Abstract
Strategically placed covalent linkages have been shown to stabilize helical conformations in short peptide sequences. Here we report the synthesis of a stabilized α-helix that utilizes an internal disulfide linkage. Structural analysis indicates that the dynamic nature of the disulfide bridge allows for the reversible formation of an α-helix through oxidation and reduction reactions.
Keywords: Foldamer, Stabilized α-helix, Helix inducer, Hydrogen bond surrogate, Disulfide bridge
1. Introduction
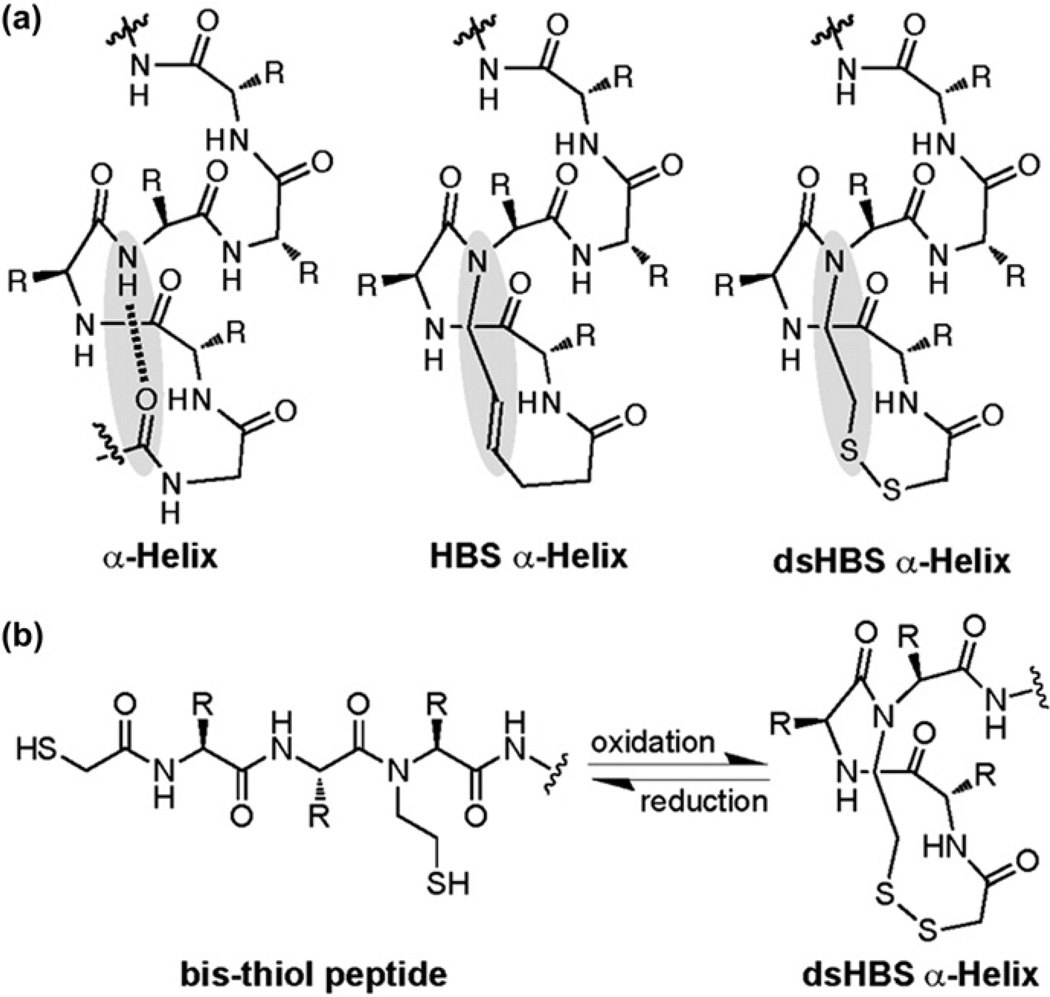
Methods that provide reversible control over protein secondary structure formation have proven to be useful for probing protein structure and interactions.1–4 In this report, we describe the design of a helix stabilized by a reversible covalent linkage. The strategy builds on our reported efforts to prepare internally-constrained α-helix mimetics, termed hydrogen bond surrogate (HBS) helices.5, 6 HBS helices contain a covalent bond in place of an intrastrand hydrogen bond between main chain atoms in canonical α-helices (Fig. 1a). We have previously demonstrated that alkene, alkane, and thioether linkages can serve to nucleate and stabilize the desired conformation in short peptides.7–9 Herein we show that replacement of the main chain hydrogen bond with a disulfide group provides facile access to reversibly stabilized α-helices (Fig. 1a).1,10,11 The disulfide linkage enforces slightly different dihedral requirements than the hydrocarbon bridges;12 however, our biophysical studies suggest that conformational stability of dsHBS helix is similar to those of HBS helices. dsHBS helices are readily formed by oxidizing bis-thiol peptides in which the two sulfhydryl groups formally occupy the i and i+4 positions (Fig.1b).
Fig. 1.
(a) Hydrogen bond surrogate (HBS) α-helices feature a covalent bond in place of the intramolecular hydrogen bond between the i and i+4 residues. (b) In dsHBS α-helices the main chain hydrogen bond is replaced with a disulfide linkage, and can be reversibly formed from a bis-thiol peptide.
2. Results and discussion
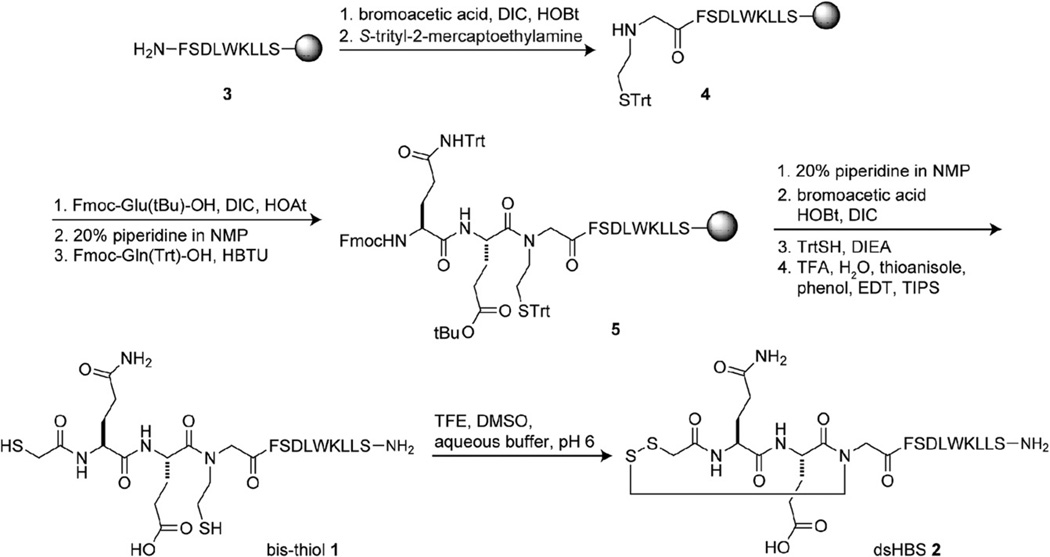
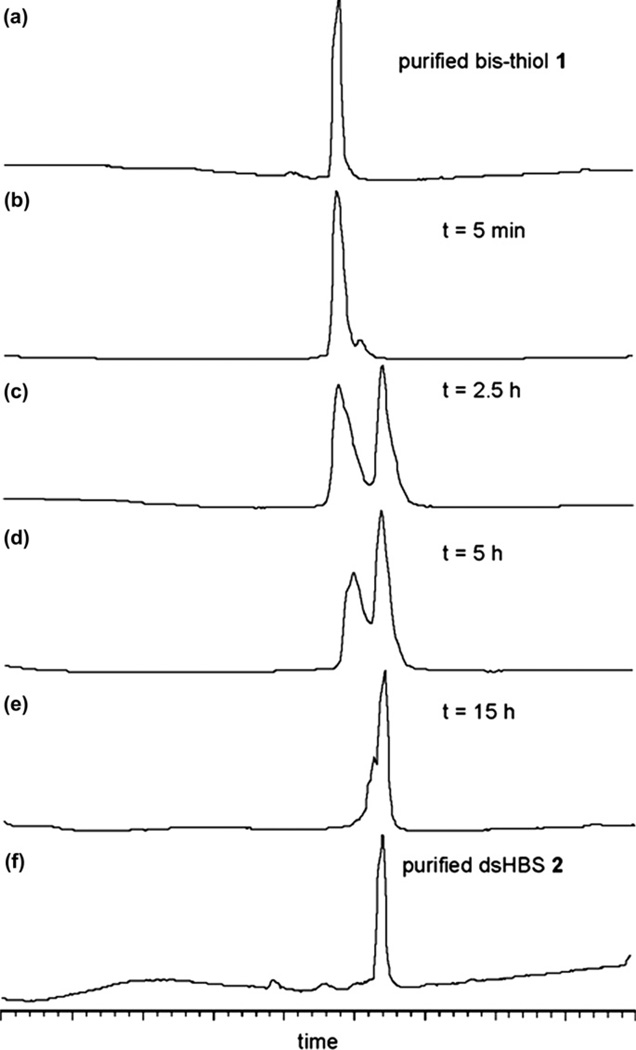
For these preliminary studies we designed a short helix based on the p53 activation domain.13,14 The bis-thiol p53 peptide 1 was synthesized on solid phase as shown in Scheme 1. Peptide 3 was prepared using Fmoc chemistry on Rink amide resin.15 Coupling of 3 with bromoacetic acid followed by treatment with S-trityl-2-mercaptoethylamine provided 4. Sequential coupling of Fmoc-Glu(OtBu)–OH, Fmoc-Gln(Trt)–OH, and bromoacetic acid, treatment with triphenylmethyl mercaptan followed by cleavage from resin afforded bis-thiol peptide 1. We evaluated the potential of iodine10 and DMSO16 to oxidize HPLC-purified 1 to dsHBS 2 and found DMSO in 10% TFE/ammonium bicarbonate buffer (pH 6) to provide efficient conversion to the desired product (Fig. 2).
Scheme 1.
Solid phase synthesis of bis-thiol 1 and dsHBS α-helix 2.
Fig. 2.
DMSO-mediated conversion of bis-thiol 1 to dsHBS 2.
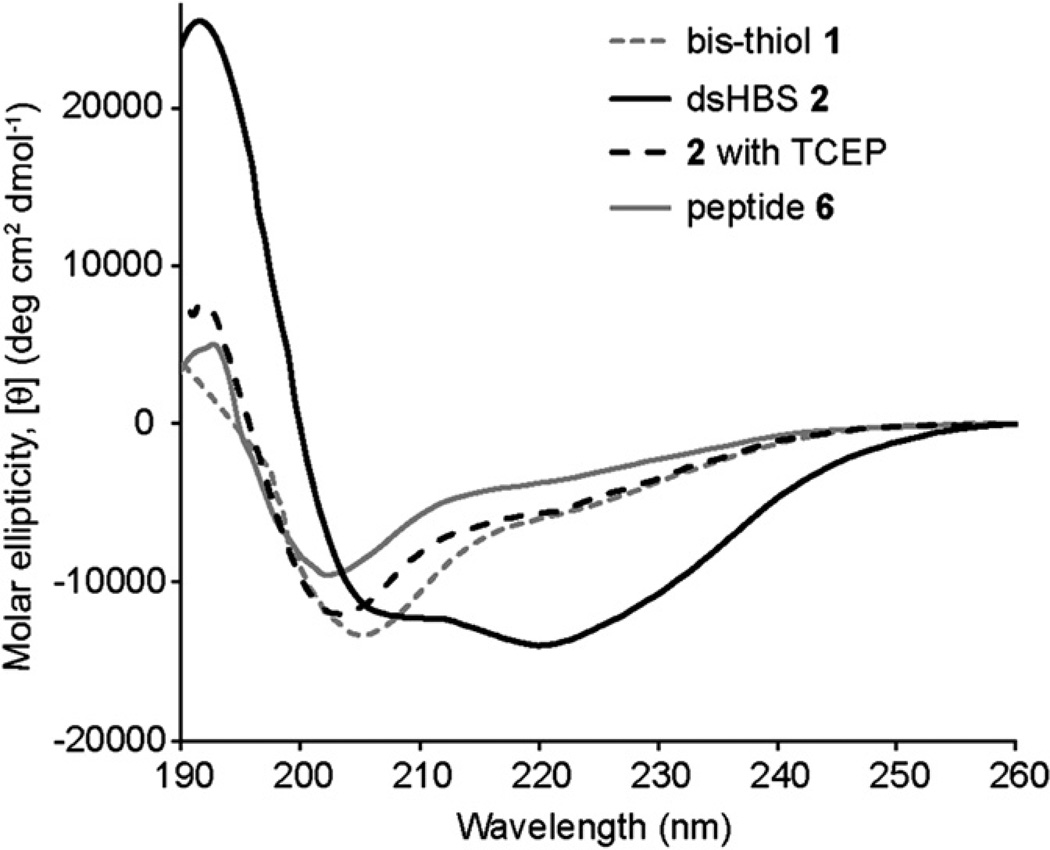
The conformation of the peptides was examined by circular dichroism and 2D NMR spectroscopies. CD studies were performed in dilute phosphate buffered saline to obtain a measure of their helical content (Fig. 3). CD spectroscopy suggests that, as expected, bis-thiol 1 is weakly helical in aqueous solutions while dsHBS 2 provides a CD signature typical of a canonical α-helix, with a double minima near 208 and 222 nm and a maximum at 190 nm.17–21 Upon treatment with 1.5 equiv of tris(2-carboxyethyl)phosphine (TCEP), a reducing agent, dsHBS 2 loses its conformational preference. The CD traces of the bis-thiol peptides are similar to that of linear p53 peptide analog (6: Ac-QEGFSDLWKLLS-NH2).
Fig. 3.
Circular dichroism spectra of peptides 1, 2, and 6. The CD spectra were obtained in phosphate buffered saline. TCEP=tris(2-carboxyethyl)phosphine. Calculated % helicity values: 1: 22%, 2: 55%, 2+TCEP: 22%, and 6: 14%.
We performed 2D NMR experiments to further establish the conformation of 2. NMR studies were performed in 20% TFE-d3 in PBS (pH 3.5) on a Bruker 500 MHz spectrometer. The organic co-solvent was needed to solubilize the compound at the low millimolar concentrations needed for NMR studies. Tri-fluoroethanol, along with other organic co-solvents, is known to enhance helicity of peptides.22–24 The %helicity of 2 increases by 1.5-fold in the presence of 20% TFE as compared to the pure aqueous solution according to CD spectroscopy (Supplementary data). The shapes of the curves, as well as the 208/222 nm ratio used to evaluate α-helicity remain constant in the two solvents.17,18 Thus, CD spectroscopy suggests that a different helical conformation is not being stabilized by the addition of the cosolvent.
A set of 2D TOCSY and NOESY spectra was used to assign 1H NMR resonances for 2. Sequential NH–NH (i and i +1) NOESY cross-peaks, a signature of helical structure, were observed for 2 as depicted in Fig. 4. The NOESY spectrum further reveals medium to weak (i, i+3) and (i, i+4) CHα–NH cross peaks that support an α-helical conformation in 2, although spectral overlap prevented assignment of some key cross-peaks. The NMR spectra, peak assignments, and the NOESY correlation chart are included in the Supplementary data.
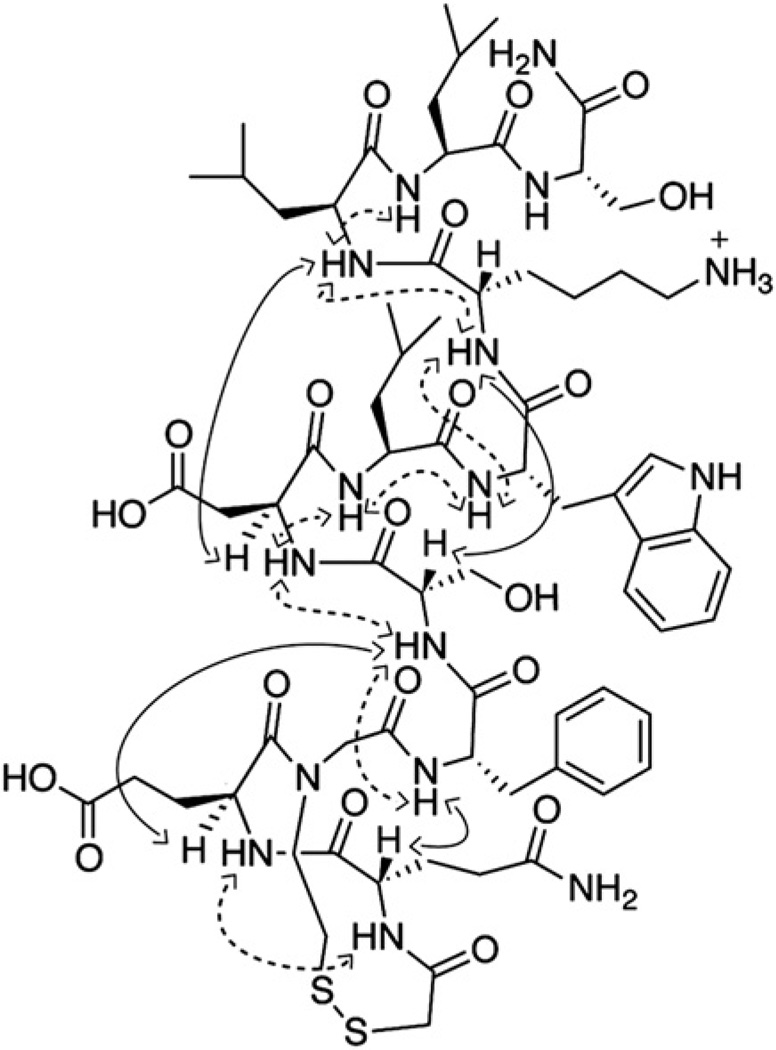
Fig. 4.
Short-range (dashed arrows) and medium-range (black arrows) NOEs observed for dsHBS 2. 2D NMR spectra were acquired in 20% trifluoroethanol-d3/PBS at 20 °C.
3. Conclusion
We have developed a new class of hydrogen bond surrogate helices in which the conformation can be reversibly controlled through oxidation of bis-thiols or reduction of the resulting disulfide bridge. We find that DMSO-mediated oxidation provides efficient conversion to the desired compound. CD and NMR spectroscopies provide strong support for our hypothesis that the disulfide bridge provides conformationally-defined α-helices. In ongoing efforts we are utilizing dsHBS helices to probe biophysical parameters related to the helix-coil transition; the results of these studies will be reported in due course.
4. Experimental section
4.1. General
Commercial-grade reagents and solvents were used without further purification, except as indicated. Dry DMF was obtained using an Innovative Technology PureSolv solvent drying system. All reactions were either stirred or mechanically shaken at room temperature, except as indicated. After each step, the resin was sequentially washed with DMF (3×5 mL), MeOH (3×5 mL), and DCM (3×5 mL). Microwave irradiation was performed in the CEM Discover single-mode reactor with controlled power, temperature, time, and stirring settings. NMR experiments were performed using a Bruker AVANCE 500 MHz spectrometer. Reversed-phase HPLC experiments were conducted with 4.6×150 mm (analytical scale) or 21.4×150 mm (preparative scale) Waters C18 Sunfire columns using a Beckman Coulter HPLC equipped with a System Gold 168 Diode array detector. HPLC buffers consisted of 0.1% aqueous trifluoroacetic acid and 0.1% trifluoroacetic acid in acetonitrile. Mass spectra were obtained by either an Agilent 1100 series LC/MSD (XCT) electrospray trap or a Bruker UltraflexXtreme MALDI-TOF/TOF. The peptide concentrations for the CD and NMR studies were calculated using absorbance of the tryptophan residue (5560 cm−1 M−1 at 280 nm).
4.2. Synthesis of bis-thiol peptide 1
Parent peptide 3 (0.25 mmol) was synthesized on a CEM Liberty microwave peptide synthesizer using standard Fmoc solid phase chemistry with Knorr Amide MBHA resin. The resin bearing 3 was transferred to a fritted polypropylene SPE tube, washed, and dried under vacuum. A solution of preactivated bromoacetic acid (0.17 g, 1.25 mmol, 5 equiv), HOBt (0.18 g, 1.25 mmol, 5 equiv), DIC (0.20 mL, 1.25 mmol, 5 equiv) in 3 mL dry DMF was added to the resin containing 3. After 3 h, the resin was washed and treated with S-trityl-2-mercaptoethylamine (0.45 g, 1.25 mmol, 5 equiv) and DIEA (0.66 mL, 3.37 mmol, 15 equiv) in 3 mL dry DMF. After 3 h, the resin containing 4 was washed. The resin was dried under vacuum for 1 h, transferred to a microwave tube, capped, and purged with N2 for 1 h.
To the microwave tube containing dried 4 on resin, a solution of preactivated Fmoc-Glu(tBu)–OH (2.13 g, 5.0 mmol, 20 equiv), HOAt (0.35 g, 2.5 mmol, 10 equiv), DIC (0.66 mL, 5.0 mmol, 20 equiv) in 4 mL dry DMF was added. The reaction mixture was subjected to microwave irradiation at 60 °C for 45 min, after which the resin was transferred to an SPE tube and washed. The resin was treated with 20% piperidine in NMP (2×20 min) for Fmoc deprotection, washed and dried under vacuum. A solution of preactivated Fmoc-Gln(Trt)–OH (0.76 g, 1.25 mmol, 5 equiv), HBTU (0.42 g, 1.13 mmol, 4.5 equiv) in 4.1 mL 5% DIEA/NMP was added to the resin. After 3 h, the resin containing 5 was washed and dried under vacuum.
Resin bound 5 was subjected to Fmoc deprotection and bromoacetylation as described above. A solution of triphenylmethyl mercaptan (0.35 g, 1.25 mmol, 5 equiv) and DIEA (0.66 mL, 3.75 mmol, 15 equiv) in 3 mL dry DMF was added to the resin. After 3 h, the resin was washed and dried under vacuum. The dried resin was treated with 81.5% TFA, 5% H2O, 5% thioanisole, 5% phenol, 2.5% EDT, 1% TIPS. After 2 h, the mixture was filtered and concentrated in vacuo. The crude solid was washed with cold ether and dried under N2. HPLC purification (gradient of 5–75 acetonitrile/water in 45 min) and lyophilization yielded bis-thiol peptide 1 as a white powder (10 mg). [M+H]+ calculated: 1555.73; [M+H]+ observed: 1555.71; analytical HPLC tR: 9.3 min (gradient of 5–95 acetonitrile/water in 15 min).
4.3. Synthesis of dsHBS 2
Bis-thiol peptide 1 (5 mg) was dissolved in 6.3 mL of 0.1 M aqueous ammonium bicarbonate solution (buffered to pH 6 with TFA) containing 20% DMSO and 10% TFE. After 15 h, the mixture was frozen and lyophilized to obtain a colorless oil. HPLC purification (gradient of 5–65 acetonitrile/water in 45 min) and lyophilization yielded dsHBS 2 (1 mg, 20%) as a white powder. [M+H]+ calculated: 1553.71; [M+H]+ observed: 1553.72; analytical HPLC tR: 9.5 min (gradient of 5–95 acetonitrile/water in 15 min).
4.4. Synthesis of S-trityl-mercaptoethylamine hydrochloride25
Cysteamine hydrochloride (1.00 g, 8.8 mmol, 1 equiv) was dissolved in DMF/DCM (1:1, 50 mL), followed by the addition of trityl chloride (3.68 g, 13.2 mmol, 1.5 equiv). After 3 h, insoluble material was filtered and washed with DCM (3×10 mL). The crude product was concentrated in vacuo and subsequently re-constituted and reconcentrated (3×50 mL DCM). Purification by flash chromatography (10% MeOH/DCM) yielded 2.35 g of off-white solid (75% yield). Rf: 0.60 (1:9 MeOH/DCM). 1H NMR (CDCl3): δ=2.36–2.60 (m, 4H), 5.25 (br s, 3H), 7.19–7.56 (m, 15H). 13C NMR (CDCl3): δ=32.08, 39.57, 67.05, 126.88, 128.08, 129.53, 144.44.
4.5. Circular dichroism spectroscopy
CD spectra were recorded on an AVIV 202SF CD spectrometer equipped with a temperature controller using 1 mm length cells and a scan speed of 5 nm/min. The spectra were averaged over 10 scans with the baseline subtracted from analogous conditions to those of the samples. The samples were prepared in 0.1× phosphate buffered saline (13.7 mM NaCl, 1 mM phosphate, 0.27 mM KCl, pH 7.4), with the final peptide concentration of 50 µM. To monitor the effects of a reducing agent on the helix conformation, a sample of dsHBS 2 was prepared with 75 µM TCEP in the buffer conditions; reduction of the disulfide bond to bis-thiol was confirmed by mass spectroscopy. The helix content of each peptide was determined from the mean residue CD at 222 nm, [θ]222 (deg cm2 dmol−1) corrected for the number of amino acids. Percent helicity was calculated from the ratio [θ]222/[θ]max, where [θ]max=(−44,000+250T)(1−k/n)=−25,170 for T=25 (°C), k=4.0, and n=12 (number of amino acid residues).7,20,23
4.6. 2D NMR spectroscopy
Spectra of dsHBS 2 were recorded on a Bruker Avance 500 at 20 °C. The sample was prepared by dissolving 1 mg of 2 in 300 µL of 20% TFE-d3 in PBS (pH 3.5) in a Shigemi NMR tube. All 2D spectra were recorded by collecting 2048 complex data points in the t2 domain by averaging 72 scans and 512 increments in the t1 domain with the States-TPPI mode. TOCSY experiments were performed with a mixing time of 80 ms on a 6000 Hz spin lock frequency. For NOESY, mixing time of 200 ms was used. The data were processed and analyzed using the Bruker TOPSPIN program. The original free induction decays (FIDs) were zero-filled to give a final matrix of 1024 by 1024 real data points. A 90° sine-square window function was applied in both dimensions.
Supplementary Material
Acknowledgements
This work was financially supported by the National Science Foundation (CHE1027009 to N.R.K.) and the National Institutes of Health (GM073943 to P.S.A.). S.E.M. thanks the New York University Department of Chemistry for a Margaret Strauss Kramer Pre-doctoral Fellowship. Support from the National Science Foundation (CHE-0958457) in the form of an instrumentation grant is gratefully acknowledged.
Footnotes
Supplementary data
2D NMR and circular dichroism spectra of 2. Supplementary data related to this article can be found online at doi:10.1016/j.tet.2011.12.068.
References and notes
- 1.Woolley GA. Acc. Chem. Res. 2005;38:486–493. doi: 10.1021/ar040091v. [DOI] [PubMed] [Google Scholar]
- 2.Ihalainen JA, Paoli B, Muff S, Backus EHG, Bredenbeck J, Woolley GA, Caflisch A, Hamm P. Proc. Natl. Acad. Sci. U.S.A. 2008;105:9588–9593. doi: 10.1073/pnas.0712099105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrader TE, Cordes T, Schreier WJ, Koller FO, Dong S-L, Moroder L, Zinth W. J. Phys. Chem. B. 2010;115:5219–5226. doi: 10.1021/jp107683d. [DOI] [PubMed] [Google Scholar]
- 4.Beharry AA, Woolley GA. Chem. Soc. Rev. 2011;40:4422–4437. doi: 10.1039/c1cs15023e. [DOI] [PubMed] [Google Scholar]
- 5.Patgiri A, Jochim AL, Arora PS. Acc. Chem. Res. 2008;41:1289–1300. doi: 10.1021/ar700264k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabezas E, Satterthwait AC. J. Am. Chem. Soc. 1999;121:3862–3875. [Google Scholar]
- 7.Wang D, Chen K, Kulp JL, III, Arora PS. J. Am. Chem. Soc. 2006;128:9248–9256. doi: 10.1021/ja062710w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahon AB, Arora PS. Chem. Commun. 2012 doi: 10.1039/c1cc14730g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Chen K, Dimartino G, Arora PS. Org. Biomol. Chem. 2006;4:4074–4081. doi: 10.1039/b612891b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson DY, King DS, Chmielewski J, Singh S, Schultz PG. J. Am. Chem. Soc. 1991;113:9391–9392. [Google Scholar]
- 11.Haney CM, Loch MT, Horne WS. Chem. Commun. 2011:10915–10917. doi: 10.1039/c1cc12010g. [DOI] [PubMed] [Google Scholar]
- 12.Ravi A, Prasad BVV, Balaram P. J. Am. Chem. Soc. 1983;105:105–109. [Google Scholar]
- 13.Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP. Science. 1996;274:948–953. doi: 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]
- 14.Henchey LK, Porter JR, Ghosh I, Arora PS. ChemBioChem. 2010;11:2104–2107. doi: 10.1002/cbic.201000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patgiri A, Menzenski MZ, Mahon AB, Arora PS. Nat. Protoc. 2010;5:1857–1865. doi: 10.1038/nprot.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tam JP, Wu CR, Liu W, Zhang JW. J. Am. Chem. Soc. 1991;113:6657–6662. [Google Scholar]
- 17.Chin DH, Woody RW, Rohl CA, Baldwin RL. Proc. Natl. Acad. Sci. U.S.A. 2002;99:15416–15421. doi: 10.1073/pnas.232591399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kallenbach NR, Lyu PC, Zhou HX. In: Circular Dichroism and the Conformational Analysis of Biomolecules. Fasman GD, editor. New York, NY: Plenum; 1996. [Google Scholar]
- 19.Driver RW, Hoang HN, Abbenante G, Fairlie DP. Org. Lett. 2009;11:3092–3095. doi: 10.1021/ol901181b. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd NE, Hoang HN, Abbenante G, Fairlie DP. J. Am. Chem. Soc. 2005;127:2974–2983. doi: 10.1021/ja0456003. [DOI] [PubMed] [Google Scholar]
- 21.Schafmeister CE, Po J, Verdine GL. J. Am. Chem. Soc. 2000;122:5891–5892. [Google Scholar]
- 22.CammersGoodwin A, Allen TJ, Oslick SL, McClure KF, Lee JH, Kemp DS. J. Am. Chem. Soc. 1996;118:3082–3090. [Google Scholar]
- 23.Luo P, Baldwin RL. Biochemistry. 1997;36:8413–8421. doi: 10.1021/bi9707133. [DOI] [PubMed] [Google Scholar]
- 24.Rajan R, Balaram P. Int. J. Pept. Protein Res. 1996;48:328–336. doi: 10.1111/j.1399-3011.1996.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 25.Di Maro S, Pong R-C, Hsieh J-T, Ahn J-M. J. Med. Chem. 2008;51:6639–6641. doi: 10.1021/jm800959f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.