Abstract
Since the introduction of the Orphan Drug Act in 1983, designed to promote development of treatments for rare diseases, at least 378 orphan drugs have been approved. Incentives include financial support, tax credits and, perhaps most importantly, extended market exclusivity. These incentives have encouraged industry interest and accelerated research on rare diseases, allowing patients with orphan diseases access to treatments. However, extended market exclusivity has been associated with unacceptably high drug costs; both for newly developed drugs and even for drugs which were previously widely available. We suggest that a paradoxical effect of orphan product exclusivity can be reduced patient access to existing drugs. In addition, the costs of each new drug are arguably unsustainable for patients and for the American health care system. Of all the specialties, neurology has the third highest number of orphan product designations, and neurological diseases account for at least one fifth of rare diseases. Citing the use of tetrabenazine for chorea in Huntington’s disease, adrenocorticotropic hormone for infantile spasms and enzyme replacement therapy with alglucosidase alpha for Pompe’s disease we highlight these paradoxical effects.
Background
The 1983 U.S. Orphan Drug Act (ODA) was designed to promote the development of drugs that demonstrate promise for the diagnosis, prevention or treatment of rare diseases1. The definition of what constitutes a rare disease varies by country but includes any disease that affects less than 200,000 individuals in the United States2. There are currently approximately 7000 rare diseases listed on the National Instititute of Health’s (NIH) Office of Rare Diseases website, affecting an estimated 25–30 million people within the United States3, most of these rare diseases are genetic and many are neurological. Approximately 250 new rare diseases are described each year4, and with advances in genetics many more common disorders will be subdivided into genetically distinct “rare” diseases.
The 1962 Kefauver-Harris amendment to the Federal Food, Drug and Cosmetic Act mandated that all drugs must be demonstrated to be safe and effective by adequate, well-controlled studies prior to receiving marketing approval5. This amendment was prompted by the thalidomide-induced birth defects in the 1950s and 1960s. Although the amendment aimed to protect the public, it increased the cost of drug development. As a result, the pharmaceutical industry focused on large target populations, thus “orphaning” individuals with rare diseases. In an attempt to encourage pharmaceutical companies to develop orphan drugs the ODA was signed into law by President Reagan in 1983, followed by similar legislation in Japan, Australia and Europe6. ODA incentives exist to encourage sponsors. In the United States, tax incentives include a 50% tax credit for development costs, with a 20 year carry forward and one year retrospective arrangement7. In addition, Food and Drug Administration (FDA) fees for review of the application for marketing are waived ($1.2 million in 2009)8. Federal grants are available for academic investigators and companies studying orphan drugs under the FDA orphan product designation (OPD) grants program; approximately $14 million is awarded among 10–15 new grants annually9. Probably the biggest incentive for sponsors is the 7-year market exclusivity awarded from the date of FDA approval. During this period the FDA cannot approve a new or generic drug application for the same product for the same indication.
The ODA succeeded in encouraging sponsors in developing drugs for rare diseases. In the decade prior to the ODA, only 10 drugs were approved for rare diseases. In the 28 years since 1983, 367 drugs have been approved10: a twelve-fold increase. Forty five of the orphan drugs approved have resulted from the Orphan Grants Program (out of over 500 studies funded). However, there is debate as to whether this increase came about purely as a result of the ODA, described by some as “one of the most successful US legislative actions in recent history”11. Over the same time period there has been a major increase in public and private investment in pharmaceutical research and development; major advances in science and technology have occurred. Other US legislation incentivized the pharmaceutical industry by increasing patent times and market exclusivity12, and small businesses were encouraged with the use of NIH funded grants13. Changes to the FDA review procedures resulted in reduced times to drug approval and prevented distribution of unapproved drugs. In addition, improved patient education, growth of patient advocacy groups and the widespread use of the internet have resulted in an increased demand for orphan drug development.
The U.S. government and Congress maintain an active interest in promoting development of orphan products. The recent Patient Protection and Affordable Care Act imposed an annual fee (based on sales) on any company manufacturing or importing branded prescription drugs14 The Preserving Access to Orphan Drugs Act of 2011 is currently referred to the Subcommittee on Health by Congress to exclude orphan product sales from the calculation of this fee15. A further Medical Innovation Prize Act has been referred to Senate committee by Congress; this Act establishes a fund (funded by health insurers) to reward research and development of drugs, biologics or manufacturing processes, specifically mentioning orphan diseases16.
In this article, we highlight issues of emerging concern to neurologists, using the examples of tetrabenazine, adrenocorticotropic hormone (ACTH) and Myozyme/Lumizyme to illustrate our concerns.
Tetrabenazine
Tetrabenazine has been used for decades in the United Kingdom17 and many other countries18 for the treatment of chorea associated with Huntington’s disease; however, until 2008 no medication had been approved for this indication in the United States. As it was not available in the United States, patients were able to buy it from abroad with a prescription from their doctor under the FDA Personal Use Import Policy19, 20, ; although they had to pay the full cost themselves. Once the FDA approved tetrabenazine as an orphan drug in August 2008, the drug could not legally be imported from other countries21. The cost per month for the initial dose of 25mg/day is estimated at $2,055 in the United States22, the same dose is available in the United Kingdom for approximately $4323. Patients with Huntington’s disease generally require 37.5 to 50 mg per day for the desired anti-choreic effect.
ACTH
ACTH is a first-line treatment for infantile spasms24, used since the 1950s in many countries including the United States; however, it was not FDA approved for this indication. In 2007, the price for one vial increased from $1,650 to $23,000, in what the company described as an “orphan-style pricing model”25. In October 2010, the FDA approved H. P. ActharGel (Questcor Pharmaceuticals) as monotherapy for infantile spasms in children under 2 years, precipitating a steep rise in Questcor’s share price26. The cost per vial in the United Kingdom is under $523.
Myozyme/Lumizyme
Genzyme is the most active of the top 10 biopharmaceutical companies. Genzyme has obtained 35 orphan drug designations27 and at least 5 Genzyme biologic agents have been approved for treating rare neurological diseases including Myozyme and Lumizyme for Pompe’s disease, Cerezyme and Ceredase for Gaucher’s disease, Fabrazyme for Fabry’s disease, and Aldurazyme for mucopolysaccharidosis I28.
Pompe’s disease is a rare autosomal recessive metabolic myopathy caused by a deficiency of acid alpha glucosidase (GAA), an enzyme that degrades lysosomal glycogen. While infantile-onset Pompe’s disease presents with hypotonia, weakness, and heart failure resulting in death before age 2 years, the late-onset form has extreme variation in disease phenotype making it is more difficult to predict the prognosis even within the same family29. For example, the age at onset ranges from 1 to 78 years and clinical presentation also varies from fatigue or muscle soreness to severe limb-girdle weakness with or without respiratory insufficiency30. In 2006, the FDA approved alglucosidase alfa (Myozyme) as an enzyme replacement therapy for infantile-onset Pompe’s disease based on a study which used historical controls31. In 2008, Myozyme was one of the most commercially profitable drugs with product sales of $296 million27. In 2009, Genzyme had manufacturing problems due to particle contamination which caused severe shortages of several biologic agents. As a result, the company had to turn over $175 million of unlawful profits to the FDA32. Lumizyme, alglucosidase alfa, was subsequently approved by the FDA in May 2010 as the first specific treatment for late-onset Pompe’s disease in the United States based on the result of a randomized, double-blind, placebo-controlled study of alglucosidase alfa33. Genzyme shares rose nearly 5 percent after Lumizyme was approved by the FDA. Due to the quality-control problems at the plant in Allston Landing, Myozyme (U.S. manufactured) has been reserved primarily for infantile-onset Pompe’s disease while Lumizyme is produced and shipped from the company’s plant in Belgium exclusively for patients with late-onset disease. It is estimated that combined sales of Myozyme and Lumizyme will reach $1- $1.5 billion annually34. Currently, the average wholesale price for Lumizyme 50 mg per single-use vial is approximately $84035. Cost of therapy is dependent upon the patient’s weight. For a 70 kg individual, the cost would be $23,520 per dose with an annual cost of $564,480 per patient.
Table 1 demonstrates the difference in price of tetrabenazine, ACTH, and alglucosidase alfa between the United States and United Kingdom.
Table 1.
Price comparison between U.S. and U.K.
Differences in review procedure of orphan drug approvals
Several issues that have arisen, some played out in the media rather than in the medical literature. First, orphan drugs have been demonstrated to have shorter development time than non-orphan drugs, despite no official difference in FDA requirements7. The FDA, however, has noted that clinical testing may not be as extensive as for non-orphan drugs36. Because most orphan drugs are approved for the treatment of serious or life-threatening diseases they are more likely to be approved using Fast Track review procedures. A review of orphan drugs approved by the European Medicines Agency found methodological limitations in the data submitted, including incomplete toxicological data, absence of repeated-dose toxicity studies in two animal species, lack of randomized controlled trials or use of placebo rather than an active comparison drug37. In addition, approval was granted for some drugs based only on uncontrolled phase II studies, the number of patients enrolled was typically small and the trials often very short. Similarly, in the United States Mitsumoto et al. found that only 32% of orphan drugs approved by the FDA met the standard of two randomized double-blind trials, many were not blinded, not randomized or not compared with a control38. A frequent weakness of orphan drug clinical trials is the small sample size, which while understandable, makes it difficult to detect adverse events that are severe but infrequent. Thirteen percent of orphan drugs caused more adverse events after approval than had been anticipated36; in addition, several products discontinued due to safety concerns (e.g., Vioxx) have been rejuvenated and approved as orphan drugs27.
Unnecessarily high cost of orphan drugs
The high cost of orphan drugs has been defended by pharmaceutical companies as necessary in order to recoup development costs39. However, this argument cannot hold for the many orphan drugs that were previously readily and cheaply available in generic form, such as colchicine. In the case of colchicine, following FDA approval for treatment of acute gout, a common disease, and familial Mediterranean fever (FMF), a rare disease in 200940, the price leapt from 9 cents to almost $5 per tablet for an expensive brand-name formulation (Colcrys). Given that the pharmaceutical company receives 7-year market exclusivity for its use as an orphan drug for FMF, the company took legal action to prevent any of the other manufacturers of colchicine from continuing to produce or market their products. The cost to state Medicaid programs of this change alone is estimated to increase from $1 million to $50 million per year41. Since cost has increased, adherence has decreased particularly in among economically disadvantaged populations as discussed in several media and patients forums42–44. This causes more suffering in patients with FMF who require daily colchicine for life to avoid the acute attacks of fever and pain and the life-threatening complication of renal amyloidosis45 than patients with gout.
Although orphan drugs were assumed initially to have limited potential for profit, 43 blockbuster drugs (annual sales over $1 billion) have orphan designations. Many orphan blockbusters have achieved blockbuster status within the 7 year market exclusivity period27. This high cost is a particular concern to neurologists. Neurology has the third highest number of orphan designations (after oncology and infectious diseases) with approximately 200 orphan designations6, 27. Of the 43 “blockbuster” orphan drugs, 12 have primary indications for neurological disorders and two (rituximab and mycophenolate mofetil) are used frequently by neurologists although the primary indication is not for a neurological indication (Table 2). Of the 16 individual orphan diseases with the most orphan drug approvals, two are neurological; multiple sclerosis and gliomas, considered relatively common diseases by neurologists, have 42 designations and 9 approvals between them8. The office of Rare Diseases Research at the NIH includes approximately 7,000 conditions in the list of rare diseases; at least one fifth are neurological conditions46. When one considers that many of the neurological diseases listed are in fact multiple genetically distinct conditions, it becomes clear that the potential for growth in orphan drugs for neurological disease is enormous.
Table 2.
Blockbuster drugs that have obtained orphan drug designation27. The highlighting drugs are primarily designated or frequently used for neurological conditions.
| Brand Name | Generic Name | Main Indications | Designation (Year) | Global Salesa 2010 (Million) |
|---|---|---|---|---|
| Humira | Adalimumab | Inflammatory bowel diseases, Juvenile rheumatoid arthritis | 2005* | 6,548 |
| Fosamax b | Alendronate | Osteogenesis Imperfecta | 2001 | - |
| Ceredase# | Alglucerase | Type I, II, III Gaucher’s disease | 1985* | 720 |
| Abilify | Aripiprazole | Tourette’s syndrome | 2006 | 2,565 |
| Avastin | Bevacizumab | Chemotherapy for cancers | 2003 | 7,178 |
| Velcade | Bortezomib | Multiple myeloma, lymphomas | 2003* | - |
| Tracleer | Bosentan | Pulmonary artery hypertension, Idiopathic pulmonary fibrosis | 2000* | - |
| Botox | Botulinum toxin | Cerebral palsy, Dystonia | 1984* | 1,414 |
| Novoseven | Factor VIIa | Hemophilia, Factor VII deficiency, Intracranial hemorrphage | 1988* | 1,483 |
| Epogen | Epoetin alfa | Anemia related to HIV infection,, Anemia in end stage renal disease, | 1986* | 2,524 |
| Procrit | Epoetin alfa | Anemia related to HIV infection,, Anemia in end stage renal disease, | 1987 | 1,934 |
| Enbrel | Etanercept | Juvenile rheumatoid arthritis, Wegener’s granulomatosis | 1998* | 7,287 |
| Neupogen | Filgrastim | Neutropenia in various conditions | 1990* | 1,286 |
| Neurontin b | Gabapentin | Treatment of ALS | 1995 | 823 |
| Copaxone | Glatiramer | Multiple sclerosis | 1987* | 3,300 |
| Gleevec | Imatinib | Acute and chronic leukemia | 2001* | 4,265 |
| Cerezyme# | Imiglucerase | Type I, II, III Gaucher’s disease | 1991* | 720 |
| Remicade | Infliximab | Inflammatory bowel diseases, | 1995* | 6,565 |
| Betaseron | IFN beta-1b | Multiple sclerosis | 1988* | 1,658 |
| Avonex | IFN beta-1a | Multiple sclerosis | 1991* | 2,518 |
| Rebif | IFN beta-1a | Multiple sclerosis | 1992 | 2,297 |
| Lamictal b | Lamotrigine | Lennox-Gastaut Sundrome | 1995* | 671 |
| Revlimid | Lenalidomide | Multiple myeloma, Lymphoma, Leukemia | 2001* | - |
| Lupron | Leuprolide | Precocious puberty | 1988* | 1,617 |
| Mobic | Meloxicam | Juvenile rheumatoid arthritis | 2002* | |
| Provigil b | Modafinil | Narcolepsy | 1993* | 999 |
| CellCept | Mycophenolate | Pemphigus vulgaris, MG | 2006 | 1,433 |
| Sandostatin | Octreotide | Acromegaly | 1998* | 1,291 |
| Kogenate | Octocog | Hemophilia | 1989* | 1,380 |
| Taxol | Paclitaxel | Kaposi’s sarcoma | 1997* | - |
| Pegasys | IFN alfa-2a | Renal cell carcinoma, CML | 1998 | 1,828 |
| Alimta | Pemetrexed | Malignant mesothelioma | 2001* | - |
| Mirapex b | Pramipexole | Tourette’s syndrome | 2008 | - |
| Evista | Raloxifene | Postmenopausal breast cancer | 2005* | - |
| Rituxan | Rituximab | NHL, chronic leukemia, vasculitis | 1994* | 7,061 |
| Vioxx | Rofecoxib | Juvenile rheumatoid arthritis | 2004 | - |
| Prograf | Tacrolimus | Graft-versus-host-disease | 1998* | - |
| Cialis | Tadalafil | Pulmonary artery hypertension | 2006 | 1,699 |
| Temodar | Temozolomide | Malignant glioma, Metastatin melanoma | 1998* | 1,065 |
| Spiriva | Tiotropium | Cystic fibrosis (conjuctive therapy) | 2008 | 3,935 |
| Topamax b | Topiramate | Lennox-Gastaut Sundrome | 1992* | - |
| Herceptin | Trastuzumab | Pancreatic cancer | 1999 | 6,032 |
| Zometa | Zoledronic acid | Tumor induced hypercalcemia | 2000* | 1,511 |
Sources: FDA, Pipeline Review, Drug Topics, and Pharmacy Times
Numbers represent million dollars
Generic formulation is now available
Received orphan approval
Similar products
ALS = Amyotrophic Lateral Sclerosis, CML = Chronic Myeloid Leukemia, HIV = Human Immunodeficiency Virus, IFN = Interferon, MG = Myasthenia Gravis, NHL = Non-Hodgkin’s lymphoma.
Costs are unsustainable for patients and for the nation
As new treatments for orphan diseases emerge the cost of orphan drugs may become unsustainable. In the United States up to 80% of the prescription cost is covered by insurance, leaving the patient responsible for the remaining 20% or more. With many orphan drugs costing thousands of dollars a month, this is unaffordable for the average individual. A review by Goldman et al. demonstrated that as the cost of medication to the individual increased, adherence reduced. In addition, hospitalizations increased and patients with chronic medical conditions sought more expensive inpatient and emergency health care47. Most neurological diseases are chronic and require long-term medication, making the high cost of orphan drugs of particular concern to neurologists. The high cost of these drugs within the United States has led to patients buying orphan drugs from abroad at a fraction of the U.S. cost. In some cases, these are drugs which are FDA-approved, have been manufactured by a U.S.-based company, exported and sold elsewhere cheaper. However, it is illegal for anyone but the manufacturer to re-import exported drugs, even if they comply with all FDA regulations48. While treatments for rare diseases are not the only costly treatments, these costs are unsustainable for patients and for the nation. Patients end up paying in multiple ways for the same drug if they pay taxes, purchase health insurance as well as paying part of the cost themselves. Moreover, the costs of Federal programs and of insurance premiums will continue to increase as more orphan drugs are approved.
Issues particular to biologics
Biologics, more complex or larger molecules that are made from living cells and tissues, also illustrate the paradoxical effects of the ODA on drug availability and sustainability. The incentives of biologic innovation have become exceedingly attractive after Congress granted 12-year market exclusivity for a new biologic agent before “biosimilar” products could be approved. Moreover, manufacturers can secure additional 12-year market exclusivity by making minor changes to an approved product despite expiration of its patent. These changes include anything from a minor change in the structure or alteration in the administration schedule49.
Among the “blockbuster drugs” which have obtained orphan drug designation,50 biologics remain one of the fastest-growing groups in the market. Their costs are invariably high, reaching over $400,000 per patient per year27. Unlike small molecule drugs, biologics are much more attractive to industry given the longer market exclusivity and the difficulty of making “biosimilars” or generic versions. Biopharmaceutical companies argue that the high cost and long period of protection from competition are reasonable because the costs of biologic innovation are much higher and biologics take longer to develop than small molecule drugs. Until recently the pathway of developing “biosimilars” was no quicker or less expensive than obtaining a full Biologic Licensing Application as the FDA required the company to conduct their own clinical trials for safety and efficacy data of the biosimilar compared to the innovator’s product49. However, President Obama signed into law The Biologics Price Competition and Innovation Act in March 2010, to reduce the time required and prevent unnecessary duplication of clinical trials. Another issue is that biologic agents in the United States are considered “tier 4” medications which indicate that the patient is responsible for at least 20% of prescription costs. Conversely, many of the countries in the European Union provide universal coverage or require only a small co-payment for prescribed medications51. The effects of the ODA on the availability and affordability of biologics clearly demonstrate the major tension which exists between encouraging innovation by industry and ensuring access of drugs to patients.
The authors of this paper are involved in clinical trials in rare diseases. If successful, these trials will result in increasing numbers of orphan drugs, having the paradoxical effect of increasing costs to patients thereby reducing compliance with medications; increasing long-term morbidity from the very diseases we wish to treat.
Future Directions
Growth of the orphan drug market
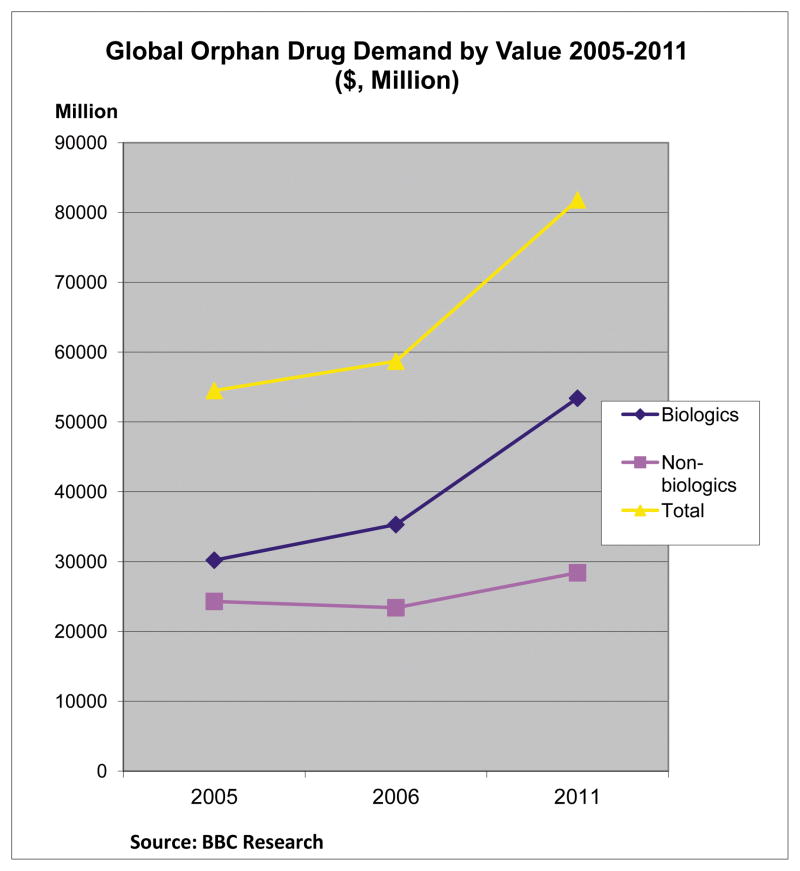
Although the magnitude of the impact of the high costs of orphan drugs is probably relatively small as the overall drug budget accounted for approximately 1% of total drug spending in 200752, 53, the growth in this market has been enormous since the ODA was passed 30 years ago. The global orphan drugs market increased from $54.5 in 2005 to $58.7 billion in 2006 and it reached $81.8 billion in 201154 (Fig 1). Currently, biologic drugs account for a major share of the orphan drug market (65%). The orphan drug market is expected to grow at a compound annual growth rate of nearly 6% to reach $112.1 billion by 201455. Likewise, the growth in global research and development into orphan drugs increased from $305 million to $1.9 billion between 2000 and 200856 and it is expected that the proportion of hospital drug costs accounted for by orphan drugs will double over the next 5-year period.57 With the increasing budget pressure from orphan products, it is likely that the orphan drug costs will increase substantially and U.S. insurers may increasingly shift costs directly to patients58; potentially reducing patient access to such drugs.
Fig 1.
The future development of orphan drugs for neurological diseases is likely to expand despite the price of orphan drugs and the accessibility of the drugs. This expansion will be due in part to 1) the identification of more genetic orphan diseases, 2) the commercial success of the market, particularly biologic drugs, 3) the fragmentation of previously large markets to become multiple distinct orphan diseases e.g. gabapentin received orphan approval for treatment of post-herpetic neuralgia even though it has been widely used in other causes of neuropathic pain, and 4) the identification of sub-populations of larger neurological disorders, for example, the average wholesale price for 20 mg daily of Palmyra (dalfampridine), a sustained release form of 4-Aminopyridine (4-AP), is approximately $12,850 per year59 since it received an approved orphan indication to improve walking in multiple sclerosis while the generic short acting 4-AP costs less than $1,000 per year60.
Addressing the high cost of orphan drugs
The basic dilemma for policy makers is where to draw the line between allocating a substantial share of limited funds to a very small number of individuals (to ensure equal opportunity) and abandoning individual patients affected by a serious but rare disease (to enable treatment of a greater number of individuals)50, 61–63. To address this, Pinxten et al suggest a combined ethical framework of 1) budgetary insulation; 2) prioritizing allocation of resources; and 3) random allocation to those individuals who do not meet rational criteria50. Universally, however, resources are limited; thus, the higher the cost of an orphan drug for an individual patient, the fewer patients will be able to avail themselves of the drug.
Several suggestions have been proposed to address these issues. Under President Bush, attempts to remove orphan drug status from products whose sales exceeded $200 million or which affected more than 200,000 individuals were vetoed. The United States remains one of the few countries that do not regulate prescription drug prices27. The effects of this can be seen from the hugely disparate prices of some of the examples mentioned earlier between the United States and the United Kingdom (Table 1). Another possible mechanism is to reduce market exclusivity for drugs which have previously approved indications, such as colchicine, or to reduce extended market exclusivity for products once they become overly profitable or their use expands, such as occurs in Europe. Even though Europe and other countries have even longer periods of market exclusivity than the United States (ten years), prices charged for drugs are much less and access is more equitable, due in part to price regulation. In Europe, if a product is sufficiently profitable, market exclusivity can be reduced to six years. This has not occurred to date partly due to fears that it may reduce investment incentives64. In other countries (e.g., Japan), once companies reach a certain profit margin, they are required to pay extra tax on that profit until the initial subsidy is repaid65. The difficulty with these solutions is that they may act as disincentives to drug companies.
Differential pricing (also known as price discrimination) has been suggested as a solution to make global medicines affordable in developing countries66; charging high prices in affluent nations to generate sufficient revenue, while charging lower costs in developing countries. This solution is at odds with the concept of free trade as it requires separation of markets to maintain the price differential. While this may be an acceptable solution for diseases endemic in the developing world, it seems less likely to solve the universal problem of the high cost of orphan drugs. In practice, despite differential pricing, drug costs in developing countries remain high relative to income so drugs remain unaffordable66. In addition, Danzon et al demonstrated that in affluent countries, insurance coverage makes consumers relatively insensitive to price, leading to unrestrained pricing67. The high costs of orphan drugs, meanwhile, predominantly affect developed countries due to differing priorities and a lack of opportunities such as screening programs or genetic testing in developing nations68, 69. Another suggestion is that orphan drug pricing contravenes competition law64; however, this would be a difficult and contentious route to a solution and would likely result in withdrawal of companies from the orphan drug market with subsequent consequences for patients.
A common solution to the rising price of medical technology is to have a separate review process. In Europe, there is extensive intervention from government in orphan drug markets70. In the United Kingdom, there is an independent organization, the National Institute for Health and Clinical Excellence (NICE; http://www.nice.org.uk). Part of its remit is to publish guidance based on the best evidence to weigh up the costs and benefits of treatments. NICE bases its decisions on (QALY) gained by a specific treatment; generally if a treatment costs more than approximately £30,000 per QALY, it is not considered cost effective. When drugs are marketed with a higher cost per QALY, in some instances NICE has entered a cost-sharing or risk-sharing agreement with the company marketing the drug71. For rare diseases the National Specialist Commissioning Advisory Group (NSCAG) advise the Department of Health and commission services on a national level; so far this has allowed prescription of enzyme replacement therapy through specialist centers61. Hence, alglucosidase alpha currently costs as much in the United Kingdom as in the United States. While NICE and NSCAG are far from perfect72, an independent body may be a useful way of attempting to reign in the spiraling costs of orphan drugs in the United States.
Conclusion
Although the ODA is recognized as one of the most successful legislations to promote pharmaceutical and biological innovation, the paradoxical effects on drug availability and unsustainable costs remain major issues that demand cautious legislative reform. The ODA should be designed primarily to benefit patients with rare diseases. Although it is necessary to promote pharmaceutical innovation and maintain reasonable profit for the investing companies, this must not be to the detriment of long-term public health and the U.S. economy.
Footnotes
Potential Conflicts of Interest:
Dr. Murphy is the recipient of a Post Doctoral training fellowship from the Inherited Neuropathy Consortium Rare Disease Clinical Research Consortium supported by the NINDS/ORD (1U54NS065712-01).
Dr. Puwanant has received funding support from the NIH (MDCRC 2 U54NS048843-08 [PI: Richard T. Moxley, III], and CINCH 2 U54 NS059065-07 [PI: Robert C. Griggs], and the Muscular Dystrophy Association: Clinical Research Training Grant.
Dr. Griggs serves as Chair of Executive Committee of the Muscle Study Group, which receives support from pharmaceutical companies; has served on scientific advisory boards for The National Hospital Queen Square and PTC Therapeutics, Inc.; serves on the editorial boards of NeuroTherapeutics and Current Treatment Opinions in Neurology; is Correspondence Editor for Neurology; receives royalties from the publication of Andreoli and Carpenter’s Cecil Essentials of Medicine (W.B. Saunders Company, 2000, 2004, 2007, and 2010) and Cecil Textbook of Medicine (Saunders, 2000, 2004, 2008, and 2010 [in press]); and has received research support from TaroPharma and support from the NIH (NINDS T32 NS07338 [PI and preceptor], 2 U54 NS059065-06 [PI], R01 NS045686-05 [PI], and NINDS 525326 [Co-PI]), the Food and Drug Administration. Dr. Griggs holds Orphan Product Designation for mexilitine for nondystrophic myotonia and deflazacort for Duchenne muscular dystrophy.
References
- 1.Department of Health and Human Services: Office of Inspector General. [Accessed February 14, 2011.];The Ophan Drug Act: Implementation and Impact. 2001 May; Available at: http://oig.hhs.gov/oei/reports/oei-09-00-00380.pdf.
- 2.Griggs RC, Batshaw M, Dunkle M, et al. Clinical research for rare disease: opportunities, challenges, and solutions. Mol Genet Metab. 2009;96:20–26. doi: 10.1016/j.ymgme.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institutes of Health: Office of Rare Diseases. [Accessed February 4, 2011.]; Available at: http://rarediseases.info.nih.gov/Resources/Rare_Diseases_Information.aspx.
- 4.Wastfelt M, Fadeel B, Henter JI. A journey of hope: lessons learned from studies on rare diseases and orphan drugs. J Intern Med. 2006;260:1–10. doi: 10.1111/j.1365-2796.2006.01666.x. [DOI] [PubMed] [Google Scholar]
- 5.Krantz JC., Jr New drugs and the Kefauver-Harris amendment. J New Drugs. 1966;6:77–79. [PubMed] [Google Scholar]
- 6.Field MJ, Boat TF. Rare diseases and orphan product. 1st. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 7.Seoane-Vazquez E, Rodriguez-Monguio R, Szeinbach SL, Visaria J. Incentives for orphan drug research and development in the United States. Orphanet J Rare Dis. 2008;3:33. doi: 10.1186/1750-1172-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun MM, Farag-El-Massah S, Xu K, Cote TR. Emergence of orphan drugs in the United States: a quantitative assessment of the first 25 years. Nat Rev Drug Discov. 2010 Jul;9:519–522. doi: 10.1038/nrd3160. [DOI] [PubMed] [Google Scholar]
- 9.United States Food and Drug Administration. [Accessed February 14, 2011.];Information on the Orphan Products Grants Program. 2010 May; Available at: http://www.fda.gov/ForIndustry/DevelopingProductsforRareDiseasesConditions/WhomtoContactaboutOrphanProductDevelopment/ucm134580.htm.
- 10.United States Food and Drug Administration. [Accessed February 12, 2011.];Search Orphan Drug Designations and Approvals. Available at: http://www.accessdata.fda.gov/scripts/opdlisting/oopd/index.cfm.
- 11.Haffner ME, Whitley J, Moses M. Two decades of orphan product development. Nat Rev Drug Discov. 2002;1:821–825. doi: 10.1038/nrd919. [DOI] [PubMed] [Google Scholar]
- 12.Kesselheim AS. Using market-exclusivity incentives to promote pharmaceutical innovation. N Engl J Med. 2010;363:1855–1862. doi: 10.1056/NEJMhle1002961. [DOI] [PubMed] [Google Scholar]
- 13.United States Department of Health & Human Services: Office of Extramural Research. [Assessed February 12, 2011.];Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) Programs. Available at: http://grants.nih.gov/grants/funding/sbirsttr_programs.htm.
- 14. [Accessed on October 12, 2011.];The Patient Protection and Affordable Care Act. Public Law 111-148, 111th Congress, H.R. 3590. 2010 Mar 23; Available at: http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdf.
- 15. [Accessed October 12, 2011.];Preserving Access to Orphan Drugs Act of 2011. H.R. 2672, 112th Congress. Available at: http://thomas.loc.gov/
- 16. [Accessed October 12, 2011.];Medical Innovation Prize Act, S. 1137, 112th Congress. Available at: http://thomas.loc.gov/cgi-bin/query/z?c112:S.1137.IS.
- 17.Dalby MA. Effect of tetrabenazine on extrapyramidal movement disorders. Br Med J. 1969;2:422–423. doi: 10.1136/bmj.2.5654.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology. 2006;66:366–372. doi: 10.1212/01.wnl.0000198586.85250.13. [DOI] [PubMed] [Google Scholar]
- 19.Pollock Daniel L. Blame Canada (and the rest of the world): the twenty-year war on imported prescription drugs. [Accessed October 12, 2011.];Bepress legal series 2005. :806. Available at: http://law.bepress.com/cgi/viewcontent.cgi?article=4130&context=expresso.
- 20. [Accessed October 12, 2011.];FDA Personal Use Import Policy. Available at: http://www.ceri.com/import.htm.
- 21.United States Food and Drug Administration. [Accessed July 28, 2011.];Xenazine NDA approval. 2008 Aug; Available at: http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2008/021894s000ltr.pdf.
- 22.Fallon Community Health Plan Department of Pharmacy Services. [Accessed March 1, 2011.];Prior Authorization Approval Criteria Xenazine (tetrabenazine) Available http://www.fchp.org/~/media/Files/FCHP/Imported/Xenazine_tetrabenazine.pdf.ashx.
- 23.British National Formulary. 61. London: BMJ Group and RPS Publishing; 2011. [Google Scholar]
- 24.Baram TZ, Mitchell WG, Tournay A, Snead OC, Hanson RA, Horton EJ. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics. 1996;97:375–379. [PMC free article] [PubMed] [Google Scholar]
- 25.Watsan R. [Accessed February 10, 2011.];Epilepsycom/Professionals: Articles and Publications. 2007 Sep; Available at: http://professionalsepilepsycom/page/ar_1189197304html.
- 26.Investorguide.com. [Accessed February 6, 2011.];Investing. Questcor Pharmaceuticals Launches Acthar for the Treatment of Infantile Spasms. 2010 Nov; Available at: http://www.investorguide.com/stock-news-show.php?story_id=35337060&topic=QCOR.
- 27.Wellman-Labadie O, Zhou Y. The US Orphan Drug Act: rare disease research stimulator or commercial opportunity? Health Policy. 2010;95:216–228. doi: 10.1016/j.healthpol.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 28. [Accessed February 10, 2011.];Genzyme Cooperation: Product Lists. Available at: http://www.genzyme.com/business/biz_home.asp.
- 29.Wagner KR. Enzyme replacement for infantile Pompe disease: the first step toward a cure. Neurology. 2007 Jan 9;68(2):88–9. doi: 10.1212/01.wnl.0000253226.13795.40. [DOI] [PubMed] [Google Scholar]
- 30.Van der Beek NA, Hagemans ML, Reuser AJ, et al. Rate of disease progression during long-term follow-up of patients with late-onset Pompe disease. Neuromuscul Disord. 2009;19:113–117. doi: 10.1016/j.nmd.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Kishnani PS, Corzo D, Nicolino M, et al. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- 32.United States Food and Drug Administration. Press Announcements. [Accessed February 4, 2011.];FDA News Release. 2010 May; Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm213212.htm.
- 33.van der Ploeg AT, Clemens PR, Corzo D, et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med. 2010;362:1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- 34.Clark T, Berkrot B. [Accessed February 8, 2011.];Reuters: Update 2-Genzyme wins FDA approval for Pompe drug. 2010 May; Available at: http://www.reuters.com/article/2010/05/25/genzyme-idUSN2513053720100525?pageNumber=1.
- 35. [Accessed April 10, 2011.];CVS Caremark: Specialty TrendsRx® Alert. Available at: https://www.caremark.com/portal/asset/SpecialtyTrendsRxAlert_Lumizyme.pdf.
- 36.Scharf SF. Orphan drugs: the question of products liability. Am J Law Med. 1985;10:491–513. [PubMed] [Google Scholar]
- 37.Joppi R, Bertele V, Garattini S. Orphan drug development is progressing too slowly. Br J Clin Pharmacol. 2006;61:355–360. doi: 10.1111/j.1365-2125.2006.02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitsumoto J, Dorsey ER, Beck CA, Kieburtz K, Griggs RC. Pivotal studies of orphan drugs approved for neurological diseases. Ann Neurol. 2009;66:184–190. doi: 10.1002/ana.21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wheadon DE. Market exclusivity for biologics. N Engl J Med. 2010;362:661. doi: 10.1056/NEJMc0912463. author reply -2. [DOI] [PubMed] [Google Scholar]
- 40.United States Food and Drug Administration. [Accessed February 12, 2011.];FDA Approves Colchicine for Acute Gout, Mediterranean Fever. 2009 Jul; Available at. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm174620.htm.
- 41.Kesselheim AS, Solomon DH. Incentives for drug development--the curious case of colchicine. N Engl J Med. 2010;362:2045–2047. doi: 10.1056/NEJMp1003126. [DOI] [PubMed] [Google Scholar]
- 42.Lowe Derek. [Accessed October 12, 2011.];In the Pipeline: Colchicine’s Price Goes Through the Roof. Available at: http://pipeline.corante.com/archives/2010/04/14/colchicines_price_goes_through_the_roof.php.
- 43.CBS News. [Accessed March 1, 2011.];FDA approval of ancient remedy sends price soaring. Available at: http://www.cbsnews.com/2100-18563_162-20118283.html.
- 44.Arthritis Today. [Accessed March 1, 2011.];The Price of Gout Drug, Colchicine, Goes Up. Available at: http://www.arthritistoday.org/news/colchicine-gout-drug-price053.php.
- 45.Grody WW, Getzug T. Colchicine’s other indication--effect of FDA action. N Engl J Med. 2010;363:2267–2268. doi: 10.1056/NEJMc1009918. [DOI] [PubMed] [Google Scholar]
- 46.National Institutes of Health: Office of Rare Diseases Research. [Accessed July 28, 2011.];Rare Diseases and Related Terms. Available at: http://rarediseases.info.nih.gov/RareDiseaseList.aspx?PageID=1.
- 47.Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007;298:61–69. doi: 10.1001/jama.298.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.United States Food and Drug Administration. Drug Safety and Availability. Hawaii: Governor Linda Lingle; Sep, 2009. [Accessed February 12, 2011.]. http://www.fda.gov/Drugs/DrugSafety/ucm179204.htm. [Google Scholar]
- 49.Engelberg AB, Kesselheim AS, Avorn J. Balancing innovation, access, and profits--market exclusivity for biologics. N Engl J Med. 2009;361:1917–1919. doi: 10.1056/NEJMp0908496. [DOI] [PubMed] [Google Scholar]
- 50.Pinxten W, Denier Y, Dooms M, Cassiman JJ, Dierickx K. A fair share for the orphans: ethical guidelines for a fair distribution of resources within the bounds of the 10-year-old European Orphan Drug Regulation. J Med Ethics. 2012;38:148–153. doi: 10.1136/medethics-2011-100094. [DOI] [PubMed] [Google Scholar]
- 51.Lee TH, Emanuel EJ. Tier 4 drugs and the fraying of the social compact. N Engl J Med. 2008;359:333–335. doi: 10.1056/NEJMp0804261. [DOI] [PubMed] [Google Scholar]
- 52.IMS Consulting. Orphan Drugs: rarity no garantee of access. [Accessed October 12, 2011.];Pricing & Market Access Review. 2006 Available at: http://www.onlymedics.com/documents/download-medics-KsCzr.pdf.
- 53.Orofino J, Soto J, Casado MA, Oyaguez I. Global spending on orphan drugs in France, Germany, the UK, Italy and Spain during 2007. Appl Health Econ Health Policy. 2010;8:301–315. doi: 10.2165/11531880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 54. [Accessed March 30, 2011.];BCC research: Global market for orphan drugs. Available at: http://www.bccresearch.com/report/orphan-drugs-market-phm038b.html.
- 55. [Accessed March 31, 2011.];BCC research: Global markets for orphan drugs. Available at: http://www.bccresearch.com/report/orphan-drugs-market-phm038c.html?tab=scope&highlightKeyword=orphan+drugs+sales+2008.
- 56.Orphan Drugs. Seven Days: The week in science. [Accessed Octoberf 11, 2011.];Nature. 2010 doi: 10.1038/468604a. Available at: http://www.nature.com/news/2010/101201/pdf/468604a.pdf. [DOI]
- 57.Denis A, Mergaert L, Fostier C, Cleemput I, Simoens S. Budget impact analysis of orphan drugs in Belgium: estimates from 2008 to 2013. J Med Econ. 2010;13:295–301. doi: 10.3111/13696998.2010.491427. [DOI] [PubMed] [Google Scholar]
- 58.Roy JN, Barama A, Poirier C, Vinet B, Roger M. Cyp3A4, Cyp3A5, and MDR-1 genetic influences on tacrolimus pharmacokinetics in renal transplant recipients. Pharmacogenet Genomics. 2006;16:659–665. doi: 10.1097/01.fpc.0000220571.20961.dd. [DOI] [PubMed] [Google Scholar]
- 59.CBS News. [Accessed March 31, 2011. .];Acorda’s MS Drug Ampyra Looks Good Now, but That Probably Won’t Last. Available at: http://www.cbsnews.com/8301-505123_162-43240646/acordas-ms-drug-ampyra-looks-good-now-but-that-probably-wont-last/
- 60. [Accessed March 31, 2011.];Multiple Sclerosis: 4-Aminopyridine. Available at: http://www.thecompounder.com/alternative-treatments/multiple-sclerosis/4-aminopyridine.
- 61.McCabe C, Claxton K, Tsuchiya A. Orphan drugs and the NHS: should we value rarity? BMJ. 2005;331:1016–1019. doi: 10.1136/bmj.331.7523.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burls A, Austin D, Moore D. Commissioning for rare diseases: view from the frontline. BMJ. 2005;331:1019–1021. doi: 10.1136/bmj.331.7523.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richards T. Orphan diseases: which ones do we adopt? BMJ. 2008;337:a1225. doi: 10.1136/bmj.a1225. [DOI] [PubMed] [Google Scholar]
- 64.Roos JC, Hyry HI, Cox TM. Orphan drug pricing may warrant a competition law investigation. BMJ. 2010;341:c6471. doi: 10.1136/bmj.c6471. [DOI] [PubMed] [Google Scholar]
- 65.Cheung RY, Cohen JC, Illingworth P. Orphan drug policies: implications for the United States, Canada, and developing countries. Health Law J. 2004;12:183–200. [PubMed] [Google Scholar]
- 66.Danzon PM. At what price? Nature. 2007;13:176–179. doi: 10.1038/449176a. [DOI] [PubMed] [Google Scholar]
- 67.Danzon PM, Towse A, Mulcahy AW. Setting cost-effectiveness thresholds as a means to achieve appropriate drug prices in rich and poor countries. Health Aff (Millwood) 2011;30:1529–1538. doi: 10.1377/hlthaff.2010.0902. [DOI] [PubMed] [Google Scholar]
- 68.Gucev ZS, Tasic V, Polenakovic M. On rare and “super-rare” diseases: an insight from the Republic of Macedonia. Prilozi. 2011;32:7–11. [PubMed] [Google Scholar]
- 69.Olivier C, Williams-Jones B. Pharmacogenomic technologies: a necessary “luxury” for better global public health? Global Health. 2011;7:30. doi: 10.1186/1744-8603-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Denis A, Mergaert L, Fostier C, Cleemput I, Simoens S. A comparative study of European rare disease and orphan drug markets. Health Policy. 2010;97:173–179. doi: 10.1016/j.healthpol.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 71.National Institute for Health and Clinical Excellence. [Accessed March 10, 2012.];Appraising Orphan Drugs Proposal. Available at: http://www.nice.org.uk/aboutnice/whoweare/seniormanagementteam/meetings/2005/12july2005/appraising_orphan_drugs.jsp.
- 72.Schlander M. The use of cost-effectiveness by the National Institute for Health and Clinical Excellence (NICE): no(t yet an) exemplar of a deliberative process. J Med Ethics. 2008;34:534–539. doi: 10.1136/jme.2007.021683. [DOI] [PubMed] [Google Scholar]
- 73. [Accessed May 10, 2012.];Drug Price Soars from $1,650 to $23,000 Per Vial to Treat Babies with Infantile Spasms: Parent and Physicians React. Available at: http://professionals.epilepsy.com/page/ar_1189197304.html.