Abstract
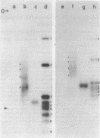
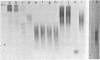
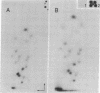
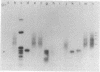
Nucleic acids isolated from uninfected and potato spindle tuber viroid-infected Rutgers tomato plants were fractionated on agarose gels under two different sets of denaturing conditions and hybridized to 125I-labeled viroid in a series of blot hybridization experiments. Complementary strand nucleic acids detected in extracts of infected plants were heterogeneous in size, with four discrete bands containing molecules approximately 700, 1050, 1500, and 1800 nucleotides long. Enzymatic studies indicated that these viroid minus strands are composed exclusively of RNA and, as extracted, are present in complexes containing extensive double-stranded regions. After treatment with several RNases under conditions favoring digestion of single-stranded regions, the high molecular weight minus strands can no longer be detected and roughly unit-length minus strands appear. A model for the structure of the viroid replication intermediate is proposed.
Keywords: potato spindle tuber viroid, blot hybridization, denaturing gel, double-stranded RNA, ribonuclease III
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bailey J. N., McAllister W. T. Mapping of promoter sites utilized by T3 RNA polymerase on T3 DNA. Nucleic Acids Res. 1980 Nov 11;8(21):5071–5088. doi: 10.1093/nar/8.21.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Vovis G. F., Zinder N. D. Insertion mutant of bacteriophage f1 sensitive to EcoRI. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2699–2702. doi: 10.1073/pnas.76.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson E., Diener T. O., Robertson H. D. Potato spindle tuber and citrus exocortis viroids undergo no major sequence changes during replication in two different hosts. Proc Natl Acad Sci U S A. 1978 Feb;75(2):951–954. doi: 10.1073/pnas.75.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson E., Pape L. K., Robertson H. D. Approaches to sequence analysis of 125I-labeled RNA. Nucleic Acids Res. 1979 Jan;6(1):91–110. doi: 10.1093/nar/6.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener T. O., Raymer W. B. Potato spindle tuber virus: a plant virus with properties of a free nucleic acid. Science. 1967 Oct 20;158(3799):378–381. doi: 10.1126/science.158.3799.378. [DOI] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Grill L. K., Semancik J. S. RNA sequences complementary to citrus exocortis viroid in nucleic acid preparations from infected Gynura aurantiaca. Proc Natl Acad Sci U S A. 1978 Feb;75(2):896–900. doi: 10.1073/pnas.75.2.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Kohne D. E., Levison S. A., Byers M. J. Room temperature method for increasing the rate of DNA reassociation by many thousandfold: the phenol emulsion reassociation technique. Biochemistry. 1977 Nov 29;16(24):5329–5341. doi: 10.1021/bi00643a026. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Sequence of interrupted and uninterrupted mRNAs and cloned DNA coding for the two overlapping nonstructural proteins of influenza virus. Cell. 1980 Sep;21(2):475–485. doi: 10.1016/0092-8674(80)90484-5. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. A., Cress D. E. Molecular cloning and characterization of potato spindle tuber viroid cDNA sequences. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5302–5306. doi: 10.1073/pnas.77.9.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Dunn J. J. A nucleotide sequence from a ribonuclease III processing site in bacteriophage T7 RNA. Proc Natl Acad Sci U S A. 1977 Mar;74(3):822–826. doi: 10.1073/pnas.74.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Jelinek W. Determination of nucleotide sequences from double-stranded regions of HeLa cell nuclear RNA. J Mol Biol. 1977 Oct 5;115(4):571–589. doi: 10.1016/0022-2836(77)90103-6. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dunn J. J. Ribonucleic acid processing activity of Escherichia coli ribonuclease III. J Biol Chem. 1975 Apr 25;250(8):3050–3056. [PubMed] [Google Scholar]
- Robertson H. D. Enzymatic synthesis of bacteriophage fl DNA: RNA hybrid and double stranded RNA. Nat New Biol. 1971 Feb 10;229(6):169–172. doi: 10.1038/newbio229169a0. [DOI] [PubMed] [Google Scholar]
- Robertson H. D. Isolation of specific ribosome binding sites from single-stranded DNA. J Mol Biol. 1975 Mar 5;92(3):363–375. doi: 10.1016/0022-2836(75)90286-7. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Webster R. E., Zinder N. D. Purification and properties of ribonuclease III from Escherichia coli. J Biol Chem. 1968 Jan 10;243(1):82–91. [PubMed] [Google Scholar]
- Semancik J. S., Weathers L. G. Exocortis virus: an infectious free-nucleic acid plant virus with unusual properties. Virology. 1972 Feb;47(2):456–466. doi: 10.1016/0042-6822(72)90281-4. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]