weeks in those not receiving therapy (Herdman et al., 2003). A number of investigators have documented advantages of visual based therapy for improving global recovery time (Chen et al., 2011; Cohen, 2006). The oculo-motor and gaze stability exercises also improve postural stability dramatically, even without posture training (Morimoto et al., 2011). Surprisingly, optokinetic (OKN) based therapy can produce a significantly better outcome than computerized dynamic posturography (CDP)-based postural rehabilitation on a number of postural measures (Rossi-Izquierdo et al., 2011). The improvement in all of these cases results directly from overcoming self-imposed limits on compensation that are a collateral or side effect of compensatory strategies adopted previously. However, ocular tilt (eye torsion) due to otolith organ loss compensates only partially after otolith organ damage and the axis of eye rotation remains misaligned with respect to the axis of head rotation.
A set of visually based vestibular-visual-cognitive interaction tests has proven to be useful as objective, functional outcome measures for customized vestibular rehabilitation programs (Gottshall and Hoffer, 2010). This test battery is performed in a darkened room with an effective viewing distance of 10 feet. Individuals give a verbal response that is recorded by the test operator. The test battery includes static visual acuity (SVA), perception time (PT), target acquisition (TA), target following (TF), dynamic visual acuity (DVA), and gaze stabilization tests (GST). The PT is measured by calculating the time in milliseconds (msec) that a randomly presented target must be on the screen before accurate subject recognition. The TA metric is the time (in msec) required for generating a saccade from the center of the screen to a new optotype positioned up, down, to the right, or to the left. The TF measures the smooth pursuit speed (in deg/sec) at which the subject can accurately track a symbol moving horizontally or vertically at constant velocity. The DVA is measured as the logarithm of the minimum angle of resolution (logMAR) from the Snellen chart optotype scale. The DVA test assesses changes in function from stable acuity to head motion acuity with active horizontal or vertical head motion (Herdman et al., 1998). The GST metric is the speed in deg/sec at which the subject could accurately hold a target and maintain recognition while performing active horizontal or vertical head motion.
Although posturography and the dynamic gait index (DGI) are useful outcome measures for severely affected individuals, their utility is hampered by ceiling effects in a wide variety of balance disorder populations. The visually based interaction tests have proved to be the most sensitive measure of outcome. The details of findings in specific groups of patients are presented elsewhere (Gottshall and Hoffer, 2010). Although each test in this battery provides valuable information, the vertical GST is the last measure to improve in our patient populations. This likely indicates that recovery of vestibular function is dependent on the frequency and velocity of motion and that it is related intimately to the prior establishment of gaze stabilization.
5. Moving forward: a translational approach
5.1 Vestibular compensation is a life-long, trainable adaptive process
The term ‘vestibular compensation’ is used most commonly to describe phenomena that occur after unilateral impairment of the vestibular periphery (Curthoys and Halmagyi, 1999). The profound effects of compensatory processes on balance control were noted during the latter decades of the nineteenth century in some of the earliest descriptions of the phenomena. The most striking was von Bechterew’s report (Bechterew, 1883) that unilateral, serial bilateral, and simultaneous bilateral damage to the vestibular periphery showed distinctly different patterns of behavioral recovery The effects of serial peripheral lesions (‘Bechterew phenomenon or compensation’) are particularly noteworthy. After functional compensation for a unilateral injury, a lesion of the intact inner ear produced the same nystagmus, eye deviation, body deviation and head torsion as if the previous destroyed vestibular organs were intact. This reproducible phenomenon was accepted as evidence for central compensatory processes for profoundly asymmetric vestibular information (Magnus, 1924 (reprint 1980)).
Experimental paradigms to study the neurobiological bases for ‘vestibular compensation’ have focused almost exclusively on naturalistic, short term sequelae of labyrinthectomy. This operational definition is advantageous for reductionist experimental design purposes, but we should not succumb to collective intellectual myopia regarding the relationship of this special case to less drastic adaptive challenges for vestibular function. Vestibulo-ocular, postural and balance control show considerable adaptive plasticity to compensate for less severe perturbations. There are extensive literatures regarding adaptive capabilities of vestibulo-ocular reflexes to altered visual inputs (Gauthier and Robinson, 1975; Gonshor and Melvill Jones, 1976a; Gonshor and Melvill Jones, 1976b; Kramer et al., 1998; Schubert et al., 2008; Shelhamer et al., 1992; Shelhamer et al., 2002) and the more complex cognitive and sensory-motor adaptative learning capabilities of postural and locomotor performance (Bhatt and Pai, 2009; Bronstein and Reynolds, 2007; Bronstein et al., 2009; Earhart et al., 2002; Pavol and Pai, 2002). Vestibular rehabilitation therapy uses these adaptive learning processes to improve functional compensation. Hence, they should be included in the purview of translationally directed basic studies of ‘vestibular compensation’.
This more expansive teleological perspective presents vestibular compensation as an adaptive process that maintains stable balance function in the face of challenges that create functional asymmetries in vestibular input. The lack of clinical manifestations of normal developmental asymmetries in the inner ear and vestibular nerve reflects such adaptive processes. Later challenges that are met by compensation mechanisms include spontaneous, age-related loss of vestibular hair cells (Lopez et al., 2005; Merchant et al., 2000; Rauch et al., 2000), age- and hair cell-related loss of vestibular ganglion cells (Park et al., 2001; Velazquez-Villasenor et al., 2000), central and peripheral effects of chemical exposures to drugs or toxins, and disease.
5.2 A Systems-of-Systems perspective of vestibular compensation
The successful outcomes of vestibular rehabilitation therapy provide strong empirical support for the hypothesis that clinical outcomes can be improved by guiding vestibular compensation through a stepwise trajectory of top-down partial recovery states. It recognizes the functional interdependency of balance-related sensorimotor processes and that some adaptive strategies may have to be unlearned because they retard the ability to achieve the next set of goals. Hence, it focuses our attention on the issue of sequentially shaping the conditions so that adaptive mechanisms can achieve a better functional outcome. This approach is tantamount to recasting the basic scientific issue of improving vestibular compensation as a question of identifying appropriate serial interventions (drugs and physical therapy) to guide efficacious functional reorganization of a ‘complex adaptive System-of-Systems’ (Holland, 1992) for balance control.
A complex, adaptive systems approach for vestibular compensation and rehabilitation can be summarized simply and intuitively. A top-down progression of compensation is a direct consequence of the location of head-referenced vestibular and visual systems atop the inverted pendulum of the neck, trunk and lower limbs, which are governed by both head-referenced and body-references sensorimotor control systems. Because motion intolerance (including nausea and fear of falling) is a consequence of inappropriate VOR, COR and gaze performance, these head-referenced behaviors are limiting factors for the magnitude and dynamics of tolerable body motion (static sway and dynamic motion). In subjects with normal VOR, COR and DP performance, static posture appears to be stabilized by minimizing muscle activation rather than by directly reducing postural sway (Kiemel et al., 2011) and two principal components of motion appear to account for more than 92% of spontaneous static postural sway (Maurer and Peterka, 2005). In the acute period after sudden unilateral vestibular dysfunction, adaptive suppression of spontaneous nystagmus is accompanied a voluntary limitation of the range and speed of head movements to avoid unpleasant symptoms such as dizziness, disequilibrium, fear of falling and nausea (Herdman and Whitney, 2007). This reluctance to extend the range and magnitude of motion is the first obstacle to patient compliance with rehabilitation exercises, which must be performed with sufficient speed and range-of-motion to elicit at least mild/moderate symptoms (Borello-France et al., 1999). Because head motion due to normal postural sway often elicits motion intolerance, the VOR, COR, gaze and DP exercises are performed initially while seated. The process is then repeated while retraining dynamic control of the inherently unstable bipedal posture of humans (Loram and Lakie, 2002; Loram et al., 2011), limited by the compensation of head-referenced intolerance that can be induced by body motions.
The clinical data raise the question of whether interim outcomes of vestibular rehabilitation therapy are aligned with quasi-stable, adapted states that are sufficient for a specified normal range of activities. This approach suggests three foci for examining neurobiological mechanisms. Firstly, it is important to identify quasi-stable operating states of balance function in normal and adapting individuals and their neural bases, which may occur at many levels of the neuraxis. For example, functional imaging correlates of cortical visual motion responses are suppressed in compensated patients 5–13 years after unilateral vestibular nerve transaction during acoustic neuroma surgery (Deutschländer et al., 2008). Secondly, it is important to examine dynamic changes in cognitive and information processing performance after vestibular dysfunction and during compensation, both in terms of the trajectory of clinical recovery and as an outcome measure for assessing interventions. Thirdly, it is important to identify and characterize the adaptive and learning mechanisms that facilitate transitions between these operating characteristics, and their interaction with cognitive performance. This strategy, motivated by vestibular rehabilitation research offers the possibility of shaping the recovery trajectory with interventions that target molecular and genetic factors in these compensatory mechanisms.
Footnotes
DISCLAIMER: The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Carey D. Balaban, Departments of Otolaryngology, Neurobiology, Communication Sciences & Disorders, and Bioengineering, University of Pittsburgh, Pittsburgh, PA USA.
Michael E. Hoffer, Department of Otolaryngology, Naval Medical Center San Diego, San Diego, CA USA.
Kim R. Gottshall, Director of Vestibular Assessment and Evaluation, Naval Medical Center San Diego, San Diego, CA USA.
References
- Allum JHJ, Yamane M, Pfaltz CR. Long-term modifications of vertical and horizontal vestibulo-ocular reflex dynamics in man. I. After acute unilateral vestibular paralysis. Acta Oto-Laryngologica. 1988;105:328–337. doi: 10.3109/00016488809097015. [DOI] [PubMed] [Google Scholar]
- Allum JHJ, Ledin T. Recover of vestibulo-ocular reflex function in subjects with an acute unilateral vestibular deficit. J Vestibular Research. 1999;9:135–144. [PubMed] [Google Scholar]
- Allum JHJ, Adkin AL. Improvements in trunk sway observed for stance and gait tasks during recovery from an acute unilateral peripheral vestibular deficit. Audiology & Neuro-Otology. 2003;8:286–302. doi: 10.1159/000071999. [DOI] [PubMed] [Google Scholar]
- Arnstein AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson GJG, Stein MB, Ireland D. A factor analytic study of the dizziness handicap inventory: does it assess phobi avoidance in vestibular referrals? J Vestibular Research. 1999;9:63–68. [PubMed] [Google Scholar]
- Balaban CD, Romero GG. A role of climbing fibers in regulation of flocculonodular lobe protein kinase C expression during vestibular compensation. Brain Research. 1998;804:253–265. doi: 10.1016/s0006-8993(98)00658-1. [DOI] [PubMed] [Google Scholar]
- Balaban CD, Freilino M, Romero GG. Protein kinase C inhibition blocks the early appearance of vestibular compensation. Brain Research. 1999;845:97–101. doi: 10.1016/s0006-8993(99)01958-7. [DOI] [PubMed] [Google Scholar]
- Balaban CD, Yates BJ. Vestibulo-autonomic interactions: a teleologic perspective. In: Highstein SN, Fay RR, Popper AN, editors. Springer Handbook of Auditory Research: The Vestibular System. Springer-Verlag; New York, NY: 2004. pp. 286–342. [Google Scholar]
- Balaban CD, Jacob RG, Furman JM. Neurologic bases for comorbidity of balance disorders, anxiety disorders and migraine: neurotherapeutic implications. Expert Rev Neurother. 2011;11:379–394. doi: 10.1586/ern.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechterew W. Ergebnisse der Durchscheidung des N. acusticus, nebst Erörterung der Bedeutung der semicirculären Canäle für das Körpergleichgewicht. Pflügers Arch f d ges Physiol. 1883;30:312–347. [Google Scholar]
- Beidel DC, Horak FB. Behavior therapy for vestibular rehabilitation. Journal of Anxiety Disorders. 2001;15:121–130. doi: 10.1016/s0887-6185(00)00046-3. [DOI] [PubMed] [Google Scholar]
- Bernard, C., 1875, c1989 Lectures on Anesthetics and on Asphyxia (1875), translated from the French by B. Raymond Fink, Vol., Wood Library-Museum of Anesthesiology, Park Ridge, Ill.
- Bhatt T, Pai YC. Generalization of gait adaptation for fall prevention: from movable platform to slippery floor. J Neurophysiol. 2009;101:948–957. doi: 10.1152/jn.91004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel L, Harlay F, Magnan J, Chays A, Lacour M. Deficits and recovery of head and trunk orientation and stabilization after unilateral vestibular loss. Brain. 2002;125:880–894. doi: 10.1093/brain/awf085. [DOI] [PubMed] [Google Scholar]
- Borello-France DF, Gallagher JD, Redfern MS, Furman JM, Carvell GE. Voluntary movement strategies of individuals with unilateral peripheral vestibular hypofunction. J Vestibular Research. 1999;9:265–275. [PubMed] [Google Scholar]
- Bottini G, Karnath HO, Vallar G, Sterzi R, Frith CD, Frackowiak RSJ, Paulesu E. Cerebral representations for egocentric space. Functional-anatomical evidence from caloric vestibular stimulation and neck vibration. Brain. 2001;124:1182–1196. doi: 10.1093/brain/124.6.1182. [DOI] [PubMed] [Google Scholar]
- Brandt T, Schautzer F, Hamilton D, Brüning R, Markowitsch HJ, Kalla R, Darlington CL, Smith PF, Strupp M. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain. 2005;128:2732–2741. doi: 10.1093/brain/awh617. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Reynolds R. The moving platform after-effect reveals dissociation between what we know and how we walk. J Neural Transm. 2007;114:1297–1303. doi: 10.1007/s00702-007-0791-8. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Bunday KL, Reynolds R. What the “broken escalator” phenomenon teaches us about balance. Annals New York Acad Sci. 2009;1164:82–88. doi: 10.1111/j.1749-6632.2009.03870.x. [DOI] [PubMed] [Google Scholar]
- Bucher S, Dieterich M, Wiesmann M, Weiss A, Zink R, Youstry T, Brandt T. Cerebral functional magnetic resonance imaging of vestibular, auditory, and nociceptive areas during galvanic stimulation. Annals Neurol. 1998;44:120–125. doi: 10.1002/ana.410440118. [DOI] [PubMed] [Google Scholar]
- Cawthorne T. Vestibular injuries. Proc Roy Soc Med. 1945;39:270–273. doi: 10.1177/003591574603900522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthorne T, Cooksey FS. Original Cawthorne Cooksey Rehabilitation Exercises 1946. Proc Roy Soc Med. 1946;39:270. [Google Scholar]
- Cawthorne T. Some observations on the pathology and surgical treatment of labyrinthine vertigo of non-infective origin. Ann R Coll Surg Engl. 1949;4:342–359. [PMC free article] [PubMed] [Google Scholar]
- Chen EW, Fu AS, Chan KM, Tsang WW. Balance control in very old adults with and without visual impairment. Eur J Appl Physiol. 2011 doi: 10.1007/s00421-011-2139-1. [DOI] [PubMed] [Google Scholar]
- Cohen HS. Disability and rehabilitation in the dizzy patient. Curr Opin Neurol. 2006;19:49–54. doi: 10.1097/01.wco.0000194373.08203.33. [DOI] [PubMed] [Google Scholar]
- Collins JJ, De Luca CJ. The effects of visual input on open-loop and closed-loop postural control mechanisms. Exp Brain Res. 1995;103:151–163. doi: 10.1007/BF00241972. [DOI] [PubMed] [Google Scholar]
- Crane BT, Demer JL. Human horizontal vestibulo-ocular reflex initiation: effects of acceleration, target distance and unilateral deafferentation. J Neurophysiol. 1998;80:1151–1166. doi: 10.1152/jn.1998.80.3.1151. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Minor LB, Beraneck M, Sadeghi SG. Neural substrates underlying vestibular compensation: contribution of peripheral versus central processing. J Vestibular Research. 2009;19:171–182. doi: 10.3233/VES-2009-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys IS, Halmagyi GM. Vestibular compensation. In: Büttner U, editor. Vestibular Dysfunction and Its Therapy. Vol. 55. Karger; Basel: 1999. pp. 82–110. [Google Scholar]
- De Zeeuw CI, Hansel C, Bian F, Koekkoek SKE, van Alphen AM, Linden DJ, Oberdick J. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron. 1998;20:495–508. doi: 10.1016/s0896-6273(00)80990-3. [DOI] [PubMed] [Google Scholar]
- Deutschländer A, Hüfner K, Kalla R, Stephan T, Dera T, Glasauer S, Wiesmann M, Strupp M, Brandt T. Unilateral vestibular failure suppresses cortical visual motion processing. Brain. 2008;131:1025–1034. doi: 10.1093/brain/awn035. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Bartenstein P, Brandt T. Dominance for vestibular cortical function in the non-dominant hemisphere. Cerebral Cortex. 2003;13:995–1007. doi: 10.1093/cercor/13.9.994. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Brandt T. Functional brain imaging of peripheral and central vestibular disorders. Brain. 2008;131:2538–2552. doi: 10.1093/brain/awn042. [DOI] [PubMed] [Google Scholar]
- Dutia MB. Mechanisms of vestibular compensation: recent advances. Curr Opin Otolaryngol & Head and Neck Surgery. 2010;18:420–424. doi: 10.1097/MOO.0b013e32833de71f. [DOI] [PubMed] [Google Scholar]
- Earhart GM, Melvill Jones G, Horak FB, Block EW, Weber KD, Fletcher WA. Transfer of podokinetic adaptation from stepping to hopping. J Neurophysiol. 2002;87:1142–1144. doi: 10.1152/jn.00588.2001. [DOI] [PubMed] [Google Scholar]
- Eikhoff SB, Weiss PH, Amunts K, Fink GR, Zilles K. Identifying human parieto-insular vestibular cortex using fMRI and cytoarchitectonic mapping. Human Brain Mapping. 2006;27:611–621. doi: 10.1002/hbm.20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enticott JC, O’Leary SJ, Briggs RJS. Effects of bilateral vestibulo-ocular reflex exercises on vestibular compensation after vestibular schwannoma surgery. Otology & Neurotology. 2005;26:265–269. doi: 10.1097/00129492-200503000-00024. [DOI] [PubMed] [Google Scholar]
- Fasold O, von Brevern M, Kuhberg M, Ploner CJ, Villringer A, Lempert T, Wenzel R. Human vestibular cortex identified with caloric stimulation in functional magnetic resonance imaging. NeuroImage. 2002;17:1384–1392. doi: 10.1006/nimg.2002.1241. [DOI] [PubMed] [Google Scholar]
- Faulstich M, van Alphen AM, Luo C, du Lac S, De Zeeuw CI. Oculomotor plasticity during vestibular compensation does not depend upon cerebellar LTD. J Neurophysiol. 2006;96:1187–1195. doi: 10.1152/jn.00045.2006. [DOI] [PubMed] [Google Scholar]
- Fetter M, Diener HC, Dichgans J. Recovery of postural control after and acute vestibular lesion in humans. J Vestibular Research. 1991;1:373–383. [PubMed] [Google Scholar]
- Gauthier GM, Robinson DA. Adaptation of the human vestibuloocular reflex to magnifying lenses. Brain Research. 1975;92:331–335. doi: 10.1016/0006-8993(75)90279-6. [DOI] [PubMed] [Google Scholar]
- Gliddon CM, Darlington CL, Smith PF. GABAergic systems in the vestibular nucleus and their contribution to vestibular compensation. Progress in Neurobiology. 2005;75:53–81. doi: 10.1016/j.pneurobio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Gonshor A, Melvill Jones G. Short-term adaptive changes in the human vestibulo-ocular reflex arc. J Physiology. 1976a;256:361–379. doi: 10.1113/jphysiol.1976.sp011329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonshor A, Melvill Jones G. Extreme vestibulo-ocular adaptation induced by prolonged optical reversal of vision. J Physiology. 1976b;256:381–414. doi: 10.1113/jphysiol.1976.sp011330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto MM, Romero GG, Balaban CD. Transient changes in flocculonodular lobe protein kinase C expression during vestibular compensation. Journal of Neuroscience. 1997;17:4367–4381. doi: 10.1523/JNEUROSCI.17-11-04367.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottshall KR, Hoffer ME. Tracking recovery of vestibular function in Iindividuals With blast-induced head trauma using vestibular –visual-cognitive interaction tests. Journal of Neurologic Physical Therapy. 2010;34:94–97. doi: 10.1097/NPT.0b013e3181dead12. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Balaban CD. Anterograde tracing of projections from the dorsal raphe nucleus to the vestibular nuclei. Neuroscience. 2006a;143:641–654. doi: 10.1016/j.neuroscience.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Balaban CD. Serotonergic and nonserotonergic neurons in the dorsal raphe nucleus send collateralized projections to both the vestibular nuclei and the central amygdaloid nucleus. Neuroscience. 2006b;140:1067–1077. doi: 10.1016/j.neuroscience.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Balaban CD. Selective anterograde tracing of the individual serotonergic and nonserotonergic components of the dorsal raphe nucleus projection to the vestibular nuclei. Neuroscience. 2007;147:207–223. doi: 10.1016/j.neuroscience.2007.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmagyi GM, Weber KP, Curthoys IS. Vestibular function after acute vestibular neuritis. Restor Neurol Neurosci. 2010;28:37–46. doi: 10.3233/RNN-2010-0533. [DOI] [PubMed] [Google Scholar]
- Hazlett RL, Tusa RJ, Waranch HR. Development of an inventory for dizziness and related factors. J Behavioural Medicine. 1996;19:73–85. doi: 10.1007/BF01858175. [DOI] [PubMed] [Google Scholar]
- Herdman SJ. Treatment of benign paroxysmal vertigo. Physical Therapy. 1990;70:381–388. doi: 10.1093/ptj/70.6.381. [DOI] [PubMed] [Google Scholar]
- Herdman SJ, Clendaniel RA, Mattox DE, Holliday MJ, Niparko JK. Vestibular adaptation exercises and recovery: acute stage after acoustic neuroma resection. Otolaryngology-Head & Neck Surgery. 1995;113:77–87. doi: 10.1016/s0194-5998(95)70148-6. [DOI] [PubMed] [Google Scholar]
- Herdman SJ, Tusa RJ, Blatt P, Suzuki A, Venuto PJ, Roberts D. Computerized Dynamic Visual Acuity Test in the assessment of vestibular deficits. Am J Otol. 1998;19:790–796. [PubMed] [Google Scholar]
- Herdman SJ, Schubert MC, Das VE, Tusa RJ. Recovery of dynamic visual acuity in unilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg. 2003;129:819–824. doi: 10.1001/archotol.129.8.819. [DOI] [PubMed] [Google Scholar]
- Herdman SJ, Whitney SL. Interventions for the patient with vestibular hypofunction. In: Herdman SJ, editor. Vesibular rehabilitation. F.A. Davis; Philadelphia: 2007. pp. 309–337. [Google Scholar]
- Hillier SL, McDonnell M. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction (Review) Cochrane Database of Systematic Reviews. 2007;2007:1–73. doi: 10.1002/14651858.CD005397.pub2. [DOI] [PubMed] [Google Scholar]
- Hillier SL, McDonnell M. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst Rev. 2011;16:CD005397. doi: 10.1002/14651858.CD005397.pub3. [DOI] [PubMed] [Google Scholar]
- Holland JH. Complex adaptive systems. Daedalus. 1992;121:17–30. [Google Scholar]
- Horak FB, Nashner LM, Diener HC. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res. 1990;82:167–177. doi: 10.1007/BF00230848. [DOI] [PubMed] [Google Scholar]
- Horlings CGC, Küng UM, Bloem BR, Honegger F, Van Alfen N, Van Engelen BGM, Allum JHJ. Identifying deficits in balance control following vestibular or proprioceptive loss using posturographic analysis of stance tasks. Clinical Neurophysiology. 2008;119:2338–2346. doi: 10.1016/j.clinph.2008.07.221. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar long-term Depression: characterization, signal transduction, and tunctional roles. Physiol Rev. 2001;81:1144–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- Jacobson GP, Newman WW. Tne development of the dizziness handicap inventory. Arch Otolaryngol Head Neck Surg. 1990;116:424–427. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- Joëls M, Baram TZ. The neuro-symphony of stress. Nature Reviews Neuroscience. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AR, Seckl JR, Dutia MB. Role of the flooculus in mediating vestibular nucleus neuron plasticity during vestibular compensation in the rat. J Physiology. 2002;545:903–911. doi: 10.1113/jphysiol.2002.024281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiemel T, Zhang Y, Jeka JJ. Identification of neural feedback for upright stance in humans: stabilization rather than sway minimization. J Neuroscience. 2011;31:15144–15153. doi: 10.1523/JNEUROSCI.1013-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PD, Shelhamer M, Peng GCY, Zee DS. Context-specific short-term adaptation of the phase of the vestibulo-ocular reflex. Exp Brain Res. 1998;120:184–192. doi: 10.1007/s002210050392. [DOI] [PubMed] [Google Scholar]
- Lacour M, Barthelemy J, Borel L, Magnan J, Xerri C, Chays A, Ouaknine M. Sensory strategies in human postural control before and after unilateral vestibular neurotomy. Exp Brain Res. 1997;115:300–310. doi: 10.1007/pl00005698. [DOI] [PubMed] [Google Scholar]
- Lacour M. Restoration of vestibular function: basic aspects and practical advances for rehabilitation. Current Medical Research and Opinion. 2006;22:1651–1659. doi: 10.1185/030079906X115694. [DOI] [PubMed] [Google Scholar]
- Lacour M, Tighilet B. Plastic events in the vestibular nuclei during vestibular compensation: the brain orchestration of a “deafferentation” code. Restor Neurol Neurosci. 2010;28:19–35. doi: 10.3233/RNN-2010-0509. [DOI] [PubMed] [Google Scholar]
- Leitges M, Kovac J, Plomann M, Linden DJ. A Unique PDZ Ligand in PKCa Confers Induction of Cerebellar Long-Term Synaptic Depression. Neuron. 2004;44:585–594. doi: 10.1016/j.neuron.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Linden DJ, Connor JA. Long-term synaptic depression. Annual Review of Neuroscience. 1995;18:319–357. doi: 10.1146/annurev.ne.18.030195.001535. [DOI] [PubMed] [Google Scholar]
- Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Nat Acad Sci. 2011;108:16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Walton K. Vestibular compensation: a distributed property of the central nervous system. In: Asanuma H, Wilson VF, editors. Integration in the Nervous System. Igaku-Shoin; Tokyo: 1979. pp. 145–166. [Google Scholar]
- Lobel E, Kleine JF, Le Bihan D, Leroy-Willig A, Berthoz A. Functional MRI of galvanic vestibular stimulation. J Neurophysiol. 1998;80:2699–2709. doi: 10.1152/jn.1998.80.5.2699. [DOI] [PubMed] [Google Scholar]
- Lopez I, Ishiyama G, Tang Y, Tokita J, Baloh RW, Ishiyama A. Regional estimates of hair cells and supporting cells in the human crista ampullaris. Journal of Neuroscience Research. 2005;82:421–431. doi: 10.1002/jnr.20652. [DOI] [PubMed] [Google Scholar]
- Loram ID, Lakie M. Human balancing of an inverted pendulum: position control by small, ballistic-like, throw and catch movements. J Physiology. 2002;540.3:1111–1124. doi: 10.1113/jphysiol.2001.013077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Gollee H, Lakie M, Gawthrop PJ. Human control of an inverted pendulum: Is continuous control necessary? Is intermittent control effective? Is intermittent control physiological? J Physiology. 2011;589.2:307–324. doi: 10.1113/jphysiol.2010.194712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Magnus, R., 1924 (reprint 1980). Body posture = Korperstellung : experimental-physiological investigations of the reflexes involved in body posture, their cooperation and disturbances ; Based on a translation by William R. Rosanoff, and on a translation by Franklin Book Programs, Inc., Cairo, for the National Library of Medicine, Vol., Amerind, Springfield, VA, USA.
- Maurer C, Peterka RJ. A new interpretation of spontaneous sway measures based on a simple model of human postural control. J Neurophysiol. 2005;93:189–200. doi: 10.1152/jn.00221.2004. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann NY Acad Sci. 2010;1204:E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant SN, Velazquez-Villasenor L, Tsuji K, Glynn RJ, Wall Cr, Rausch SD. Temporal bone studies of the human peripheral vestibular system. Normative vestibular hair cell data. Annals of Otology, Rhinology, & Laryngology - Supplement. 2000;181:3–13. doi: 10.1177/00034894001090s502. [DOI] [PubMed] [Google Scholar]
- Morimoto H, Asai Y, Johnson EG, Lohman EB, Khoo K, Mizutani Y, Mizutani T. Effect of oculo-motor and gaze stability exercises on postural stability and dynamic visual acuity in healthy young adults. Gait Posture. 2011;33:600–603. doi: 10.1016/j.gaitpost.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Morris AE, Lutman ME, Yardley L. Measuring outcome from vestibular rehabilitation, part I: quantitative development of a new self-report measure. International Journal of Audiology. 2008;47:169–177. doi: 10.1080/14992020701843129. [DOI] [PubMed] [Google Scholar]
- Morris AE, Lutman ME, Yardley L. Measuring outcome from vestibular rehabilitation, part II: refinement and validation of a new self-report measure. International Journal of Audiology. 2009;48:24–37. doi: 10.1080/14992020802314905. [DOI] [PubMed] [Google Scholar]
- Norré ME, Beckers AM. Vestibular rehabilitation training. Specificity of adequate exercise. Arch Otolaryngol Head Neck Surg. 1988;1988:883–886. doi: 10.1001/archotol.1988.01860200067020. [DOI] [PubMed] [Google Scholar]
- Odkvist I, Odkvist LM. Physiotherapy in vertigo. Acta Oto-Laryngologica. 1988;455:74–76. [PubMed] [Google Scholar]
- Park JJ, Tang Y, Lopez I, Ishiyama A. Age-related change in the number of neurons in the human vestibular ganglion. Journal of Comparative Neurology. 2001;431:437–443. doi: 10.1002/1096-9861(20010319)431:4<437::aid-cne1081>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Pavol MJ, Pai YC. Feedforward adaptations are used to compensate for a potential loss of balance. Exp Brain Res. 2002;145:528–538. doi: 10.1007/s00221-002-1143-4. [DOI] [PubMed] [Google Scholar]
- Perez N, Garmendia I, Garcia-Granero M, Martin E, Garcia-Tapia R. Factor analysis and correlation between Dizziness Handicap Inventory and dizziness characteristics and impact on quality of life scales. Acta Oto-Laryngologica Suppl. 2001;545:145–154. doi: 10.1080/000164801750388333. [DOI] [PubMed] [Google Scholar]
- Peterka RJ, Benolken MS. Role of somatosensory and vestibular cues in attenuating visually induced human postural sway. Exp Brain Res. 1995;105:101–110. doi: 10.1007/BF00242186. [DOI] [PubMed] [Google Scholar]
- Peterka RJ, Statler KD, Wrisley DM, Horak FB. Postural compensaton for unilateral vestibular loss. Frontiers in Neurology. 2011;2:Article 57, 1–13. doi: 10.3389/fneur.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Velazquez-Villasenor L, Dimitri PS, Merchant SN. Decreasing hair cell counts in aging humans. Annals New York Acad Sci. 2000;942:220–227. doi: 10.1111/j.1749-6632.2001.tb03748.x. [DOI] [PubMed] [Google Scholar]
- Rossi-Izquierdo M, Santos-Pérez S, Soto-Varela A. What is the most effective vestibular rehabilitation technique in patients with unilateral peripheral vestibular disorders? Eur Arch Otorhinolaryngol. 2011:268. doi: 10.1007/s00405-011-1532-z. [DOI] [PubMed] [Google Scholar]
- Schonewille M, Gao Z, Boele HJ, Veloz MFV, Amerika WE, SImek AAM, De Jeu MT, Steinberg JP, Takamiya K, Hoebeek FE, Linden DJ, Huganir RL, De Zeeuw CI. Reevaluating the role of LTD in cerebellar motor learning. Neuron. 2011;70:43–50. doi: 10.1016/j.neuron.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert MC, Della Santina CC, Shelhamer M. Incremental angular vestibulo-ocular reflex adaptation to active head rotation. Exp Brain Res. 2008;191:435–446. doi: 10.1007/s00221-008-1537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerger RJ, Balaban CD. Organization of the coeruleo-vestibular pathway in rats, rabbits and monkeys. Brain Research Reviews. 1999;30:189–217. doi: 10.1016/s0165-0173(99)00015-6. [DOI] [PubMed] [Google Scholar]
- Shelhamer M, Robinson DA, Tan HS. Context-specific adaptation of the gain of the vestibulo-ocular reflex in humans. J Vestibular Res. 1992;2:89–96. [PubMed] [Google Scholar]
- Shelhamer M, Peng GCY, Ramat S, Patel V. Context-specific adaptation of the gain of the oculomotor response to lateral translation using roll and pitch head tilts as contexts. Exp Brain Res. 2002;146:388–393. doi: 10.1007/s00221-002-1235-1. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Horak FB. Rehabilitation strategies for patients with vestibular deficits. Neurol Clin. 1990;8:441–457. [PubMed] [Google Scholar]
- Shutoh F, Katoh A, Ohki M, Itohara S, Tonegawa S, Nagao S. Role of protein kinase C family in the cerebellum-dependent adaptive learning of horizontal optokinetic response eye movements in rats. Eur J Neurosci. 2003;18:134–142. doi: 10.1046/j.1460-9568.2003.02717.x. [DOI] [PubMed] [Google Scholar]
- Simon HA. A behavioral model of rational choice. The Quarterly Journal of Economics. 1955;69:99–118. [Google Scholar]
- Simon HA. Rational choice and the structure of the environment. Psychological Review. 1956;63:129–138. doi: 10.1037/h0042769. [DOI] [PubMed] [Google Scholar]
- Smith-Wheelock M, Shepard NT, Telian SA. Physical therapy program for vestibular rehabiliitation. Am J Otol. 1991;12:218–225. [PubMed] [Google Scholar]
- Smith PF, Curthoys IS. Mechanisms of recovery following unilateral labyrinthectomy: a review. Brain Res Rev. 1989;14:155–180. doi: 10.1016/0165-0173(89)90013-1. [DOI] [PubMed] [Google Scholar]
- Spiegel EA, Sommer I. Vestibular mechanisms. In: Glasser O, editor. Medical Physics. Vol. 1. Year Book Publishers; Chicago: 1944. pp. 1638–1653. [Google Scholar]
- Suzuki M, Kitano H, Ito R, Kitanishi T, Yazawa Y, Ogawa T, Shiino A, Kitajima K. Cortical and subcortical vestibular response to caloric stimulation detected by functional magnetic resonance imaging. Cognitive Brain Res. 2001;12:441–449. doi: 10.1016/s0926-6410(01)00080-5. [DOI] [PubMed] [Google Scholar]
- Talkowski ME, Redfern MS, Jennings JR, Furman JM. Cognitive requirements for vestibular and ocular motor processing in healthy adults and patients with unilateral vestibular lesions. Journal of Cognitive Neuroscience. 2005;17:1432–1441. doi: 10.1162/0898929054985419. [DOI] [PubMed] [Google Scholar]
- Tamber AL, Wilhelmsen KT, Strand LI. Measurement properties of Dizziness Handicap Inventory by cross-sectional and longitudinal designs. Health and Quality of Life Outcomes. 2009;7:101. doi: 10.1186/1477-7525-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez-Villasenor L, Merchant SN, Tsuji K, Glynn RJ, Wall Cr, Rauch SD. Temporal bone studies of the human peripheral vestibular system. Normative Scarpa’s ganglion cell data. Annals of Otology, Rhinology, & Laryngology - Supplement. 2000;181:14–19. doi: 10.1177/00034894001090s503. [DOI] [PubMed] [Google Scholar]
- Vereeck L, Truijen S, Wuyts FL, Van De Heyning PH. Internal consistency and factor analysis of the Dutch version of the Dizziness Handicap Inventory. Acta Oto-Laryngologica. 2007;127:788–795. doi: 10.1080/00016480601075464. [DOI] [PubMed] [Google Scholar]
- Vereeck L, Wuyts FL, Truijen S, De Valck C, Van De Heyning PH. The effect of early and customized vestibular rehabilitation on balance after acoustic neuroma resection. Clinical Rehabilitation. 2008;22:698–713. doi: 10.1177/0269215508089066. [DOI] [PubMed] [Google Scholar]
- Whitney SL, Sparto PJ. Principles of vestibular physical therapy rehabilitation. NeuroRehabilitation. 2011;29:157–166. doi: 10.3233/NRE-2011-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley L, Masson E, Verschuur C, Haacke N, Luxon L. Symptoms, anxiety and handicap in dizziness patients: development of the Vertigo Symptom Scale. J Psychosomatic Research. 1992;36:731–741. doi: 10.1016/0022-3999(92)90131-k. [DOI] [PubMed] [Google Scholar]
- Yardley L, Putman J. Quantitative analysis of factors contributing to handicap and distress in vertiginous patients. Clin Otolaryngol Allied Sci. 1992;17:231–236. doi: 10.1111/j.1365-2273.1992.tb01833.x. [DOI] [PubMed] [Google Scholar]
- Yardley L, Gardner M, Bronstein AM, Davies R, Buckwell D, Luxon L. Interference between postural control and mental task performance in patients with vestibular disorder and healthy controls. J Neurol Neurosurg Psychiat. 2001;71:48–52. doi: 10.1136/jnnp.71.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]