Abstract
A hydrophobic two-photon absorbing (2PA) red emitter (R) was successfully incorporated into micelles formed from two block copolymers, poly(ε-caprolactone)-block-poly(ethylene glycol)s, for imaging and toxicity studies. In micelles, the chromophore R exhibits a 2PA cross-section of 400 GM (1 GM =1×10−50 cm4 s photon−1 molecule−1) at 820 nm, which is among the highest values reported for red 2PA emitters. The micelles with a cationic amino moiety-containing poly(ethylene glycol) corona showed an enhancement of cell internalization and delivered the dye into the cytoplasmic regions of the mouse macrophage RAW 264.7 cells. In comparison, the dye in micelles with neutral poly(ethylene glycol) as corona could not be delivered into the cells. Cytotoxicity of the micelle-R constructs was studied using a 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. More than 90% of the cells were viable after they were stained with the dye-containing micelles at different concentrations (dye concentrations of 2 – 6 μM and polymer concentrations of 0.05 – 0.15 mg/mL) for 16 hours. This is the first reported application of a hydrophobic 2,1,3-benzothiadiazole-containing 2PA red emitter delivered into the cytoplasm of cells for bioimaging and toxicity assessment.
Keywords: Block copolymers, Molecular imaging, Cytotoxicity, Nanoparticles, Two-photon absorbing materials
1. Introduction
Substantial progress has been made over the past few years in the development of two-photon absorbing (2PA) materials that can be excited in the near infrared (NIR) spectral region (700 nm to 1000 nm). The strong research interests are inspired by the potential of using these materials for frequency up-conversion lasing, high-density optical storage, two-photon fluorescence microscopy, and photodynamic therapy applications.1,2 However, commercially available hydrophilic fluorescent molecular probes, such as fluoresceins, rhodamines, and green fluorescence protein usually have quite low 2PA cross-sections (δ, expressed in GM = 1×10−50 cm4 s photon−1 molecule−1) less than 200 GM.3 Recently, 2PA dyes based on the D-π-D, D-π-A-π-D, D-π-A, A-π-A (D: donor; π: conjugates; A: acceptor) structures have been shown to exhibit δ values higher than 2000 GM.4 Nevertheless, most of these 2PA materials are hydrophobic. Only a few water-soluble 2PA green emitters have been reported with high 2PA cross-sections (2050 GM) very recently.5 In addition to the commonly used blue and green emitters, red emitters are highly desirable for bioimaging because of the reduced auto-fluorescence of bio-substrates within the red spectral window.6 Until now, studies of the synthesis of efficient red 2PA emitters are quite limited.7 Red emission can be obtained either by direct excitation of chromophores7 or via indirect excitation using fluorescence resonance energy transfer (FRET) mechanism.8 Among the red 2PA emitters reported, the 2,1,3-benzothiadiazole (BTD)-containing hydrophobic D-π-A-π-D type dyes have been shown to exhibit high δ values of about 800 GM at 780 nm.7a. But, their poor solubility in water still limits their biological applications.
As most of the efficient 2PA chromophores are hydrophobic, the application of these materials in biological environment is a major challenge. The approach of using dye-doped silica particles9 has been demonstrated as an efficient solution to disperse 2PA materials in aqueous solutions. These particles have been used for bioimaging,9a, c, d, e pH sensing9f, and a potential photodynamic therapy to HeLa cancer cells.9g. Another potential method for the delivery of 2PA compounds is to use micelles formed from amphiphilic block copolymers (ABCs).10 The ABC, composed of at least one hydrophobic block and one hydrophilic block, can form micelle nanostructures with the hydrophobic blocks as the cores and hydrophilic blocks as the corona in aqueous solutions.10 These nanoparticles with sizes ranging from a few tens to hundreds of nanometers in water have been shown to act as nanocarriers or reservoirs to deliver hydrophobic therapeutic and diagnostic agents such as drugs,11 superparamagnetic iron oxides,12 and quantum dots13 into cells either through a non-specific endocytosis14 or receptor-mediated endocytosis (e.g. targeted delivery).15 Tian et al has recently demonstrated that hydrophobic 2PA emitters could be incorporated efficiently into ABC micelles16a or covalently linked onto ABCs16b to show reasonable 2PA efficiency for use in aqueous solutions. Moreover, the micelles with 2PA chromophores can be added with photosensitizers to enhance singlet oxygen generation efficiency through FRET.16b Zhang et al has also demonstrated the incorporation of hexa-peri-hexabenzocoronene core-containing 2PA chromophores and magnetic nanoparticles into the micelles of diacylphospholipid-b-poly(ethylene glycol) for magnetically guided two-photon cellular imaging.17 However, until now, most of the 2PA chromophores used in either dye-doped silica nanopaticles or micelles were based on fluorescent molecular probes that emit within the blue to green spectral window.
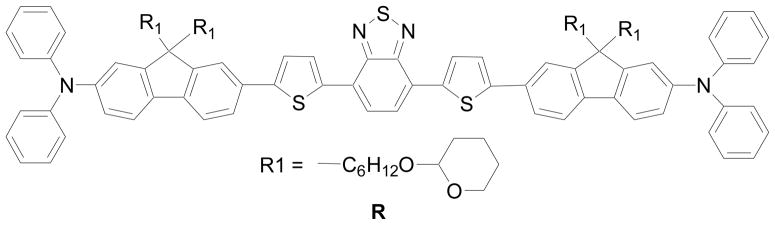
Herein, we report the synthesis of a new red 2PA emitter (R, see Figure 1 and S-Scheme 1 in supplementary materials), the incorporation of this emitter in micelles formed from either a commercially available methoxy-poly(ethylene glycol)-block-poly(caprolactone) (PEG45-b-PCL23) or a newly synthesized PEG-b-PCL type block copolymer with a ionic CF3COO−NH3+ moiety (P4, see Scheme 1). We also demonstrated the successful delivery of R into mouse macrophage RAW 264.7 cells using P4 and compared its photophysical properties in different environments.
Figure 1.
Molecular structure of the red emitter R.
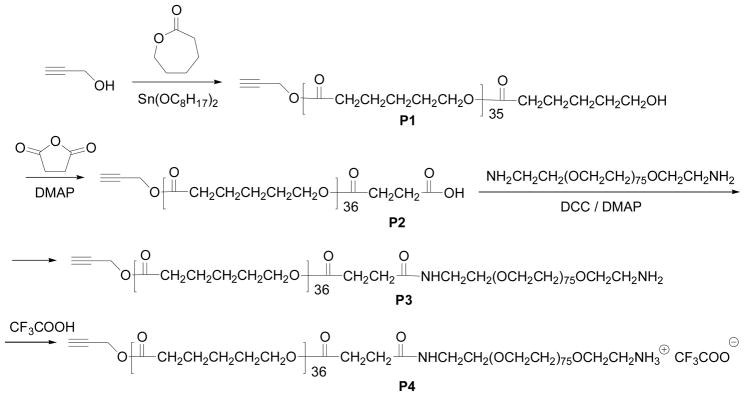
Scheme 1.
The synthesis of P4.
2. Experimental Section
2.1. Materials
α,ω-Diamino-(polyethylene glycol) (Mn = 3400) was prepared according to the procedures described in the literature.18 Propargyl alcohol, ε-caprolactone, stannous octoate, 4-N,N′-diaminomethylpyridine (DMAP), succinic anhydride, N,N′-dicyclohexylcarbodiimide (DCC), N-hydroxysuccinimide (NHS), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay were purchased from Aldrich (St. Louis, MO, USA) and used without further purification. Methoxy-poly(ethylene glycol)-b-poly(ε-caprolactone) (PEG45-b-PCL23, Mn (NMR) = 4600, Mw/Mn = 1.19. Its chemical structure was given in S-Figure 1 of Supporting Materials) was purchased from Polymer Source Inc (Montreal, Canada). Dialysis membranes (regenerate cellulose, Mw cut off 7000 and 10000) were purchased from Pierce (Rockford, IL, USA).
Dulbecco’s Modified Eagle’s Medium (DMEM) and Hoechst 33342 were acquired from Invitrogen (Carlsbad, CA, USA).
2.2. General methods
1H NMR spectra were measured using a Bruker 300 instrument operating at 300 MHz with TMS internal standard as a reference for chemical shifts. Molecular weights of polymers were determined using a Waters 1515 GPC coupled with UV and RI detectors, in reference with a series of polystyrene standards with THF as the eluent. UV-Vis spectra were recorded with a Hewlett Packard 8452A Diode Array UV-Vis Spectrophotometer. One-photon fluorescence spectra were recorded with a Perkin Elmer Luminescence Spectrometer LS 50B using a Xenon lamp as a light source with the emission and excitation slit of 5 nm.
Atomic force microscopy (AFM, NanoScope III, Digital Instrument) equipped with an integrated silicon tip/cantilever with resonance frequency ~240 KHz in height and phase image models were utilized for the observation of morphologies. Polymer solutions (4 μL) were dropped on a mica substrate and dried at room temperature for morphological observation. The AFM topographies showed no evidence of tip-induced modification during successive scans.
Dynamic light scattering (DLS) measurements for micelle diameters were performed using a Malvern Nano-ZS instrument equipped with a 4mW He-Ne laser (633 nm) with an output at a scattering angle of 90°. The solution was passed through a 0.45 μm Nylon micro-filter (VWR, Batavia, IL, USA) to remove dust before the DLS measurements.
2.3. Preparation of micelles and incorporation of the 2PA red emitter R
Stock solution of micelle 1
PEG45-b-PCL23 (5.0 mg) and R (0.54 mg) were dissolved in THF (350 μL) and stirred for 30 minutes. The THF solutions were added slowly into 1.5 mL of a 10 mM HEPES MilliQ distilled water solution (pH : 7.2) under vigorous stirring. The micelles were transferred to a dialysis bag and dialyzed against 10 mM HEPES MilliQ water for 3 days with a water change of about every 8 hours. The solution was then filtered through a 200 nm micro-filter to eliminate excess non-incorporated 2PA dyes. The content of R in the micelles was determined from a standard curve for R using an absorbance spectrometer. Detailed procedures of the establishment for the standard curves were given in S-Figure 2 of Supporting Materials. The concentration of R in micelle 1 was determined to be 120 μM.
Stock solution of micelle 2
Although the preparation procedure is similar to micelle 1, the polymers used here are PEG45-b-PCL23 (1.2 mg) and P4 (3.8 mg, see Scheme 1 for its structure). The concentration of R in micelle 2 was determined to be 112 μM.
2.4. The dye (R) release from micelles
The profile of the release kinetics of the 2PA molecules was investigated at 37 °C by dialysis. Briefly, 3 mL of each micelle-based nanocarrier, loaded as previously described, was placed in a dialysis membrane. Dialysis was performed against 0.3 L of 10 mM HEPES solutions or DMEM. Samples (50 μL) were drawn at time intervals from the nanocarrier dispersion, further diluted with DMSO and then used to determine the dye concentration with a UV-Vis spectrophotometer. The release percentage was calculated based on the absorbance change.
2.5. Critical micelle concentration (CMC) determination
The CMC of the block copolymer in double distilled water was determined by a fluorescence probe technique using pyrene as a hydrophobic fluorescent probe.19 Pyrene dissolved in acetone (1.8 × 10−4 M) was added to 20 mL vials and the acetone was removed by evaporation. Block copolymer with concentrations of 1.0 × 10−5 to 1.0 mg/mL in water was added to the vials containing pyrene. The final concentration of pyrene in the block copolymer aqueous solution was fixed at 6.0 × 10−7 M. These solutions were stirred at room temperature for 24 hr before measurement. Excitation spectra of the pyrene molecules were measured by using a fluorescence spectrometer at room temperature under 390 nm light irradiation. The intensity ratio of I337 to I334 was analyzed as a function of block copolymer concentration.
2.6. Determination of quantum yields
Fluorescence quantum yield measurements were carried out on a Perkin-Elmer LS 50B luminescence spectrometer. The fluorescence quantum yields (η) of samples in solutions were recorded by using rohdamine B (η = 0.65 in ethanol) 20 excited at 510 nm and were calculated according to the following equation:21
where (ηr) and (ηs) are the fluorescence quantum yields of standards and the samples, respectively. Ar and As are the absorbance of the standards and the measured samples at the excitation wavelength, respectively. Ir and Is are the integrated emission intensities of standards and the samples, respectively. nr and ns are the refractive indices of the corresponding solvents of the solutions, respectively. The experimental error was ~ 10%.
2.7. Determination of fluorescence lifetimes
The fluorescence lifetimes were measured by time-correlated single-photon counting on PicoQuant FluoTime 200 avalanche photodiode (Berlin, Germany) using 470 nm light for excitation. Intensity decay curves obtained were fitted as a sum of exponential terms22
where Ai is a preexponential factor representing the functional contribution to the time-resolved decay of the component with a lifetime τi. The mean lifetime 〈τ〉 for biexponential decays of fluorescence were calculated from the decay times and exponential factors using the following equation:
2.8. Determination of two-photon cross-sections
Two-photon excitation spectra were measured using a two-photon induced fluorescence technique23 using a mode-locked Ti:Sapphire laser excitation source (Coherent; Mira 900; Bloomfield, Connecticut, USA). The laser provides a pulse of approximately 120 femtosecond pulse width at a pulse repetition frequency of 76 MHz in the wavelength range of 710 to 1000 nm. The pumping wavelengths were determined by a monochromator-CCD system. Fluorescein in pH 11 water, which has been well characterized in literature23,was used as a reference (r). The two-photon absorption cross section of a sample compound (s) can be calculated at each wavelength according to
where S is the detected two-photon induced fluorescence signal, η is the fluorescence quantum yield, C is the concentration of the chromophore, and φ is the overall fluorescence collection efficiency of the experimental apparatus. The 2PA chromophore concentrations of the aqueous solutions for the 2PA cross-section determinations were 5 × 10−6 M. The measurements were conducted in an intensity regime where the fluorescence signal showed a quadratic dependence on the intensity of the excitation beam. The uncertainty in the measured cross sections was ~ 15%.
2.9. Cell culture and imaging
Mouse macrophage RAW 264.7 cells (from the American Type Culture Collection (ATCC), Manassas, VA) were cultured in DMEM supplemented with 10% fetal bovine serum, 5% penicillin, 2 mM L-glutamine (Sigma), and incubated at 37 °C in a 5% CO2 atmosphere. The cells were seeded onto 96-well plates at 10,000 cells per well, and incubated for 1 day. The R loaded micelles made from PEG45-b-PCL23 and P4 were dissolved and diluted in DMEM growth medium to give final R concentrations of 2 – 12 μM and polymer concentrations of 0.05 - 0.3 mg/mL for cell staining. The media in the wells were replaced with 100 μL of the pre-prepared samples containing R loaded micelles. The plates were then returned to the incubator and maintained in 5% CO2 at 37 °C for 1 – 16 h. Upon removal of the micelles solutions, the cells were washed once with 100 μL of PBS solution. 10 μL of Hoechst 33342 with a concentration of 1 mM in 100 μL of fresh DMEM medium was then added into the wells and incubated for 30 minutes for nuclei staining. Live cells were imaged with a Zeiss LSM 510 confocal microscope (Thornwood, NY, USA). The blue emission of the Hoechst 33342 was generated by a 405 nm laser and the emission was collected from 420–480 nm. The red emission from R was excited at 488 nm and the emission was collected from 600–700 nm. Negligible background fluorescence of cells was detected under the setting used.
2.10. Cytotoxicity study
The assay was performed by an in vitro MTT based toxicology assay kit (Sigma). The cells incubated with R loaded micelles for 16 hours in the 96-well plate were washed with PBS buffer and then incubated in fresh DMEM medium (100 μL) and 10 μL of MTT solution (5 mg/mL) in 5% CO2 at 37°C for another 3 h. 60 μl of the culture medium was taken out and 50 μl of DMSO was added to each well to dissolve the internalized purple formazan crystals by gentle pipetting up and down. The absorbance was measured at a wavelength of 490 nm using SpectraMax 190 from Molecular Devices (Downingtown, PA, USA). Each experiment was conducted twice in triplicate. The result was expressed as a percentage of the absorbance of the blank control.
2.11. Preparation of the new block copolymer P4
2.11.1. Preparation of P1
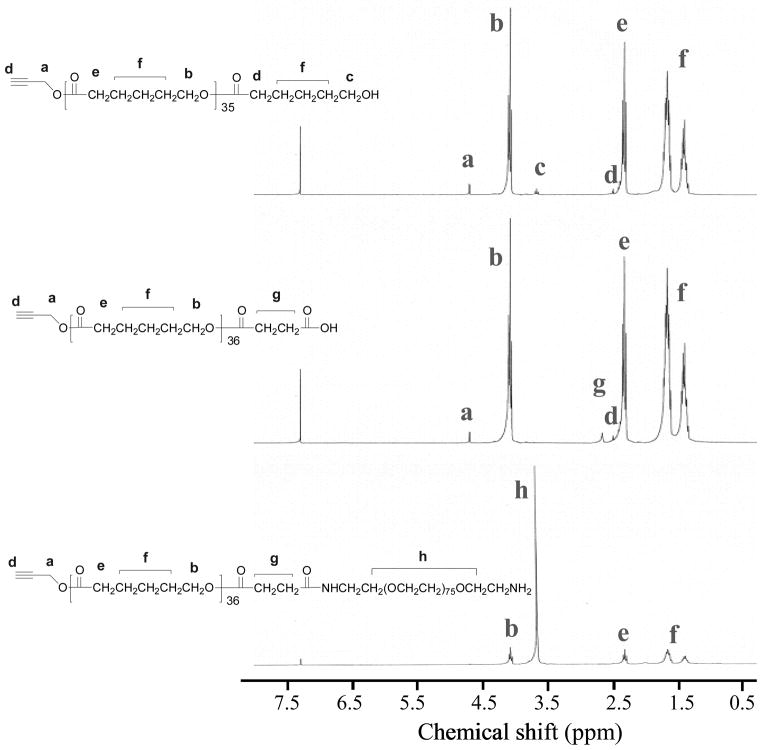
The dried propargyl alcohol (16.4 mg, 0.29 mmol) was weighed into a dry flask and ε-caprolactone (1.0 g, 8.76 mmol) was subsequently added. The reaction mixture was stirred for 5 min at 110 °C in a preheated oil bath before the catalyst (stannous octoate, 1 drop) was added. The polymerization was performed for 12 h. The resulting viscous solution was rapidly cooled, upon which it solidified. The crude polymer was dissolved in dichloromethane and precipitated in cold methanol. The isolated yield was 80%. Mn (GPC) = 6200, Mw/Mn = 1.30. 1H NMR (CDCl3): 4.68 (s, 2H), 4.07 (t, 72H), 2.33 (t, 72H), 1.61 (m. 144H), 1.42 (m, 72H). DP = 36. Mn (NMR) = 4800.
2.11.2. Preparation of P2
P1 (1.60 g, 0.33 mmol of the corresponding hydroxyl group), 100 mg of succinic anhydride (1 mmol), and 100 mg of DMAP (0.82 mmol) were dissolved in 10 mL of anhydrous methylene chloride and stirred at room temperature for 24 hours. The mixture was washed with water and then dried over Na2SO4. The polymer was finally precipitated from the methylene chloride into cold methanol, filtrated, and dried under vacuum oven to yield a powder of 1.5 g (94%). 1H NMR (CDCl3): 4.68 (s, 2H), 4.07 (t, 72H), 2.68 (m, 4H), 2.33 (t, 72H), 1.61 (m. 144H), 1.42 (m, 72H).
2.11.3. Preparation of P3
P2 (1.50 g, 0.30 mmol of the corresponding carboxylic acid), 60 mg of NHS (0.52 mmol), 10 mg of DMAP (0.082 mmol), and 110 mg of DCC (0.54 mmol) were dissolved in 10 mL of CH2Cl2 and the solution was stirred at room temperature for 5 hours. The precipitated DCU was filtrated. The filtrate was cooled to 0–5 °C and then an excess amount of α,ω-diamino-polyethylene glycol (2.7 g, 0.8 mmol) in 20 mL of methylene chloride was added drop by drop into the mixture and the reaction was kept at room temperature overnight. After the solvent was removed, the residue was suspended in 200 mL of water for 20 hours. The suspension was filtrated, re-dissolved into 100 mL of methylene chloride and washed with 100 mL of water twice. The organic layer was dried over Na2SO4. After the removal of organic solvent, the white powder was dissolved in CH2Cl2 and precipitated into ether. The yield was 79% (1.90 g). Further purification was carried out using dialysis. The powder (100 mg) was dissolved in 6 mL of THF and the solution was added to 25 mL of water slowly. The mixture was dialyzed against water in the regenerated cellulose membrane with a molecular weight cut-off of 10,000–12,000 for 4 days and then lyophilized. 1H NMR (CDCl3): 4.68 (s, 2H), 4.07 (t, 72H), 3.78 (m, 310H), 2.68 (m, 4H), 2.33 (t, 72H), 1.61 (m. 144H), 1.42 (m, 72H). Mn (GPC) = 13200, Mw/Mn = 1.29. Mn (NMR) = 8300.
2.11.4. Preparation of P4
P3 (100 mg) was dissolved into 10 mL of methylene chloride, and then 300 mg of CF3COOH was added. The mixture was stirred at room temperature for 30 minutes. After the solvent was removed the residue (100 mg) was used directly without further purification. Mn (GPC) = 13300, Mw/Mn = 1.29.
3. Results and Discussion
3.1. Synthesis of the new block copolymers P3 and P4
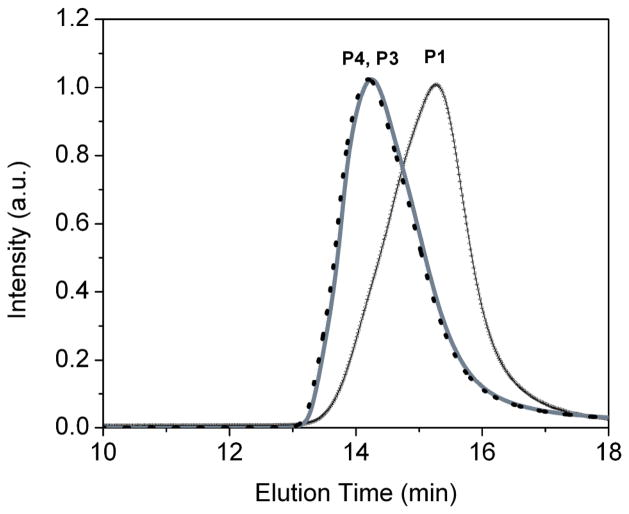
A new PEG-b-PCL type block copolymer (P3), having an active amino-group at the end of the polymer for further chemical modifications, was prepared as shown in Scheme 1. The final polymer and its intermediates were characterized using NMR (Figure 2) and GPC (Figure 3). It has been shown that the PEG-b-PCL type block copolymers have low CMC and can be biocompatible.13 Propargyl alcohol was chosen as the initiator for the polymerization of ε-caprolactone through the typical ring-opening polymerization24 to prepare P1. P1 was then reacted with succinic anhydride to afford a carboxylic acid-containing P2. The P2 was then reacted with a polyethylene oxide that has two amino end groups using DCC as the dehydration agent. In order to get the mono-amino-substituted polymer P3, a large excess of the diamino-PEG was used. The purified P3 shows a single-modal GPC curve (Figure 3), indicating its purity, which is further confirmed by 1H NMR. In order to enhance the delivery efficiency using the new PEG-b-PCL derivative, P3 was mixed with trifluoacetic acid (TFA) and dried to yield P4 with a cationic amino group. The use of polymers that contain cationic amino groups has been reported to effectively enhance the intracellular delivery of dyes and particles.25 The CMC of P4 was measured to be 0.37 uM (3.39 × 10−3 mg/mL), which is close to the CMC of the PEG45-b-PCL23 (0.28 uM, 1.20 × 10−3 mg/mL).13
Figure 2.
1H NMR spectra of P1, P2, and P3.
Figure 3.
GPC curves of P1, P3, and P4.
3.2. The micelle preparation and stability studies
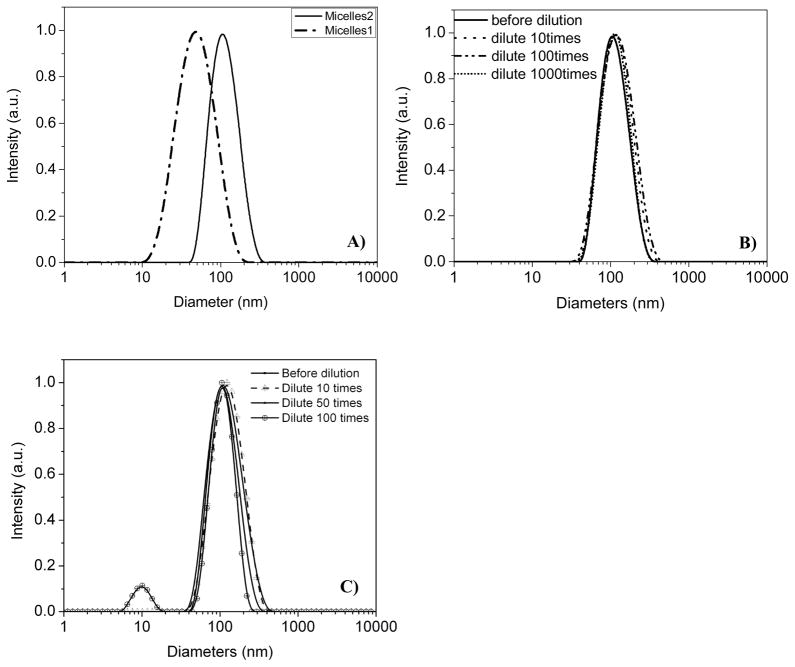
Although R is completely insoluble in water, it can be incorporated into the micelles formed from block copolymers (Scheme 2), which allows the hydrophobic compound to be used in an aqueous solution. For the PEG-b-PCL type block copolymers, the PCL segment acts as the hydrophobic core and the hydrophilic PEG segment functions as the shell. The core of the micelle (PCL) can function as a reservoir to accommodate the hydrophobic 2PA chromophore. The shell (PEG), having a brush-like protective corona, can ensure a complete dispersion of the micelles in water. Micelles were prepared using the dialysis approach in 10 mM HEPES buffer solutions and characterized using DLS. Depending on the polymers used, the micelles have average diameters of 60 and 110 nm (Figure 4A). The characterization using AFM and TEM for these micelles is unsatisfactory. The morphologies were quite unclean with large clusters probably due to significant amounts of HEPES present (data not shown).
Scheme 2.
Schematic drawing of the preparation of micelles. For micelle 1, all of the block copolymers were PEG45-b-PCL23. For micelle 2, the block copolymers used were PEG45-b-PCL23 (24 wt-%) and P4 (76 wt-%). Therefore, the corona surface of the micelle 2 contains the CF3COO−NH3+ moieties.
Figure 4.
The DLS profiles of Micelle 1 and Micelle 2 (A), and the dilutions of the micelle 2 with 10mM HEPES aqueous solution (B) and DMEM medium (C).
Stability of the micelles was studied while dissolved in HEPES buffer or DMEM medium. The stock solutions of the micelles (with a dye concentration of ~ 120 μM and a polymer concentration of ~ 3 mg/mL) were extremely stable in HEPES, even with a 1000x dilution with 10 mM HEPES (Figure 4B) solution. The micelles were less stable in DMEM medium than in HEPES solution, most likely because the medium has additional supplements such as sugars, salts, and phenol red. In DMEM, the micelles were stable enough to survive with 50x dilution. However, when the original micelles were diluted 100 times with DMEM medium, small particles in the range of ~10 nm were observed (Figure 4C), indicating some micelles had dissociated to generate unimers of P4, or some of the R molecules had released from micelles. Based on this observation, we could only dilute the original micelle stock solutions to 10 – 50 folds using DMEM medium for bioimaging.
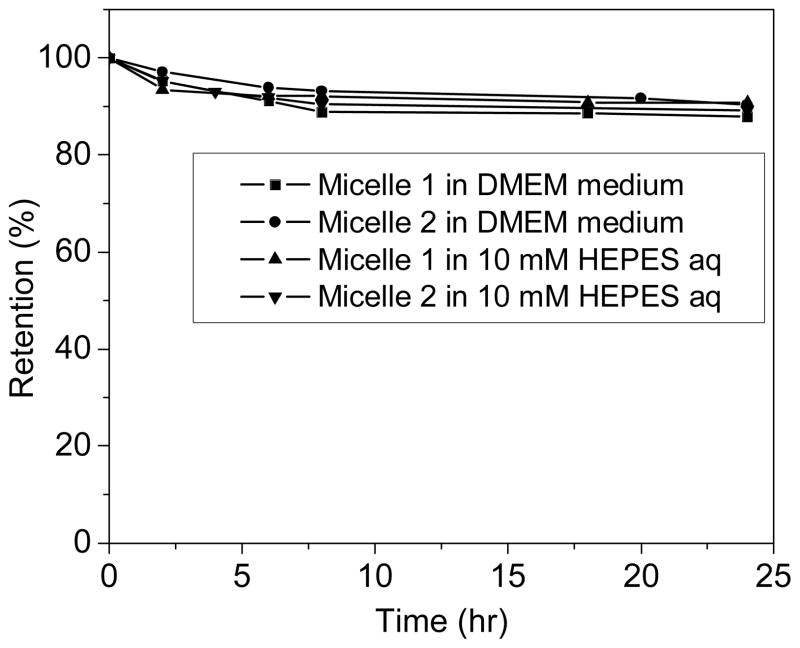
The release of dyes from micelles was further studied in HEPES buffer solution and DMEM medium26 at 37°C. It was found that the release of the dye from the micelles was slow (Figure 5). Less than 11% of the R was released from the micelles after 24 hours, indicating sufficient stability of the micelles under the biological environments.
Figure 5.
Release of R from the micelles.
3.3. Photophysical properties
The photophysical properties of the R were investigated in both organic solvents (toluene, THF, dichloromethane (DCM), and N,N′-dimethylformamide (DMF)) and micellar aqueous solutions. The data are summarized in Table 1.
Table 1.
Typical photophysical properties of R in organic solvents and in micelles.
| λmax abs [a] (nm) | λmax em [b] (nm) | η[c] | Lifetime (ns)[d]
|
δ (GM) | λmax 2PA [g] (nm) | ηδ at 820 nm (GM) | |||
|---|---|---|---|---|---|---|---|---|---|
| τ1 | τ2 | <τ> | |||||||
| In toluene | 524 | 628 | 0.44 | 4.08 | 930[e] | 820 | 409 | ||
| In THF | 524 | 658 | 0.27 | 3.77 | 520[e] | 830 | 143 | ||
| In DCM | 523 | 670 | 0.09 | 2.06 | 370[e] | 830 | 30 | ||
| In DMF | 527 | 674 | 0.01 | --- | --- | ---[h] | ---[h] | ||
| In micelle 1 | 534 | 672 | 0.07 | 1.70 (0.54) | 4.61 (0.46) | 3.70 | 310[f] | 22 | |
| In micelle 2 | 534 | 671 | 0.08 | 1.79 (0.63) | 4.36 (0.37) | 3.31 | 400[f] | 32 | |
absorbance maximum;
emission maximum;
quantum yield;
the numbers in the parenthesis of τ1 and τ2 are their corresponding A1 and A2;
the maximum of the 2PA cross-sections;
the 2PA cross-sections at 820 nm;
the wavelength of the maximum 2PA cross-sections;
too weak to measure.
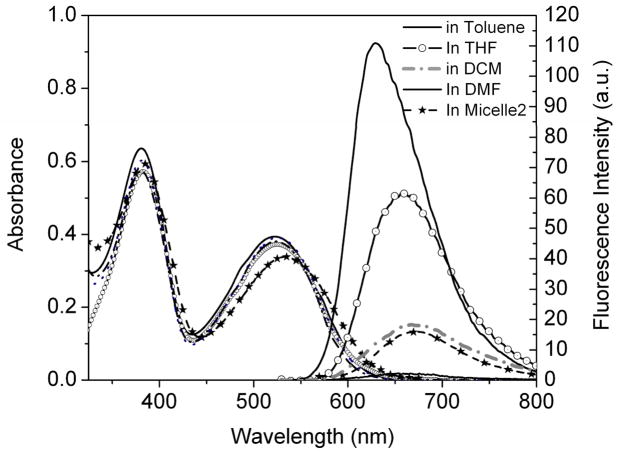
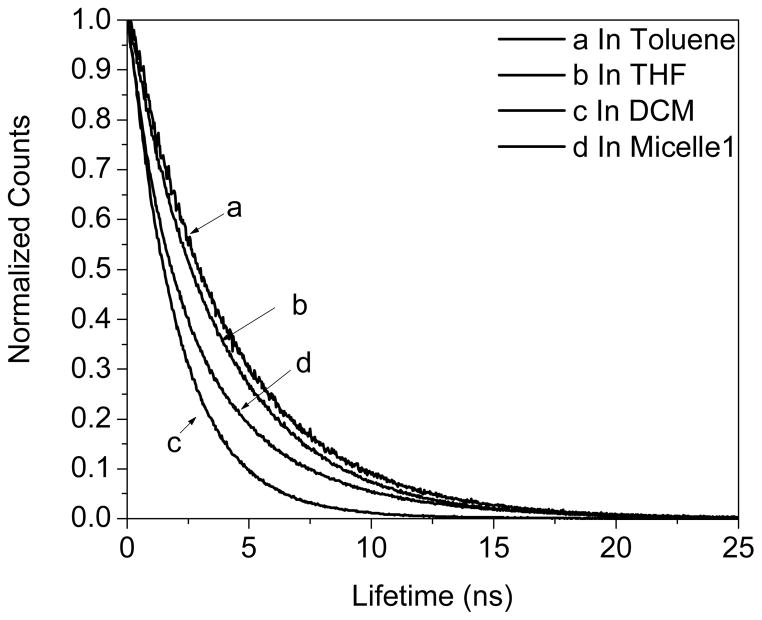
The structure of the red 2PA emitter (R) is based on a D-π-A-π-D motif as shown in Figure 1. The two amino groups act as the electron-donating units while the BTD functions as the electron-accepting moiety in the center. Almost no shift of its absorbance maximum in the above listed organic solvents was observed (Figure 6), indicating that the solvent polarity didn’t affect its electronic states. The absorption spectrum of the compound has two bands around 330–425 and 425–625 nm arising from π-π* and charge-transfer transitions, respectively.7a The material emits in the red spectral widow at the wavelength region of 550–800 nm, and the emission spectrum in a certain solvent is static regardless of excitation wavelengths. This independence of excitation wavelength might result from the spontaneous and efficient intramolecular charge transfer (ICT) between the donor and acceptor groups.7a, 27 The red emission exhibits significant solvatochromism contrary to the absorption process. With increasing solvent polarity, the fluorescence maxima displayed remarkable bathochromic shifts with significantly decreased fluorescence intensities (Figure 6 and Table 1). The quantum yield decreased from 0.44 in toluene to 0.01 in DMF. These results indicated that the excited state (assumed to be the first excited state S1) is far more polar than that of the ground state, showing the ICT occurred between the terminal amino group and the BTD ring. The results also indicated that the ICT excited state is much more sensitive to the polarity of its environment.7a, 28 Lifetimes were also consistent with the quantum yields, decreasing with the increased solvent dielectric constants (The dielectric constants of Toluene, THF, and DCM are 2.4, 7.52, and 9.08 respectively). Single exponential decays of lifetime were observed in toluene, THF, and DCM, corresponding to the lifetime of 4.08, 3.77, and 2.06 ns respectively (Figure 7).
Figure 6.
UV-Vis and fluorescence emission spectra of R in toluene, THF, DCM, and diluted micelle 2. For these measurements, the dye concentration was 5μM.
Figure 7.
Typical lifetime decays of R.
Clearly red-shifted absorbance of R in micelles was observed as compared to that of R in organic solvents. The emission maxima and quantum yields in micelles were quite close to those in DCM. The quantum yields of R in the two micelles were 0.07 and 0.08, respectively. The lifetime decays of R in micelles were biexponential with a fast decay of 1.70 ns and a slow decay of 4.61 ns for micelle 1, compared with 1.79 ns and 4.36 ns for micelle 2 (Table 1). The corresponding mean lifetimes of micelle 1 and micelle 2 were 3.7 and 3.3 ns, respectively. The different lifetime decays of the dye in micelles and in organic solvents indicated their different environments. The exhibition of a faster decay process in the micelles than in DCM indicated a more polar environment of the polyester segment (the PCL) than in DCM. The slow decay process suggested strong aggregations of the chromophores formed in the micelles because the aggregations may have delayed the relaxation process.9b Since the quantum yields and lifetimes of the two micelles were quite close, we may hypothesize that the microenvironments of the red emitters inside the micelle cores have no significant difference despite the fact that the variation of the sizes of the two micelles was large.
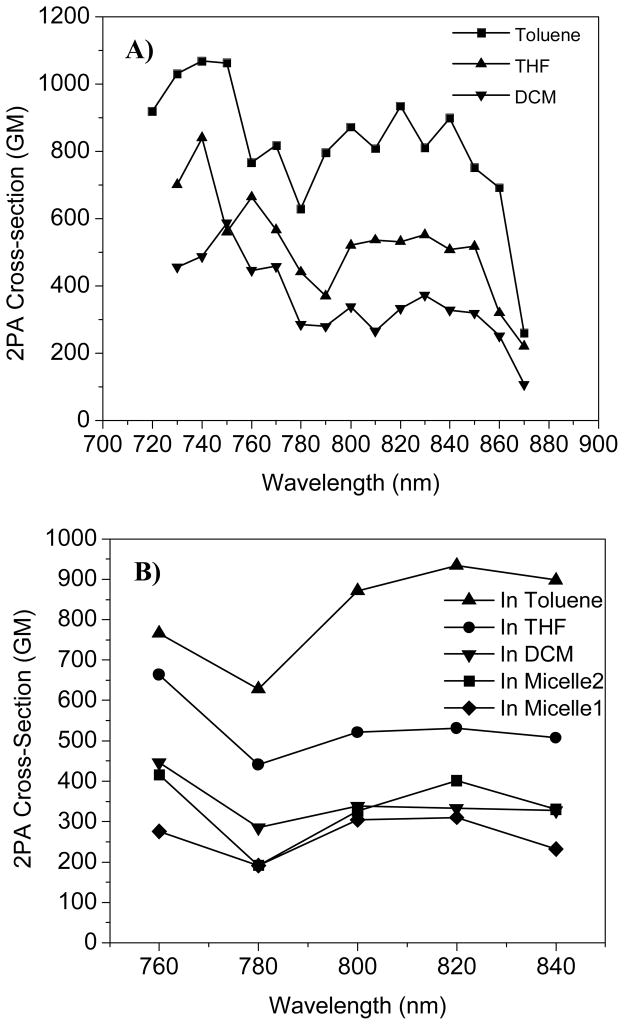
The 2PA cross-sections of R (Figure 8) were measured by using the two-photon-induced fluorescence method with fluorescein in 1N NaOH aqueous solution as the reference. The plots of the 2PA cross-sections against the excitation wavelength exhibited two maxima around 740 and 820 nm corresponding to the π-π* and ICT transitions observed in the 1PA spectra, which indicates the spectral similarity between the 1PA and 2PA. However, when the solvent polarity increased, 2PA cross-sections decreased. The solvent effect on 2PA cross-section is also a complicated process. Many factors, such as the solute-solvent interactions, polarity and viscosities of the solvents, changes in the chromophores geometry, and the possible multi-chromophore aggregations may all affect the ICT states,5a, 28 which influence the 2PA processes. The red emitter in the micelle shows smaller cross-section than those in toluene and THF, but quite close to that in DCM and much higher than that in DMF. Although there were limitations to get the cross-sections as high as that in the less polar organic solvents, such as toluene and THF, using these two micelles, this approach provides a feasible way of using hydrophobic 2PA chromophores in a biological setting. In addition, the red emitter in the micellar aqueous solutions exhibited a reasonably large 2PA cross-section of 400 GM at 820 nm, which was among the highest reported values of water-soluble red 2PA emitters (For example, the commonly used rhodamine B has a maximum 2PA cross-sections of ~ 210 GM23b).
Figure 8.
2PA cross-sections of R in organic solvents (A) and in micelles (B).
3.4. Imaging and cytotoxicity
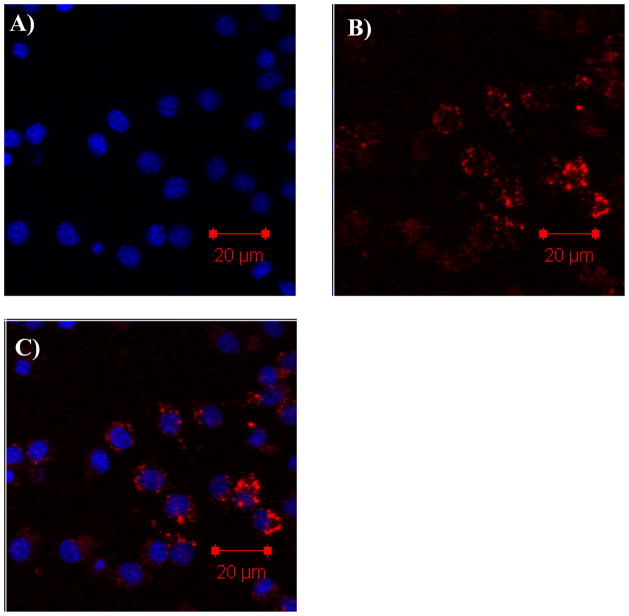
The cellular uptake of micelles containing R by mouse macrophage RAW 264.7 cells was studied by using confocal laser scanning microscopy. The RAW 264.7 cells were incubated with the micelle 1 and micelle 2-containing DMEM medium to study the effect of peripheral groups of the micelles (e.g. the shells of the micelles) on the uptake. It was found that micelle 1 with the neutral PEG peripheries did not stain the cells (data not shown) even after 24 hours incubation at a high dye concentration of 12 μM. Thus, the R incorporated in the micelles was cell impermeable or the neutral PEG segment didn’t have sufficient internalization with the RAW 264.7 cell membranes to deliver the R. However, the cells could be successfully stained by micelle 2 with the cationic corona (Figure 9). The fluorescence intensities inside the cells became strong and strong with the increase of the uptake time or concentrations (see S-Figure 3 in supporting materials). The different staining results between the two micelles were most likely due to the difference in the micelle surfaces. It has been shown that the cationic amino group can enhance the non-specific endocytosis process.25 Based on the difference of the internalization results, it is hypothesized that the whole polymeric micelle 2 were taken up by the cells. It should be noted that R by itself could not be dissolved in water, and this result has demonstrated the potential of using this approach to deliver hydrophobic agents into live cells.
Figure 9.
The confocal images of RAW 264.7 cells incubated with R in micelle 2 (dye concentration is 4 μM and polymer concentration is 0.1 mg/mL) showing the particulate distribution and localization of the R after 16 h internalization. Blue emission represents the Hoechst 33342 (A). Red emission represents the R (B). The superimpose of the blue and red fluorescence is given in C.
In order to understand the subcellular locations of the red emitter incorporated in the micelles, a nuclei staining dye of Hoechst 33342 was used following the cell uptake of the micelles. We observed only very minor colocalization of the red emitter R with nuclear-selective blue emitter Hoechst 33342, which indicated cytoplasmic localization of the red emitter.
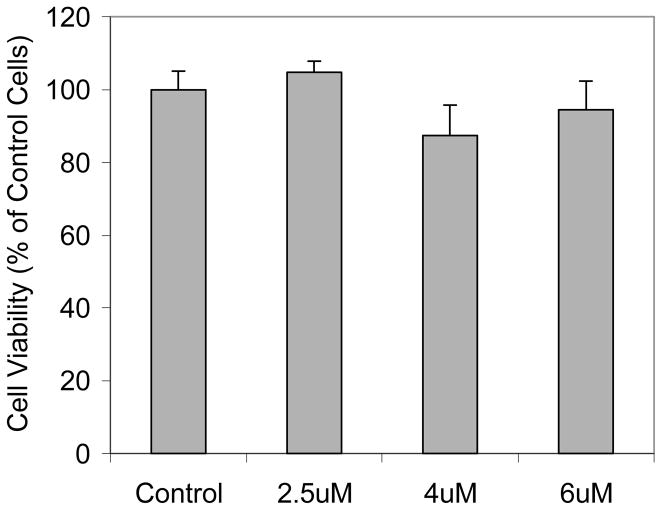
Cytotoxicity to the RAW 264.7 cells was investigated using the MTT assay. Greater than 90% of the cells were viable after the cells were stained for 16 hours using the dye concentrations of 2 – 6 μM. These observations demonstrated the biocompatibility of the dye-incorporated micelles using the new synthesized P4.
4. Conclusion
We have developed a new PEG-b-PCL type block copolymer with a cationic amino ending group for enhancing the non-specific endocytosis process. Using this new polymer, a completely water-insoluble 2PA red emitter was incorporated into micelles and used in cell culture medium to successfully stain the cytoplasmic regions of the mouse macrophage RAW 264.7 cells. We have also demonstrated the biocompatibility of the dye-incorporating micelles with RAW 264.7 cells by showing greater than 90% cell viability under various dye and polymer concentrations. Moreover, the influence of the solvent polarity and micelle environments on the absorption, emission, quantum yield, and lifetime of the red emitter R has been systematically studied. Further investigation using these micelles for in-vivo and 3D imaging under the two-photon microscopy is in progress.
Supplementary Material
Figure 10.
Cell viability [3-(4,5-dimethylthiazo-2-yl)-2,5-diehenyl tetrazolium bromide (MTT) assay] of RAW 264.7 cells incubated in the presence of R incorporated with micelle 2 in DMEM medium for 16 hours. The concentrations of R are given in the X-axis. The 2.5, 4 and 6 uM of the R in micelles correspond to polymer concentrations of 0.0625, 0.1, and 0.15 mg/mL, respectively.
Acknowledgments
Support from the Microscale Life Sciences Center (MLSC)-A Center of Excellence in Genome Sciences funded by NIH is acknowledged. Alex K.-Y. Jen thanks the Boeing-Johnson Foundation for its support.
References
- 1.see a recent review about 2PA materials and their applications He G, Tan LS, Zheng Q, Prasad PN. Multiphoton Absorbing Materials: Molecular Designs, Characterizations, and Applications. Chemical Reviews. 2008;108:1245–330. doi: 10.1021/cr050054x.
- 2.(a) Denk W, Strickler JH, Web WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–6. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]; (b) Cahalan MD, Parker I, Wei SH, Miller MJ. Two-photon tissue imaging: seeing the immune system in a fresh light. Nature Rev Immun. 2002;2:872–80. doi: 10.1038/nri935. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rubart M. Two-Photon Microscopy of Cells and Tissue. Circ Res. 2004;95:1154–66. doi: 10.1161/01.RES.0000150593.30324.42. [DOI] [PubMed] [Google Scholar]; (d) Sánchez SA, Gratton E. Lipid-protein interactions revealed by two-photon microscopy and fluorescence correlation spectroscopy. Acc Chem Res. 2005;38:469–77. doi: 10.1021/ar040026l. [DOI] [PubMed] [Google Scholar]
- 3.(a) So PTC, Dong CY, Masters BR, Berland KM. Two-photon excitation fluorescence microscopy. Annu Rev Biomed Eng. 2000;2:399–429. doi: 10.1146/annurev.bioeng.2.1.399. [DOI] [PubMed] [Google Scholar]; (b) Xu C, Webb WW. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. J Opt Soc Am B. 1996;13:481–91. [Google Scholar]; (c) Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nature Biotechnology. 2003;21:1369–77. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
- 4.(a) Albota M, Beljonne D, Brédas JL, Ehrlich JE, Fu JY, Heikal AA, Hess SE, Kogej T, Levin MD, Marder SR, McCord-Maughon D, Perry JW, Röckel H, Rumi M, Subramaniam G, Webb WW, Wu XL, Xu C. Design of organic molecules with large two-photon absorption cross sections. Science. 1998;281:1653–6. doi: 10.1126/science.281.5383.1653. [DOI] [PubMed] [Google Scholar]; (b) Rumi M, Ehrlich JE, Heikal AA, Perry JW, Barlow S, Hu ZY, McCord-Maughton D, Parker TC, Röckel H, Thayumanavan S, Marder SR, Beljonne D, Brédas JL. Structure-Property Relationships for Two-Photon Absorbing Chromophores: Bis-Donor Diphenylpolyene and Bis(styryl)benzene Derivatives. J Am Chem Soc. 2000;122:9500–10. [Google Scholar]; (c) Ventelon L, Charier S, Moreaux L, Mertz J, Blanchard-Desce M. Nanoscale push-push dihydrophenanthrene derivatives as novel fluorophores for two-photon-excited fluorescence. Angew Chem Int Ed. 2001;40:2098–101. doi: 10.1002/1521-3773(20010601)40:11<2098::AID-ANIE2098>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]; (d) Yoo J, Yang SK, Jeong MY, Ahn HC, Jeon SJ, Cho BR. Bis-1,4-(p-diarylaminostyryl)-2,5-dicyanobenzene Derivatives with Large Two-Photon Absorption Cross-Sections. Org Lett. 2003;5:645–8. doi: 10.1021/ol027343h. [DOI] [PubMed] [Google Scholar]
- 5.(a) Woo HY, Liu B, Kohler B, Korystov D, Mikhailovsky A, Bazan GC. Solvent effects on the two-photon absorption of distyrylbenzene chromophores. J Am Chem Soc. 2005;127:14721–9. doi: 10.1021/ja052906g. [DOI] [PubMed] [Google Scholar]; (b) Woo HY, Hong JW, Liu B, Mikhailovsky A, Korystov D, Bazan GC. Water-soluble [2.2]paracyclophane chromophores with large two-photon action cross sections. J Am Chem Soc. 2005;127:820–1. doi: 10.1021/ja0440811. [DOI] [PubMed] [Google Scholar]; (c) Woo HY, Korystov D, Mikhailovsky A, Nguyen TQ, Bazan GC. Two-Photon Absorption in Aqueous Micellar Solutions. J Am Chem Soc. 2005;127:13794–5. doi: 10.1021/ja054911q. [DOI] [PubMed] [Google Scholar]
- 6.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nature Medicine. 2003;9:123–8. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 7.(a) Kato SI, Matsumoto T, Shigeiwa M, Gorohmaru H, Maeda S, Ishii T, Mataka S. Novel 2,1,3-benzothiadiazole-based red-fluorescent dyes with enhanced two-photon absorption cross-sections. Chem Eur J. 2006;12:2303–17. doi: 10.1002/chem.200500921. [DOI] [PubMed] [Google Scholar]; (b) Thomas KRJ, Lin JT, Velusamy M, Tao Y, Chuen C. Color tuning in benzo[1,2,5]thiadiazole-based small molecules by amino conjugation/deconjugation: Bright red-light-emitting diodes. Adv Funct Mater. 2004;14:83–90. [Google Scholar]; (c) Kato S, Matsumoto T, Ishii T, Thiemann T, Shigeiwa M, Gorohmaru H, Maeda S, Yamashita Y, Mataka S. Strongly red-fluorescent novel donor-pi-bridge-acceptor-pi-bridge-donor (D-pi-A-pi-D) type 2,1,3-benzothiadiazoles with enhanced two-photon absorption cross-sections. Chem Commun. 2004:2342–3. doi: 10.1039/b410016f. [DOI] [PubMed] [Google Scholar]; (d) Brousmiche DW, Serin JM, Fréchet JMJ, He GS, Lin TC, Chung SJ, Prasad PN, Kannan R, Tan LS. Fluorescence Resonance Energy Transfer in Novel Multiphoton Absorbing Dendritic Structures. J Phys Chem B. 2004;108:8592–600. [Google Scholar]; (e) Margineanu A, Hofkens J, Cotlet M, Habuchi S, Stefan A, Qu J, Kohl C, Muellen K, Vercammen J, Engelborghs Y, Gensch T, De Schryver FC. Photophysics of a Water-Soluble Rylene Dye: Comparison with Other Fluorescent Molecules for Biological Applications. J Phys Chem B. 2004;108:12242–51. [Google Scholar]; (f) Fu LM, Wen XF, Ai XC, Sun Y, Wu YS, Zhang JP, Wang Y. Efficient two-photon-sensitized luminescence of a europium(III) complex. Angew Chem Int Ed. 2005;44:747–50. doi: 10.1002/anie.200462382. [DOI] [PubMed] [Google Scholar]
- 8.For selected reviews, see: Adronov A, Fréchet JMJ. Light-harvesting dendrimers. Chem Commun. 2000:1701–10.Barigelletti F, Flamigni L. Photoactive molecular wires based on metal complexes. Chem Soc Rev. 2000;29:1–12.Choi MS, Yamazaki T, Yamazaki I, Aida T. Bioinspired molecular design of light-harvesting multiporphyrin arrays. Angew Chem Int Ed. 2004;43:150–8. doi: 10.1002/anie.200301665.
- 9.Bertazza L, Celotti L, Fabbrini G, Loi MA, Maggini M, Mancin F, Marcuz S, Menna E, Muccini M, Tonellato U. Cell penetrating silica nanoparticles doped with two-photon absorbing fluorophores. Tetrahedron. 2006;62:10434–40.Lebret V, Raehm L, Durand JO, Smaïhi M, Gérardin C, Nerambourg N, Werts MHV, Blanchard-Desce M. Synthesis and Characterization of Fluorescently Doped Mesoporous Nanoparticles for Two-Photon Excitation. Chem Mater. 2008;20:2174–83.Surface functionalization of two-photon dye-doped mesoporous silica nanoparticles with folic acid: cytotoxicity studies with HeLa and MCF-7 cancer cells. Lebret V, Raehm L, Durand JO, Smaihi M, Werts MHV, Blanchard-Desce M, Méthy-Gonnod D, Dubernet C. J Sol-Gel Sci Technol. doi: 10.1007/s10971-008-1724-1.Law WC, Yong KT, Roy I, Xu G, Ding H, Bergey EJ, Zeng H, Prasad PN. Optically and Magnetically Doped Organically Modified Silica Nanoparticles as Efficient Magnetically Guided Biomarkers for Two-Photon Imaging of Live Cancer Cells. J Phys Chem C. 2008;112:7972–7.Kim S, Pudavar HE, Bonoiu A, Prasad PN. Aggregation-enhanced fluorescence in organically modified silica nanoparticles: a novel approach toward high-signal-output nanoprobes for two-photon fluorescence bioimaging. Adv Mater. 2007;19:3791–5.Kim S, Pudavar HE, Prasad PN. Dye-concentrated organically modified silica nanoparticles as a ratiometric fluorescent pH probe by one- and two-photon excitation. Chem Comm. 2006:2071–3. doi: 10.1039/b600926c.Kim S, Ohulchanskyy TY, Pudavar HE, Pandey RK, Prasad PN. Organically Modified Silica Nanoparticles Co-encapsulating Photosensitizing Drug and Aggregation-Enhanced Two-Photon Absorbing Fluorescent Dye Aggregates for Two-Photon Photodynamic Therapy S. J Am Chem Soc. 2007;129:2669–75. doi: 10.1021/ja0680257.
- 10.Hadjichristidis N, Pispas S, Floudas GA. Block Copolymers-Synthetic Strategies, Physical Properties, and Applications. NJ: Wiley-Interscience: Hoboken; 2002. [Google Scholar]
- 11.(a) Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Delivery Rev. 2001;47:113–31. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]; (b) Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Controlled Release. 2001;73:137–72. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]; (c) van Nostrum CF. Polymeric micelles to deliver photosensitizers for photodynamic therapy. Adv Drug Delivery Rev. 2004;56:9–16. doi: 10.1016/j.addr.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 12.(a) Jain TK, Morales MA, Sahoo SK, Leslie-Pelecky DL, Labhasetwar V. Iron Oxide Nanoparticles for Sustained Delivery of Anticancer Agents. Mol Pharmaceutics. 2005;2:194–205. doi: 10.1021/mp0500014. [DOI] [PubMed] [Google Scholar]; (b) Kim BS, Qiu JM, Wang JP, Taton TA. Magnetomicelles: Composite Nanostructures from Magnetic Nanoparticles and Cross-Linked Amphiphilic Block Copolymers. Nano Lett. 2005;5:1987–91. doi: 10.1021/nl0513939. [DOI] [PubMed] [Google Scholar]
- 13.Gao XH, Yang L, Petros JA, Marshall FF, Simons JW, Nie S. In vivo molecular and cellular imaging with quantum dots. Current Opinion in Biotechnology. 2005;16:63–72. doi: 10.1016/j.copbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Savic R, Luo L, Eisenberg A, Maysinger D. Micellar Nanocontainers Distribute to Defined Cytoplasmic Organelles. Science. 2003;300:615–18. doi: 10.1126/science.1078192. [DOI] [PubMed] [Google Scholar]
- 15.Shin Junhwa, Thompson David H. Receptor-mediated endocytosis. ACS Symposium; 2004. p. 879. [Google Scholar]
- 16.(a) Tian YQ, Chen CY, Cheng YJ, Young AC, Tucker NM, Jen AKY. Hydrophobic chromophores in aqueous micellar solution showing large two-photon absorption cross sections. Adv Funct Mater. 2007;17:1691–7. [Google Scholar]; (b) Chen CY, Tian YQ, Cheng YJ, Young AC, Ka JW, Jen AKY. Two-Photon Absorbing Block Copolymer as a Nanocarrier for Porphyrin: Energy Transfer and Singlet Oxygen Generation in Micellar Aqueous Solution. J Am Chem Soc. 2007;129:7220–1. doi: 10.1021/ja071057p. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Q, Ohulchanskyy TY, Sahoo Y, Prasad PN. Water-Dispersible Polymeric Structure Co-encapsulating a Novel Hexa-peri-hexabenzocoronene Core Containing Chromophore with Enhanced Two-Photon Absorption and Magnetic Nanoparticles for Magnetically Guided Two-Photon Cellular Imaging. J Phys Chem C. 2007;111:16846–51. [Google Scholar]
- 18.Liu M, Xu W, Xu LJ, Zhong GR, Chen SL, Lu WY. Synthesis and Biological Evaluation of Diethylenetriamine Pentaacetic acid-Polyethylene Glycol-Folate: A New Folate-Derived, (99m)Tc-Based Radiopharmaceutical. Bioconjugate Chem. 2005;16:1126–32. doi: 10.1021/bc050122m. [DOI] [PubMed] [Google Scholar]
- 19.(a) Inoue T, Chen G, Nakamae K, Hoffman AS. An AB block copolymer of oligo(methyl methacrylate) and poly(acrylic acid) for micellar delivery of hydrophobic drugs. J Control Release. 1998;51:221–9. doi: 10.1016/s0168-3659(97)00172-7. [DOI] [PubMed] [Google Scholar]; (b) Lo C, Huang C, Lin K, Hsie GH. Mixed micelles formed from graft and diblock copolymers for application in intracellular drug delivery. Biomaterials. 2007;28:1225–35. doi: 10.1016/j.biomaterials.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 20.Kubin RF, Fletcher ANJ. Fluorescence quantum yields of some rhodamine dyes. J Luminescence. 1982;27:455–462. [Google Scholar]
- 21.Joshi HS, Jamshidi R, Tor Y. Conjugated 1,10-phenanthrolines as tunable fluorophores. Angew Chem Int Ed. 1999;38:2722–5. doi: 10.1002/(sici)1521-3773(19990917)38:18<2721::aid-anie2721>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Joseph R, Lakowicz . Principles of Fluorescence Spectroscopy. Plenum Press; New York: 1983. [Google Scholar]
- 23.(a) So PTC, Dong CY, Masters BR, Berland KM. Two-photon excitation fluorescence microscopy. Annu Rev Biomed Eng. 2000;2:399–429. doi: 10.1146/annurev.bioeng.2.1.399. [DOI] [PubMed] [Google Scholar]; (b) Xu C, Webb WWJ. Opt Soc Am B. 1996;13:481. [Google Scholar]
- 24.(a) He XH, Liang L, Xie M, Zhang Y, Lin S, Yan D. Synthesis of Novel Linear PEO-b-PS-b-PCL Triblock Copolymers by the Combination of ATRP, ROP, and a Click Reaction. Macromol Chem Phys. 2007;208:1797–802. [Google Scholar]; (b) Meier MAR, Aerts SNH, Staal BBP, Pasa M, Schubert US. PEO-b-PCL Block Copolymers: Synthesis, Detailed Characterization, and Selected Micellar Drug Encapsulation Behavior. Macromol Rapid Commun. 2005;26:1918–24. [Google Scholar]
- 25.(a) Song HT, Choi JS, Huh YM, Kim S, Jun YW, Suh JS, Cheon J. Surface Modulation of Magnetic Nanocrystals in the Development of Highly Efficient Magnetic Resonance Probes for Intracellular Labeling. J Am Chem Soc. 2005;127:9992–3. doi: 10.1021/ja051833y. [DOI] [PubMed] [Google Scholar]; (b) Lee J, Kim J, Park E, Jo S, Song R. PEG-ylated cationic CdSe/ZnS QDs as an efficient intracellular labeling agent. Phys Chem Chem Phys. 2008;10:1739–42. doi: 10.1039/b801317a. [DOI] [PubMed] [Google Scholar]
- 26.The medium doesn’t contain phenol red because of the significant absorption of the phenol red at 365 and 530 nm region, which has a strong interference on the red emitter’s absorbance.
- 27.Jenekhe SA, Lu L, Alam MM. New Conjugated Polymers with Donor-Acceptor Architectures: Synthesis and Photophysics of Carbazole-Quinoline and Phenothiazine-Quinoline Copolymers and Oligomers Exhibiting Large Intramolecular Charge Transfer. Macromolecules. 2001;34:7315–24. [Google Scholar]
- 28.Hong JW, Woo HY, Liu B, Bazan GC. Solvatochromism of Distyrylbenzene Pairs Bound Together by [2.2]Paracyclophane: Evidence for a Polarizable “Through-Space” Delocalized State. J Am Chem Soc. 2005;127:7435–43. doi: 10.1021/ja044326+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.