Abstract
Our understanding of the use of caloric (CS) and non-caloric sweeteners (NCS) in the US food supply is limited. This study utilizes full ingredient list and nutrition facts panel (NFP) data from Gladson Nutrition Database, and nationally representative purchases of consumer packaged foods from Nielsen Homescan in 2005 through 2009 to understand the use of CS (including FJC) and NCS in CPG foods.
Of the 85,451 uniquely formulated foods purchased during 2005–2009, 75% contain sweeteners (68% with CS only, 1% with NCS only, 6% with both CS and NCS). CS are in >95% of cakes/cookies/pies, granola/protein/energy bars, ready-to-eat cereals, sweet snacks, and sugar-sweetened beverages. NCS are in >33% of yogurts and sports/energy drinks, 42% of waters (plain or flavored), and most diet sweetened beverages. Across unique products, corn syrup is the most commonly listed sweetener, followed by sorghum, cane sugar, high fructose corn syrup and FJC. Also, 77% of all calories purchased in the US in 2005–2009 contained CS and 3% contained NCS, while 73% of the volume of foods purchased contained CS and 15% contained NCS. Trends during this period suggest a shift towards the purchase of NCS-containing products.Our study poses a challenge toward monitoring sweetener consumption in the US by discussing the need and options available to improve measures of CS and NCS, and additional requirements on NFPs on CPG foods.
Keywords: Caloric sweeteners, added sugars, fruit juice concentrate, packaged foods, United States
INTRODUCTION
There is growing scientific interest in the role of sweeteners, both caloric (CS) and non-caloric (NCS), in the global food supply. In the US, there are a large variety of CS and NCS that are used in foods 1. Their potential adverse health effects have been suggested by numerous health authorities and scholars 2–5. CS or added sugars (i.e., caloric or caloric sweeteners added to foods or beverages during processing or preparation) 6, 7, represent sources of energy with little nutritional value. Dietary recommendations include efforts to reduce surplus energy intake, in particular, “energy from foods and beverages that provide empty calories” 8. In addition to contributing excess calories, there is concern that increased exposure to sweeteners in the diet may influence taste preferences and dietary patterns 9, 10. Controversy surrounds the effects of repeated exposure to NCS on appetite and energy intake 11–13.
While sweeteners have become a focal point of dietary recommendations and policy initiatives, little is known about the use of sweeteners in foods and beverages sold and consumed in the US. Currently there is no direct measurement of sweetener contents of foods and beverages. Since added sugars, or CS, cannot be chemically distinguished from intrinsic (naturally occurring) sugars, the primary food-composition databases in the US do not contain composition information for either intrinsic or added sugars. There are two supplementary sources of added sugars information available from the US Department of Agriculture (USDA). The USDA Database for the Added Sugars Content of Selected Foods, Release 1 (2006) 14 provides data for the added sugars content of 2,038 commonly consumed foods, excluding brand name foods. A second source, the MyPyramid Equivalents Database (MPED), provides data on added sugars content of foods reported in Continuing Survey of Food Intakes of Individuals 1994–1996 and 1998, and the National Health and Nutrition Examination Survey 2001–2002 and 2003–2004 15. Unfortunately neither source is available beyond 2006 and therefore unsuitable for monitoring the most current intakes and purchases of CS in the US. To our knowledge there is no monitoring of NCS in consumer packaged goods (CPG) foods and beverages sold and consumed in the US.
The goal of the current paper is to examine the extent of CS and NCS sweetener use in the CPG foods and beverages purchased by US households. We utilize unique commercial data that provides the full set of ingredients and nutrition facts panel information on 85,451 uniquely formulated CPG foods and beverages purchased by a nationally representative sample of households. We identify the most commonly used sweeteners and the foods that are sweetened with CS and NCS in US CPGs. In addition, we raise a number of questions regarding the measurement and labeling requirements of sweeteners in the US.
METHODS
Data
Nutrition Facts Panel (NFP) label and ingredient information for each uniquely barcoded food
The 2007 and 2010 versions of the commercial Gladson Nutrition Database 16 have national brands and private label CPG items at the Universal Product Codes (UPCs) level with data on the nutrition facts panel and full ingredients lists 17. Per the Food and Drug Administration (FDA) requirements, the NFP label needs to include serving measurement, total calories, calories from fat, total fat, saturated fat, trans fats, total sugars, total carbohydrate, protein, dietary fiber, sodium, cholesterol, vitamin A, vitamin C, calcium and iron.
Commercial Scanner Food Purchase data
The Nielsen Homescan (The Nielsen Co.) 18 is a commercial data set that contains information on food products with a UPC that a household purchases over a year (acquired using scanners provided to participating households), along with important socio-demographic information and sampling weights. The households are sampled and weighted to be nationally representative and include between 40,000 to 55,000 households per year in the 2005–2009 panels. This data has been frequently used by researchers (particularly agricultural and marketing economists) to analyze food demand, consumption, branding and promotion strategies 19–21.
Linking Gladson NFP and Nielsen Homescan Food Purchase data
We matched the Gladson NFP data with the 2005–2009 Homescan data on household purchases at the UPC level in order to create a more complete measure of the nutritional content of UPCs reported purchased. This was successful for over 98% of the volume and dollar sales of foods reported purchased in Homescan. UPCs were then categorized into 41 food and 21 beverage groups based on critical dietary behaviors and consumption patterns as explained in Supplementary Table A1.
Identifying the use of sweeteners in US CPG products
To identify CPGs containing various types of CS and NCS, we conducted searches for key terms in the ingredients lists (listed in Supplementary Table A2). In this study, we include fruit juice concentrate (FJC) (not reconstituted) as a CS.
We then determined for each food and beverage group the proportion of the unique food products with various combinations of CS, NCS, and the average total sugar calories per 100g for unique products with various combinations. We are defining unique food products as those with unique formulations (e.g., a 1.5 liter bottle of Coca-cola Classic will be nutritionally equivalent to a 12 fl oz can of Coca-cola Classic and a 20 fl oz bottle of Coca-cola Classic, so even though they will have different barcodes, they only count as one food product). These sweetener categories are: no sweeteners (in which case total sugars are equal to intrinsic sugars); CS only (including FJC); NCS only; and both CS and NCS. To measure how frequently the various kinds of CS and NCS are used we ranked the top five sweetener types used within each food group. The categorization of the CS and NCS are provided in Supplementary Table A2.
Lastly, to understand how much of the US processed and packaged foods and beverages purchased contain CS and NCS, we determined the total calories and volume (or gram weight) of each product using the Homescan purchase and NFP data. We then calculated the proportion of total calories and total volume purchased by Americans that contain any CS, and any NCS for each food group and all food groups. We conducted z-tests to determine if the change in these proportions over time were statistically significant. All statistical work was conducted using Stata version 11 22.
RESULTS
The use of sweeteners in unique US CPG products
From the commercial databases described above, we identified 85,451 unique processed and packaged food and beverage products that were not raw or single ingredient foods (see Table 1). Among these, 75% contain some sweetener (68% with CS only, 1% with NCS only, 6% with both CS and NCS).
Table 1.
Percentage of uniquely formulated consumer packaged food products purchased during 2005–2009 by sweetener category for select food groups
| Select Food or Beverage Group | Number of Unique Products | % among all unique products | % Unique Products within Food Group* containing | |||
|---|---|---|---|---|---|---|
| No sweetener | CS (including FJC) only | NCS only | both CS & NCS | |||
| Baby food, formula | 993 | 1.2% | 47.5% | 52.5% | 0.0% | 0.0% |
| Cakes, cookies, pies | 5592 | 6.5% | 0.7% | 95.3% | 0.1% | 4.0% |
| Fruit, fresh, frozen, canned or dried | 1722 | 2.0% | 36.6% | 59.9% | 2.7% | 0.7% |
| Granola, protein or energy bars | 2526 | 3.0% | 3.0% | 78.2% | 0.0% | 18.8% |
| Ready-to-eat cereals | 1378 | 1.6% | 3.6% | 94.0% | 0.3% | 2.1% |
| Salad dressings and dips | 3305 | 3.9% | 26.9% | 71.0% | 1.5% | 0.7% |
| Savory Snacks | 5734 | 6.7% | 28.8% | 69.9% | 0.7% | 0.6% |
| Sweet breads and pastries | 1440 | 1.7% | 17.3% | 79.3% | 0.5% | 2.9% |
| Sweet snacks | 6710 | 7.9% | 0.6% | 84.6% | 0.6% | 14.1% |
| Yogurt | 1152 | 1.3% | 6.4% | 60.8% | 6.8% | 26.0% |
| Sports and Energy drinks | 506 | 0.6% | 1.2% | 63.4% | 6.9% | 28.5% |
| Sugar sweetened beverage | 2513 | 2.7% | 1.5% | 83.6% | 1.0% | 13.9% |
| Diet sweetened beverage † | 974 | 1.4% | 9.9% | 17.2% | 14.6% | 58.3% |
| Milk & milk/yogurt/soy drinks | 1483 | 1.7% | 32.4% | 56.6% | 2.4% | 8.6% |
| 100% Fruit juice | 1404 | 1.6% | 33.6% | 66.0% | 0.0% | 0.4% |
| Vegetable juice | 230 | 0.3% | 22.2% | 69.1% | 0.4% | 8.3% |
| Water, plain or flavored | 799 | 0.9% | 36.4% | 21.7% | 22.3% | 19.7% |
| All Food and Beverage Groups | 85451 | 100% | 25.4% | 67.9% | 1.0% | 5.6% |
Sources: Nielsen Homescan 2005–2009, Gladson Nutrition Database 2007 and 2010
Notes: CS = Caloric Sweetener; FJC = Fruit Juice Concentrate; NCS = Non-Caloric Sweetener
The % of unique products within this food group (e.g., yogurt) with various types of sweeteners
Not strictly products that contain zero-calories, rather items that are marketed as ‘non-regular’ or less calories than the regular version.
Among vegetable juices (n=230), 22% have no sweeteners, 69% contain CS only, less than 1% contain NCS only and 8% contain both CS and NCS. There were 2,513 unique sugar sweetened beverages, of which 84% contained CS only, 1% contained NCS only and 14% contained both CS and NCS.
Among diet-sweetened beverages (n=974), the majority (58%) contained both CS and NCS, with another 15% containing NCS only and 17% containing CS only. Among the 1,722 unique CPG fruit products (fresh 8%, frozen 6%, canned 34% or dried 52%), 37% are unsweetened, but 60% contain CS, 3% contain NCS and 1% contain both CS and NCS. As for the 2,526 uniquely formulated granola, protein or energy bars and 1,378 unique ready-to-eat cereals, 78% and 94% contain CS only, while less than 1% of contain NCS only, and 20% and 2% contain both CS and NCS. Of the 993 unique baby food formulas, nearly half do not have any sweeteners, but all of the other 53% contain CS. For salads dressings and dips (n=3,305), 73% are sweetened − 71% with CS only, 1.5% with NCS only, and <1% with both CS and NCS.
In Supplementary Table A3, we present additional breakdowns of the use of FJC compared to other CS across select food groups. Overall, 2% of unique products contain FJC as the only source of CS, and another 5% of unique products contain FJC combined with other sweetener/s, for a total of 7% containing any FJC.
In ranking the top five sweetener types included in ingredient lists within each food group, we found that between 2005 and 2009, across all unique food products corn syrup is the most commonly listed sweetener, followed by sorghum, cane, HFCS and FJC. Corn syrup is the most common sweetener used in baby food/formula, salad dressings and dips, sweet snacks, milk and milk/yogurt/soy drinks. HFCS is the most common for cakes/cookies/pies, fruit products, yogurt and sugar sweetened beverages and FJC came up top for fruit juice and vegetable juice. The NCSs, acesulfame potassium, aspartame and sucralose were the three most common sweeteners found in diet sweetened beverages, and both acesulfame potassium and sucralose were also highly ranked among water products. Unfortunately, since each unique food product’s formulation is proprietary information, it is not possible to determine exactly how much of each sweetener is used, and therefore these rankings are based on frequency of occurrence, rather than volume.
Measuring how much CPG products with sweeteners are purchased in the US
While understanding the number of products with CS and NCS is important, each product is not purchased equally. Supplementary Table A4 provides the proportion of total calories and total volume of select food and beverage groups purchased during 2005-2009 that contain CS only, NCS only, and both CS and NCS. We found that 77% of all calories purchased from CPGs in the US in 2005–2009 contained CS, 3% contained NCS, and 23% did not contain any sweeteners. The use of CS is common across many food groups. Of all the calories purchased from processed and packaged baby foods and fruit juices, 60% and 63% respectively are from products that contain CS. Of all the calories purchased from processed and packaged fruit (fresh/frozen/canned/dried) and savory snacks, nearly three-quarters are from products that contain CS. Also, virtually all processed and packaged cakes/cookies/pies, granola/protein/energy bars, sports and energy drinks, sugar-sweetened beverages products contain CS. As for products that contain NCS, we found that of all the calories purchased from yogurt, 25% are from products that contain NCS. The use of NCS appears to be more common among beverages —of all the calories purchased from sports/energy drinks, vegetable juice, water (plain or flavored), and diet-sweetened beverage, 13%, 28%, 29% and 44% respectively are from products that contain NCS. We also found that certain food and beverage groups are less likely to contain sweeteners. For example, we see that 41% and 27% of calories in baby food and fruit (fresh/frozen/canned/dried) products respectively contain neither CS nor NCS. Among beverages, 60% and 37% of calories in milk and milk/yogurt/soy drinks, and from fruit juices, 60% and 37% respectively do not have either CS or NCS.
Supplementary Table A4 show results based on volume of CPGs purchased during this period to better capture the prevalence of NCS, since products containing NCS will contribute no or fewer calories. Across all foods and beverages, 73% of the volume of foods purchased contained CS and 15% contained NCS. Of all sports/energy drinks, vegetable juice, water (plain or flavored), and diet-sweetened beverage purchases, 17%, 28%, 22% and 70% respectively are from products that contain NCS. Among food groups, we see that other than yogurt and granola/protein/energy bars, a very low proportion of purchases from other food groups contain NCS. In the case of baby food/formula and vegetable juice, it appears that there are no processed and packaged products that contain NCS.
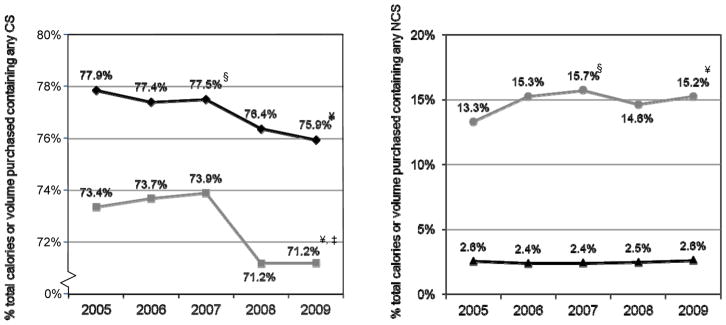
Figure 1 shows the trends in the proportion of total calories and total volume of CPGs purchased during 2005–2009 that contain any CS (panel a) and any NCS (panel b). We see that both the proportion of calories and volume from products containing CS have fallen over time (p<0.05). Meanwhile, the proportion of calories from products containing NCS has not changed markedly, however we observe an upward trend in the volume of products containing NCS being purchased (13.3% in 2005 vs. 15.2% in 2009, z=−9.01, p<0.01).
Figure 1.
Total calories and volume of consumer packaged food and beverages purchased in the United States containing caloric sweeteners (CS) and non-caloric sweeteners (NCS), 2005–2009
a. Containing any Caloric Sweeteners (CS)
 % total calories purchased containing any CS (including FJC)
% total calories purchased containing any CS (including FJC)
 % total volume purchased containing any CS (including FJC)
% total volume purchased containing any CS (including FJC)
b. Containing any Non-Caloric Sweeteners (NCS)
 % total calories purchased containing any NCS
% total calories purchased containing any NCS
 % total volume purchased containing any NCS
% total volume purchased containing any NCS
Sources: Nielsen Homescan 2005-2009, Gladson Nutrition Database 2007 and 2010
§ denotes statistical difference between 2005 and 2007 using two-tailed z-test (p<0.05)
¥ denotes statistical difference between 2005 and 2009 using two-tailed z-test (p<0.05)
‡ denotes statistical difference between 2007 and 2009 using two-tailed z-test (p<0.05)
DISCUSSION
To our knowledge, this is the first study to report the extent of sweetener use in the US CPG food supply. Three-quarters of uniquely formulated CPG foods and beverages in the US contain sweeteners, of which 73.5% contains CS and 1.5% contains NCS. From 2005–2009, 77% of the calories from CPGs purchased in the US contain CS and 3% contain NCS; while 73% of the volume purchased contained CS and 15% contained NCS. Trends suggest a shift towards the purchase of products containing NCS.
The large proportion of CPGs containing CS and/or NCS sweeteners is concerning. In 2009, the American Heart Association released a scientific statement calling for reductions in added sugar intake as a means of reducing obesity and cardiovascular disease risk 7. Caloric sweeteners represent empty calories, or sources of energy with little nutritional value. Health authorities emphasize that prevention of obesity must include strategies to reduce surplus energy intake from foods and beverages that provide empty calories 8, 23–26.
In addition to the evidence on the relationship between intake of sweeteners and cardiovascular health7, 27, there is concern that increased exposure to sweeteners in the diet may influence taste preferences, energy intake and dietary patterns9, 10. While the use of NCS in processed and packaged foods and beverages has gained attention for its ability to reduce the energy content while maintaining the sweet taste of products 13, 28, the relationship between NCS consumption and energy intake is unclear 8, 13, 29–32. Previous research has shown that the greater the sweetness of a product, the higher the consumption of sweet foods or beverages 33. Further repeated exposure to NCS uncoupled with energy has been hypothesized to affect appetite and energy intake by disrupting hormonal and neurobehavioral pathways that control hunger and satiety 11.
Currently, US nutrition labels contain information on total sugars but do not distinguish between naturally occurring (intrinsic) sugars and those that are added. Consequently it is difficult for consumers, researchers and other health professionals to monitor sweetener intakes. Efforts to include added sugars as part of the NFP label have been considered by the FDA but never implemented 34. Given that food manufacturers have full knowledge of the amount of sweeteners they include in their products, this information can be easily derived and added to the existing list of required nutrition information available to consumers.
Such a requirement will also likely spur food manufacturers to reduce the amount of CS (and possibly also NCS) that are added to their products. Such a requirement will also mean that definitions of what are considered CS or added sugars be properly defined. For example, even though FJC is currently recognized as a type of CS, FJC used as a sweetener is not measured as one in neither MPED nor national aggregates of consumption of sweeteners. Yet as this study shows, FJC is used as a sweetener in 7% of the 85,451 unique CPG products, and is used frequently in juices, sugar sweetened beverages, diet sweetened beverages, salad dressings, yogurt, granola/energy bars, ready-to-eat cereals and baby food (see Supplementary Table A3). It is also the fifth most commonly listed sweetener. Therefore, together with improvements in NFP labeling, clear official definitions of CS will also be crucial.
From a monitoring standpoint, given the lack of sweetener measurement in existing food composition databases, one way to estimate the use of sweeteners among CPGs in the US is to use commercial databases as we have done here. Should the addition of the added CS and/or NCS content be included in the NFP, our approach here will also still be applicable. Nonetheless, we note a number of limitations to this approach. While the commercial databases allow for identification of products that contain CS and NCS there are limitations with the use of NFP data. Nutrient declarations on nutrition labels may be rounded according to FDA rounding rules, limiting the precision of information provided (e.g., foods with <0.5 gram of sugar per serving meet the definition of "sugar free")35; and by law the nutrient information only needs to be within 20% of the ‘truth’ 35. Further, while we are able to identify items that contain CS and NCS, with ingredient level data we are unable to know the precise amount of an ingredient.
In addition, commercial NFP label data are not updated equally, and their proprietary nature limits researchers’ abilities to determine the frequency and comprehensiveness of collection and updating. Finally, due to the propriety nature of commercial data, this is an expensive proposition and can be challenging to undertake.
While currently, US nutrition labels do not contain information on added sweeteners a possible solution for researchers is to estimate the amount of CS via mathematical optimization techniques of linear programming (LP). LP nutrient estimation is performed by organizations such as USDA, the University of Minnesota Nutrition Coordinating Center (NCC), and Yale-Griffin. For example, USDA uses LP nutrient estimation to impute many of the nutrient values used for calculation by the Food and Nutrient Database for Dietary Studies and the NCC uses LP nutrient estimation to estimate added sugars 36, 37. We are fine-tuning these LP methods to estimate the amount of sweetener in each unique food product in our commercial data.
In summary, given that 77% of all calories purchased from CPGs in the US from 2005 to 2009 period contain CS, and that there is a trend towards purchase of NCS containing products, further research and regulatory focus on these topics are needed. While we have not yet derived the proportion of calories from CS, this paper lends support to what is seen by the nutrition profession as a critical issue – excessive consumption of foods with CS. Moreover, given the lack of current knowledge about the effects of NCS on sweet preferences and energy intake, it may also be important to start monitoring the prevalence of products containing NCS. This paper represents a call to focus legislative efforts and research on an important component of our diet and to improve both consumer knowledge and current public measurements of sweeteners consumed by Americans.
Supplementary Material
Table 2.
Most common sweeteners used in unique consumer packaged food products purchased during 2005–2009 for select food groups
| Select Food or Beverage Group | Most common | 2nd most common | 3rd most common | 4th most common | 5th most common |
|---|---|---|---|---|---|
| Baby food, formula | Corn syrup | FJC | Lactose | Sorghum | Cane |
| Cakes, cookies, pies | HFCS | Sorghum | Corn syrup | Cane | Molasses |
| Fruit, fresh, frozen, canned or dried | HFCS | Cane | FJC | Corn syrup | Sucralose |
| Granola, protein or energy bars | Sorghum | Cane | Corn syrup | Honey | Alcohol |
| Ready-to-eat cereals | Sorghum | Cane | Honey | Corn syrup | Molasses |
| Salad dressings and dips | Corn syrup | FJC | Cane | Sorghum | HFCS |
| Savory snacks | Sorghum | Corn syrup | Cane | HFCS | Lactose |
| Sweet breads and pastries | Sorghum | Corn syrup | Cane | HFCS | Honey |
| Sweet snacks | Corn syrup | Sugar alcohol | Sorghum | Lactose | Honey |
| Yogurt | HFCS | Fructose | Aspartame | Sucralose | FJC |
| Sports and Energy drinks | cane | sucrose | HFCS | sucralose | corn syrup |
| Sugar sweetened beverage | HFCS | FJC | Cane | Corn syrup | Fructose |
| Diet sweetened beverage† | Acesulfame potassium | Aspartame | Sucralose | Cane | FJC |
| Milk & milk/yogurt/soy drinks | Corn syrup | Cane | HFCS | Sucralose | Sorghum |
| 100% fruit juice | FJC | HFCS | Cane | Fructose | Sorghum |
| Vegetable juice | FJC | HFCS | Sucralose | Cane | Fructose |
| Water, plain or flavored | Sucralose | Acesulfame potassium | Fructose | Cane | Sugar alcohol |
|
| |||||
| All Food and Beverage Groups | Corn syrup | Sorghum | Cane | HFCS | FJC |
Sources: Nielsen Homescan 2005–2009, Gladson Nutrition Database 2007 and 2010.
Notes: CS = Caloric Sweetener; FJC = Fruit Juice Concentrate; NCS = Non-Caloric Sweetener; Sweeteners were categorized based on these search terms listed in Supplementary Table A2.
Not strictly products that contain zero-calories, rather it reflect products that are marketed as ‘non-regular’ or less calories than the regular versions
Acknowledgments
We thank the Robert Wood Johnson Foundation (Grant 67506) and the National Institutes of Health (R01 HL104580) for financial support. We also wish to thank Ms Izabela Annis for exception assistance with the data management and programming, Ms. Frances L. Dancy for administrative assistance, and Mr. Tom Swasey for graphics support for assistance in this effort. None of the authors have conflict of interests of any type with respect to this manuscript.
Abbreviations
- CPG
Consumer Packaged Goods
- CS
Caloric sweeteners
- FDA
Food and Drug Administration
- FJC
Fruit juice concentrate
- HFCS
High fructose corn syrup
- LP
Linear programming
- MPED
MyPyramid Equivalents Database
- NCC
University of Minnesota Nutrition Coordinating Center
- NCS
Non-caloric sweeteners
- NFP
Nutrition Facts Panel
- UPC
Universal product code
- USDA
United States Department of Agriculture
Footnotes
AUTHOR CONTRIBUTIONS
SWN, MMS, BMP all contributed to the conceptualization and jointly wrote components of the manuscript. SWN, MMS and BMP drafted the methods section; SWN prepared the tables and figures and wrote the results section; MMS, BMP and SWN jointly wrote the introduction and discussion; and all contributed to reviews and revisions of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shu Wen Ng, Email: shuwen@unc.edu.
Meghan M. Slining, Email: slining@email.unc.edu.
Barry M. Popkin, Email: popkin@unc.edu.
References
- 1.Fitch C, Keim KS. Position of the Academy of Nutrition and Dietetics: Use of nutritive and nonnutritive sweeteners. J Am Diet Assoc. 2012;112(5):739–758. doi: 10.1016/j.jand.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Popkin BM, Nielsen SJ. The sweetening of the world’s diet. Obesity. 2003;11(11):1325–1332. doi: 10.1038/oby.2003.179. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Agriculture. [Accessed May 1, 2012];Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010. 2010 http://www.cnpp.usda.gov/DGAs2010-DGACReport.htm.
- 4.WHO/FAO. Technical Report Series. Vol. 916. Geneva: World Health Organization; 2003. Diet, Nutrition and the Prevention of Chronic Diseases. Report of a joint WHO/FAO expert consultation. [PubMed] [Google Scholar]
- 5.Lustig RH, Schmidt LA, Brindis CD. Public health: The toxic truth about sugar. Nature. 2012;482:27–29. doi: 10.1038/482027a. [DOI] [PubMed] [Google Scholar]
- 6.Reedy J, Krebs-Smith S. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. JADA. 2010;110(10):1477–1484. doi: 10.1016/j.jada.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, et al. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120(11):1011–1020. doi: 10.1161/CIRCULATIONAHA.109.192627. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans 2010. 7. Washington, DC: U.S. Government Printing Office; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beauchamp GK, Moran M. Dietary experience and sweet taste preference in human infants. Appetite. 1982;3(2):139–152. doi: 10.1016/s0195-6663(82)80007-x. [DOI] [PubMed] [Google Scholar]
- 10.Liem DG, de Graaf C. Sweet and sour preferences in young children and adults: role of repeated exposure. Physiol Behav. 2004;83(3):421–429. doi: 10.1016/j.physbeh.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Tordoff MG. How do non-nutritive sweeteners increase food intake? Appetite. 1988;11(Supplement 10):5–11. [PubMed] [Google Scholar]
- 12.Ford HE, Peters V, Martin NM, Sleeth ML, Ghatei MA, Frost GS, et al. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur J Clin Nutr. 2011;65(4):508–513. doi: 10.1038/ejcn.2010.291. [DOI] [PubMed] [Google Scholar]
- 13.Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89(1):1–14. doi: 10.3945/ajcn.2008.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Department of Agriculture. [Accessed May 1, 2012];USDA Database for the Added Sugars Content of Selected Foods. Release 1 2006; http://www.ars.usda.gov/services/docs.htm?docid=12107.
- 15.U.S. Department of Agriculture. [Accessed May 1, 2012];MyPyramid Equivalents Database. 2012 Jan 31; http://www.ars.usda.gov/Services/docs.htm?docid=17558.
- 16.Gladson. [Accessed May 1, 2012];Gladson Nutrition Database. [cited Lisle, IL May 1, 2012]; http://www.gladson.com/SERVICES/NutritionDatabase/tabid/89/Default.aspx.
- 17.Ng SW, Popkin BM. Monitoring foods and nutrients sold and consumed in the US: dynamics and challenges. J Acad Nutr Diet. 2012;112(1):41–45.e4. doi: 10.1016/j.jada.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Nielsen Co. [Accessed May 1, 2012];Nielsen Retail Measures. 2012 May 1; http://en-us.nielsen.com/
- 19.Zhen C, Taylor JL, Muth MK, Leibtag E. understanding differences in self-reported expenditures between household scanner data and diary survey data: a comparison of Homescan and Consumer Expenditure Survey. Rev Agr Econ. 2009;31(3):470–492. [Google Scholar]
- 20.Einav L, Leibtag E, Nevo A. Recording discrepancies in Nielsen Homescan data: Are they present and do they matter? Quant Market Econ. 2010;8(2):207–239. [Google Scholar]
- 21.Einav L, Leibtag E, Nevo A. On the accuracy of Nielsen Homescan Data. Washington, DC: USDA; 2008. [Google Scholar]
- 22.StataCorp. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 23.Committee on Nutrition Standards for Foods in Schools. Nutrition Standards for Foods in Schools: Leading the Way toward Healthier Youth. Washington DC: National Academy Press; 2007. [Google Scholar]
- 24.Fuster VK, Bridget B, editors. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health. Washington DC: National Academy Press; 2010. Committee on Preventing the Global Epidemic of Cardiovascular Disease: Meeting the Challenges in Developing Countries (Institute of Medicine) [Google Scholar]
- 25.Wartella EA, Lichtenstein AH, Boon CS, editors. Examination of Front-of-Package Nutrition Rating Systems and Symbols: Phase 1 Report. Washington DC: National Academy Press; 2010. [PubMed] [Google Scholar]
- 26.Food and Nutrition Board. Preventing childhood obesity: health in the balance. Washington, DC: National Academy Press; 2004. [Google Scholar]
- 27.Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang D-H, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86(4):899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 28.Brown RJ, De Banate MA, Rother KI. Artificial sweeteners: A systematic review of metabolic effects in youth. Int J Pediatr Obes. 2010;5(4):305–312. doi: 10.3109/17477160903497027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blundell JE, Hill AJ. Paradoxical effects of an intense sweetener (aspartame) on appetite. Lancet. 1986;1(8489):1092–1093. doi: 10.1016/s0140-6736(86)91352-8. [DOI] [PubMed] [Google Scholar]
- 30.Black RM, Leiter LA, Anderson GH. Consuming aspartame with and without taste: Differential effects on appetite and food intake of young adult males. Physiol Behav. 1993;53(3):459–466. doi: 10.1016/0031-9384(93)90139-7. [DOI] [PubMed] [Google Scholar]
- 31.Tordoff M, Alleva A. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am J Clin Nutr. 1990;51(6):963–969. doi: 10.1093/ajcn/51.6.963. [DOI] [PubMed] [Google Scholar]
- 32.Anton SD, Martin CK, Han H, Coulon S, Cefalu WT, Geiselman P, et al. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite. 2010;55(1):37–43. doi: 10.1016/j.appet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holt SHA, Cobiac L, Beaumont-Smith NE, Easton K, Best DJ. Dietary habits and the perception and liking of sweetness among Australian and Malaysian students: a cross-cultural study. Food Quality Preference. 2000;11(4):299–312. [Google Scholar]
- 34.Food and Drug Administration; Department of Health and Human Services. Food labeling: added sugars. 2000. pp. 39414–39415. Federal Register: Docket No. 99P-2630. [Google Scholar]
- 35.Food and Drug Administration. [Accessed January 31, 2012];Code of Federal Regulations, - Title 21, Food Labeling 21. Code of Federal Regulations http://www.access.gpo.gov/cgi-bin/cfrassemble.cgi?title=200821.
- 36.Westrich BJ, Buzzard IM, Gatewood LC, McGovern PG. Accuracy and efficiency of estimating nutrient values in commercial food products using mathematical optimization. J Food Composition Analysis. 1994;7(4):223–239. [Google Scholar]
- 37.Westrich BJ, Altmann MA, Potthoff SJ. Minnesota's Nutrition Coordinating Center uses mathematical optimization to estimate food nutrient values. Interfaces. 1998;28(5):86–99. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.