Abstract
150–200 nm diameter capsules containing 60–70 wt % of poorly soluble drugs, paclitaxel and camptothecin, were produced by layer-by-layer (LbL) assembly on drug nanocores in a solution containing uncharged stabilizers. Optimization of capsule shell architecture and thickness allowed for concentrated (3–5 mg/mL) colloids that are stable in isotonic salt buffers. Nanoparticle aggregation during the washless LbL-assembly was prevented by using low molecular weight block-copolymers of poly(amino acids) (poly-L-lysine and poly-L-glutamic acid) with polyethylene glycol (PEG) in combination with heparin and bovine serum albumin at every bilayer building step. Minimal amounts of the polyelectrolytes were used to recharge the surface of nanoparticles in this non-washing LbL process. Such PEGylated shells resulted in drug nanocapsules with high colloidal stability in PBS buffer and increased protein adhesion resistance. The washless LbL polyelectrolyte nanocapsule assembly process, colloidal stability and nanoparticle morphology were monitored by dynamic light scattering and electrophoretic mobility measurements, UV-vis spectroscopy, TEM, SEM and laser confocal microscopy imaging.
Introduction
Nanoparticulation is one of the techniques allowing increasing concentration of poorly soluble drugs in pharmaceutical solution formulations.1 Modern manufacturing processes produce concentrated, fine, insoluble drug dispersions in aqueous and non-aqueous media by mechanically disintegrating drug powder (high-pressure homogenization, pearl/ball milling, and ultrasonication) or by admixing these drugs in organic solvents to aqueous solutions containing a stabilizer or a mixture of surfactants to produce submicron drug dispersions.2 These irremovable and unexchangeable without losing colloidal stability surfactants present in up to 5–10 wt % in the milling media and are a weak point of such formulations.3 There are a number of nanocrystalline drug formulations, such as Triglige®, Rapamune®, Emend®, Tricore®, Megas ES®, and Invega®, that have been approved for medical usage or are in clinical trials, however only few of them are intended for intravenous injections (e.g., Abraxane®).4 Among other strategies for delivery of poorly soluble drugs that promote bioavailability and facilitate controlled release, there are several lipid and polymer based nanoparticles5 but most of them are in early stages of development.
Polyelectrolyte layer-by-layer (LbL) encapsulation6 is an interesting extension of these efforts. Layer-by-layer shell formation is based on sequential adsorption of polycation and polyanions on tiny drug cores. Due to electrostatic interaction between oppositely charged polyelectrolyte chains, such LbL multilayer assembly provides stable and very thin polyelectrolyte shells encapsulating drugs. The following 2–5 micrometer diameter drug particles were used for LbL encapsulation: ibuprofen, furosemide, nifedipine, naproxen, biotin, vitamin K3, insulin, dexamethasone, tamoxifen, paclitaxel, camptothecin, and curcumin.7 However, applications of LbL technique to drug encapsulation have a few challenges: 1) Most of the works on LbL encapsulation were performed on microcores in water and at very low concentration of the drug particles without special attention to colloidal stability at physiological conditions. For medical practice, one needs 3–5 mg/mL drug capsules with diameter of 100–200 nm dispersed in an isotonic salt solution (like PBS buffer or 0.9 % NaCl) which often induces aggregation. 2) Surface PEGylation is considered as a necessary step for increased in vivo circulation time of LbL capsules. 3) Targeting of LbL nanocapsules is a feasible goal due to the possibility of constructing any composition of multilayer shells, including, for example, monoclonal antibodies or other specific biomolecules.
A practical question is how to combine the nano-sized drug dispersing techniques with LbL shell formation and design injectable ~200 nm diameter particles. An important aspect is the necessity to form LbL shell on nanocores directly in the milling media that contains a sufficient amount of uncharged pharmaceutical excipients (e.g., sucrose, glycerol, polysorbates, polyvinylpyrrolidone, polyethylene glycol or others) which are applied in the mixtures with ionic surfactants for better performance.
Such charged amphiphilic (e.g., sodium lauryl sulfate, sodium docusate) or polymeric (e.g. carboxymethyl cellulose, chitosan) surfactants already on the grinding stage form drug core-attached layer which further anchors the LbL shell.8
We focused here on LbL-stabilization and surface modification of nanoparticulated labile crystalline drugs to get highly concentrated and stable in pharmaceutical buffers drug colloids. However, main methods related to LbL assembly on 100–200 nm cores are applicable to any other nanoparticles (e.g. low soluble dyes or quantum dots) that are difficult to handle by common centrifugation based LbL encapsulation.
Paclitaxel (PTX) and camptothecin (CPT) are poorly water-soluble drugs (solubility is below 0.01 mg/mL) with proven anticancer activity.9–10 LbL-coated particles of these drugs are of interest as promising intravenously injectable forms that are aqua-based with a particle diameter less than 200 nm and good colloidal stability in phosphate buffered saline (PBS) and biological fluids (blood serum). Newly developing drug nano-formulations must comply with the following criteria: the content of the drugs in a capsule has to exceed 30 wt % and drug concentration in a stable nanocolloid for injection has to be above 2 mg/mL.1–5
Our architectural approach to build-up nanocapsules includes preparation of hydrophobic PTX and CPT drug nanocores in the presence of amphiphilic or polymeric surfactants followed by deposition of thin multilayer shells of PEGylated polycations/polyanions using the washless LbL assembly technique (Fig.1). Amphiphilic surfactants prevent initial drug nanocrystals from coalescing upon core preparation and serve as anchors for attaching polycation/polyanion shell that plays a dual role in the nanoparticle stabilization. The inner layers further improve nanocore inaccessibility for recrystallization into larger particles, whilst the outermost layers enhance colloid stability due to high hydrophilicity of the polyelectrolytes. Incorporation of polyethylene glycol (PEG) moieties in LbL shell by using PEG-modified polyelectrolytes further increases colloidal stability of the dispersions and attains some protein-resistant properties. The LbL shell architecture can be developed further for anchoring tumor targeting agents though amine groups available at the outermost polyelectrolyte layer.
Fig. 1.
Scheme of drug nanocore preparation and washless LbL coating.
Due to nano-scaled cores, the choice of components for LbL shell assembly is limited to low molecular weight polyelectrolytes (less than 65 kDa) because nanoparticles have limited space for polymer chain adsorption, contrary to microparticles which have properties closer to a flat surface.11
Both PEGylated and non-PEGylated polyelectrolytes and proteins were tested to improve the formulation colloidal stability. We chose combination of cationic block-copolymers of poly-L-lysine with polyethylene glycol of different molecular weights (PLB) and anionic heparin (Hep) or bovine serum albumin (BSA) for the LbL shells on nanoparticles, with possibility of its further modification with layers including block-copolymer of PEG and poly-L-glutamic acid (PGB).
Therefore, we elaborate the concept of designing architectural shell on drug nanocores via pre-selected sequence of biodegradable polyelectrolytes with different degree of PEGylation: design of multilayer coating with shorter and longer PEG-chains located at different levels of the shell wall and preventing nanoparticle aggregation (kind of fur/ under-fur coating composition).
1. Experimental
1.1 Materials
Paclitaxel (PTX) and camptothecin (CPT) were obtained from LC Laboratories, USA. Poly-L-lysine (PLL), heparin sodium salt (Hep), bovine serum albumin (BSA), BSA-fluorescein labelled (BSA-FITC), PLL-fluorescein labelled (PLL-FITC), polystyrene sulfonate (PSS), polyethyleneimine (PEI), polyvinylpyrrolidone (PVP), Polysorbate 80, sodium docusate (AOT), phosphate buffered saline (PBS), pinacyanol chloride, ethanol, acetonitrile, dimethyl sulfoxide (DMSO) and acetone were obtained from Sigma-Aldrich and used as received. Fetal bovine serum (FBS) was obtained from Atlanta Biologicals, USA.
Block-copolymers of poly-L-lysine or poly-L-glutamic acid with polyethylene glycol (PEG) of different molecular weights were obtained from Alamanda Polymers, USA. The copolymers have a different PEG/PLL units ratio varying from 1.1 to 4.5 for the first two block co-polymers with smaller PLL molecular weight and 2.8 for higher molecular weight block copolymers: 1) PLL[16kDa]-b-PEG[5kDa] (PLB16-5), 2) PLL [16kDa]-b-PEG[20kDa] (PLB16-20), 3) PLL[33kDa]-b-PEG[20kDa] (PLB33-20), 4) PGA[30kDa]-b-PEG[20kDa] (PGB30-20). Grafted copolymer of PLL 65kDa and PEG 5kDa with a grafting ratio of ca. 4.5 (PLG65[4.5]5) was synthesized.12 Copolymer of PLL 20kDa and PEG 2kDa with a grafting ratio of 3.3 (PLG 20[3.3]2) was obtained from SuSoS, Switzerland.
1.2 Drug nanocapsules preparation
1.2.1 Paclitaxel nanocores
PTX crystals were dissolved in acetone at 80 mg/mL and 63 µL of the concentrated solution was added to 2.8 mL of PBS buffer containing 2.9 mg/mL PVP, 1.2 mg/mL AOT, and 0.36 mg/mL Polysorbate 80 at 10 °C under continuous sonication in a Branson ultrasound bath. The mixture was sonicated for 20 min to allow less than 180 nm PXT cores to be formed.
1.2.2 Camptothecin nanocores
200 µL of a 7 mg/mL camptothecin solution in DMSO was added to 2.6 mL of PBS buffer containing 0.7 mg/mL BSA and 1.6 mg/mL PVP. The buffer was titrated with HCl to pH 3. The mixture was sonicated for 20 min to obtain cores in the desired size range, less than 150 nm.
1.2.3 Formation of polyelectrolyte shell by non-washing LbL method
The drug nanocores were coated in a supernatant containing the excipients mentioned above with polyelectrolytes using a non-washing LbL procedure.13 Typically, each polyelectrolyte in small 15–25 µL aliquots of a concentrated 60 mg/mL solution was added to 2.5 mL dispersion of nanocores under constant sonication. The amount of a polyelectrolyte needed to recharge surface of the nanoparticles was evaluated as described below. After addition of each polyelectrolyte, the nanoparticles were kept for 5 min and then the next polyelectrolyte was added. No intermediate washing of nanoparticles was done until the shell was complete. Typically, 3–7 polycation / polyanion double coatings were deposited.
The LbL assembly was followed by ζ-potential (in water) and hydrodynamic diameter (in PBS) measurements (ZetaPlus Brookhaven). After the LbL shell was complete, the coated nanoparticles of ca. 200 nm diameter for PXT and 150 nm for CPT were separated from the supernatant by centrifugation, re-dispersed in PBS buffer, and kept as stable colloids of 2–5 mg/mL concentration. The yield of the LbL-coated nanoparticles varies with the applied coating and was ca. 50–90 % of the initial drug amount.
To preserve the lactone form of CPT during LbL assembly, a Hep/(PLB16-5/Hep)3 shell was assembled at pH 3.0 (with a negative charge on the top), then the dispersion was transferred to PBS buffer with pH 7.4 and at least one more polyelectrolyte bilayer was assembled to ensure nanoparticles colloidal stability.
Scanning and transmission electron microscopy (SEM, TEM) and laser confocal scanning microscopy (CLSM) images of LbL-coated nanoparticles were taking with a Hitachi S-4800, a Zeiss EM912, and a Leica DMI RE2 instruments. UV absorbency was measured with an Agilent- 8453 UV-vis spectrophotometer.
1.3 Characterization of non-washing LbL assembly of polyelectrolytes
1.3.1 Evaluation of polyelectrolyte amounts needed to reverse surface charge
Dispersions of PTX nanoparticles coated with a (PLL/Hep)n or (PLB16-5/Hep)n, n=0–1.5 shell in the supernatant were prepared as described above. A 6 mg/mL polyelectrolyte charged oppositely to the nanoparticles in 20 µL aliquots was added to the dispersion under short sonication until the surfaces of the nanoparticles were completely recharged. Every time, 0.12 mL was withdrawn from the dispersion for ζ-potential and pinacyanol assay evaluation of the amount of negative charges in the dispersion. The partially coated nanoparticles were separated from the probe by centrifugation and were redispersed in DI water. The number of negative sites in a sample was evaluated per 1 mg of the drug using the pinacyanol assay.14
1.3.2 PVP influence
To evaluate the PVP influence on the amount of polyelectrolytes to be added to recharge surface of nanoparticles, 1.5 mL of uncoated PTX nanoparticles in the supernatant containing the surfactants was diluted either with 1.5 mL of PBS buffer (pH 7.4) or with 1.5 mL of 2.8–11.2 mg/mL PVP solution in the buffer. The aliquots containing 30 µg of PLB16-5 were step-wise added to the mixture until the ζ-potential of LbL-coated nanoparticles reached a high positive (+30 mV) value. After that Hep was added in the same way. The formation of two PLB16-5/Hep bilayers followed.
1.4 Analytical techniques
1.4.1 Pinacyanol assay for negatively charged sites
Typically, 20–80 µL of a diluted sample in water was added to 2 mL of a pinacyanol solution with A0 = 0.50±0.05 at 600 nm.14 The UV-Vis spectra were recorded 2 min after mixing. The concentration of negative sites (in µmol/mg PTX) was calculated.
1.4.2 Drug concentration
PTX concentration in the LbL-coated colloids was estimated after extraction of the drug with ethanol, using UV-vis spectroscopy (227 nm). CPT concentration in a sample was estimated after extraction with a 1:1 DMSO / acetonitrile mixture. All colloids were redispersed in PBS before the extractions. The content of drugs on a dry weight basis was evaluated in lyophilized LbL-coated dispersions.
1.5 Characterization of colloidal stability of drug nanocapsules
Colloidal stability of concentrated 3 mg/mL dispersions of LbL-coated paclitaxel nanoparticles in PBS buffer was evaluated in time. The samples were kept at room temperature, 10–25 µL aliquots were taken, dispersed in 2 mL PBS and the apparent hydrodynamic diameter of nanoparticles was measured. For evaluation of stability of LbL-coated nanoparticles in serum, 100 µL of a sample under investigation was added to 2 mL of FBS and the position of nanoparticles peak in the mixture size distribution was measured in time.
1.6 Characterization of drug release from nanocolloids
The in vitro release studies for the paclitaxel nanocolloids were done at sink conditions at 37 °C in PBS buffer containing 0.2 mg/mL Polysorbate 80, which was added to increase drug solubility limit. For in vitro release studies of camptothecin nanocolloids, PBS buffer containing 2 % Polysorbate 80 was used. The LbL coated drug nanoparticles were added to the release medium at a concentration three times lower than the drug solubility limit; aliquots were withdrawn after certain time intervals and the drug concentration in supernatant was measured after removing nanoparticles by centrifugation.
1.7 Quart Crystal Microbalance analysis
To understand protein-resistant properties of PEGylated polyelectrolyte films, layer-by-layer assembly of several combination of Hep and PLL or PLB on flat surface has been studied by quartz crystal microbalance technique with motional resistance monitoring (QCM-R) in a liquid flow cell (QCM200, Stanford Research Systems).15 Aqueous 0.1 or 0.5 mg/mL solutions of polymers were used. The solutions (1 mL) and DI water (2mL) were injected into the liquid flow cell with an inset 5 MHz resonator, having an additional (PEI/PSS)3 precursor layer on its gold working surface, in the following order (H2O/polycation/H2O/heparin)n. Each solution was kept in the cell for 5 min until stable readings for resonance frequency (F) and equivalent resistance (R) were obtained. Usually it takes less than 3 min to reach the saturation of polyelectrolyte adsorption on a polyelectrolyte-modified surface.15 All changes of F and R were considered relatively to those for the resonator without film immersed in DI water. To determine the film mass all data were processed using the Sauerbrey equation for rigid deposits.15
To evaluate protein adsorption on LbL coating of different architecture, 1 mL of FBS or a 40 mg/mL BSA solution in water was injected in the liquid flow cell with a resonator coated with different combinations of PEGylated and non-PEGylated polyelectrolytes for 5 min. Then the cell was washed with 2 mL of DI water and resonator parameters were monitored for additional 10 min.
2. Results and Discussion
2.1. Drug nanocores
Paclitaxel nanocores were prepared by precipitation of drug nanocrystals from a water-miscible solvent. This bottom-up method is a convenient approach for preparation of hydrophobic drug nanoparticles with a high yield, although this technigue requires the usage of surfactants that prevent crystal growth and provide temporal nanoparticles stabilization.3 Aiming for the long term stability of concentrated LbL-coated dispersions in PBS, the cores preparation was began in this buffer (Supporting Information, Fig. A). Typical paclitaxel nanocores as prepared have a hydrodinamic diameter of 170–180 nm, while camptothecin cores are less than 150 nm.
PTX cores prepared in the presence of the surfactants and coated with PEG-modified polyelectrolyte shells have elongated shape of max 250 nm and a width of 50–70 nm (Fig. 2). On TEM images of a nanoparticle, one can distinguish crystal faces and borders. Camptothecin nanocores prepared using BSA and coated with a shell of polyelectrolytes have less pronounced crystalline structure (SI, Fig. B).
Fig. 2.
a–d) TEM images of PTX nanoparticles coated with a (PLB16-5/Hep)3/PLG65[4.5]5 shell (a, c, d, stained with (NH4)2Mo2O7) and with a (PLB16-5/Hep)5.5 shell (b, stained with UO2(CH3COO)2); e) SEM image of PTX nanoparticles with a (PLB16-5/Hep)3.5 shell; f) CLSM images of 240 nm PTX nanoparticles with a (PLB33-20/BSA-FITC)2.5 shell
2.2 Anchoring layer: surfactants
Among tested water-soluble negatively charged amphiphilic (non-lipid and non-protein) surfactants, AOT excels against others in the recovery of paclitaxel nanoparticles of the desired size and the amount of surfactant remaining adsorbed on the drug surface (SI, Table A). It forms a layer more strongly adhered to the drug nanocrystals and providing a higher negative charge surface density than the others. Besides, AOT is FDA approved for medical usage and considered as safe in food products.
Paclitaxel is well known to recrystallize very fast;9 thus the uncoated dispersion is stable only for a limited time (less than 30 min). The nanoparticles are semi-stable if the amphiphilic surfactants/PTX ratio is 0.5–0.7 g/g (with a higher than 0.48 g/g AOT/PTX ratio and 0.30–0.33 g/g Polysorbate 80/AOT ratio). Despite that 2.9 mg/mL concentrations of PVP, along with AOT and Polysorbate 80, are required for obtaining paclitaxel of less than 200 nm, its presence does not affect LbL assembly. PVP is removed from concentrated LbL-capsule dispersions by rinsing with PBS. Crystalline paclitaxel cores as prepared contain 1.03±0.23 µmol AOT per 1 mg of drug. This surfactant coats hydrophobic nanoparticles and anchors adsorption of the first cationic layer of LbL shell. 15–20 % of initially added AOT remains unbound to the surface as free dissolved molecules or small micelles that interact with PLB forming complexes with a 1: 1 positive to negative charge binding ratio. The complexes are further coated in the same way as PTX nanoparticles, but have no PTX core.
The usage of BSA in the amount of 1.4 mg per 1 mg of camptothecin allows stabilizing nanocores with a diameter of 120–140 nm (for comparison, albumin-stabilized paclitaxel nanoparticles, known as Abraxane, have size of 130 nm4). Albumin attaches to camptothecin with hydrophobic parts, while its hydrophilic parts provide colloidal stability in aqueous solutions and anchor LbL shell. Recent evidences of high paclitaxel content in micelles of alkyl-modified hyaluronic acid and chitosan 16 suggest that such polyelectrolytes can replace surfactants and allow further assembly of stable multilayer shell.
Other hydrophobically modified polyelectrolytes, such as imidazolyl, cholesteryl, deoxycholic acid conjugates of chitosan16 can be good candidates for anchoring layer in such shell architecture.
2.4 LbL shell formation
2.4.1 Amount of polyelectrolyte to reverse surface charge
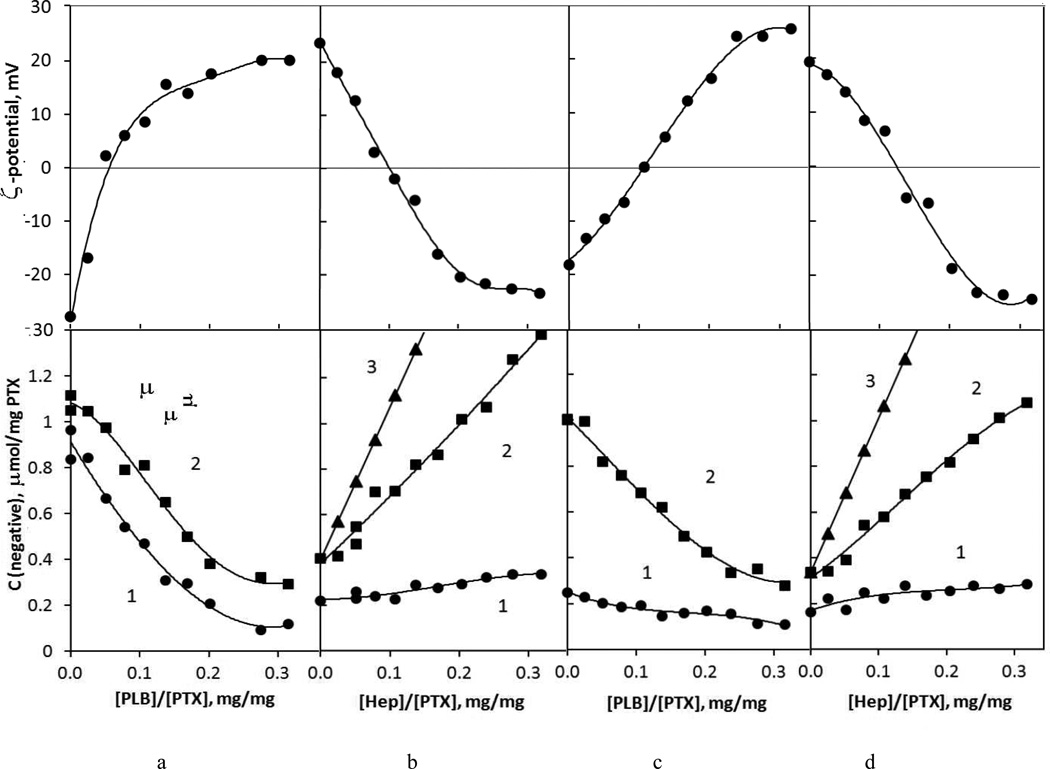
For semi-stable drug cores of a 100–200 nm apparent diameter we could not use the traditional LbL assembly based on sequential colloid precipitation and washing but developed a non-washing assembly procedure. The amounts of polyelectrolytes needed to reverse the charge of drug nanoparticles in each coating step were evaluated with the help of titration for the two inner bilayers and then used as determined. The data shown are on LbL assembly of cationic PEG-modified PLB16-5 and Hep on PTX nanocores because this formulation provided the best colloidal stability (Fig. 3). Unmodified polylysine in alternation with anionic heparin gives similar results, with the exception of much lower colloidal stability. With gradual addition of positively charged PLB16-5 to drug nanocores their ζ-potential increases (Fig. 3a). We also monitored the total amounts of negatively charged sites in the dispersion using the pinnacyanol assay. The number of negative sites decreases due to attachment of PLB16-5 chains to AOT sulfate groups previously available for the dye. Both increase of positive surface charge and decrease of available negative sites correlate and show particle recharging. 0.26 mg of PLB16-5 per 1 mg of PTX nanoparticles is sufficient for their recharging (Table 1).17
Fig. 3.
Changes of ζ-potential (upper row) and amounts of negative sites estimated by pinacyanol assay (lower row) in the process of step-wise addition of a) PLB16-5 to PTX/AOT cores, b) Hep to PTX/AOT/PLB16-5 nanoparticles, c) PLB16-5 to PTX/AOT/PLB16-5/Hep nanoparticles, d) PLB16-5 to PTX/AOT/PLB16-5/Hep nanoparticles. Lower row: 1 – nanoparticles, 2- total experimental, 3 – calculated 17
Table 1.
Amounts of polyelectrolytes needed to compensate (zero) or completely reverse surface charge of nanoparticles, mg/mg PTX
| Coating step |
Layer | PLB16-5 | PLL | ||
|---|---|---|---|---|---|
| zero | recharged | zero | recharged | ||
| 1 | PLB on AOT | 0.05 | 0.26 | 0.08 | 0.26 |
| 2 | Hep on AOT/PLB | 0.08 | 0.27 | 0.23 | 0.35 |
| 3 | PLB on AOT/PLB/Hep | 0.11 | 0.26 | - | - |
| 4 | Hep on AOT/(PLB/Hep)1.5 | 0.14 | 0.27 | - | - |
A moderate increase of negative sites on nanoparticles at the second assembly step, coating with anionic heparin, was nevertheless sufficient to support a decrease in their ζ-potential of the same magnitude as it in the first assembly step. In this step, 0.27 mg of Hep per 1 mg of the PTX colloid is needed.17
By comparing the amount of heparin added to the dispersion and assayed by pinacyanol (Fig.2b, curves 3 and 2) one sees that a part of polymer negative sites is not accessible for the indicator dye because they are strongly bound to amine groups of PLB. At the same time, a sufficient amount of heparin remains in supernatant unbound to the nanoparticles (difference between curves 2 and 1) and its negative charge is compensated by PLB16-5 added on the third step. An increase of nanoparticles surface charge and a decrease of the amount of shell negative sites clearly indicate attachment of the polycation (Fig.3c). The total amount of negative groups in solution also decreases confirming PLB16-5/Hep complexation. The polyelectrolytes, which remained unbound at every step of non-washing LbL assembly, form free floating PLB/Hep complexes observed in selected samples with ca. 40–70 nm diameter (SI, Fig. C). We assume that they are getting attached to the surface upon further LbL coating as confirmed by TEM (Fig.2).
For PLB16-5/Hep coating, the amounts of PLB16-5 and Hep needed to recharge the surface in the 1st – 3rd and 2nd – 4th coating step does not depends on the layer number, but the amount needed to compensate an opposite charge increases for thicker films (Table 1). We assume that it is related to different surface charge density of nanoparticles coated with precursor surfactant AOT and film Hep layers that promote different attachment of polylysine residues.
When non-PEGylated polylysine is used for the assembly (SI, Fig. D), higher amounts of both positive and negative polyelectrolytes are needed on each step as compared with PLB16-5. Various conformation of polymer chains (helix or coil)18 due to PEG presence may explain this difference.
In all further experiments, polyelectrolytes were added to the dispersion in one shot in the above determined amounts near to provide “recharging”, but to minimize PLB/heparin complexation apart the nanoparticles’ surface.
2.4.2 PVP influence
Polyvinylpyrrolidone, a non-ionic polymeric surfactant which is widely used in pharmacy, is applied at core preparation stage to enhance stability of formed drug nanocrystals. It remains in the supernatant during LbL-shell assembly. In the concentration range up to 7 mg/mL, the PVP does not affect the PLB16-5/Hep multilayer assembly (SI, Fig.E). Similarly, 3–10 layers of polyelectrolytes on insulin microrocores were formed in aqueous solutions of polyethylene glycol.19 Uncharged polymeric additives change viscosity and density of the medium but in relatively small concentrations they do not affect its dielectric properties to a degree that prevents electrostatic interaction of polyelectrolyte chains in LbL process.
2.4.3 LbL shell building-up
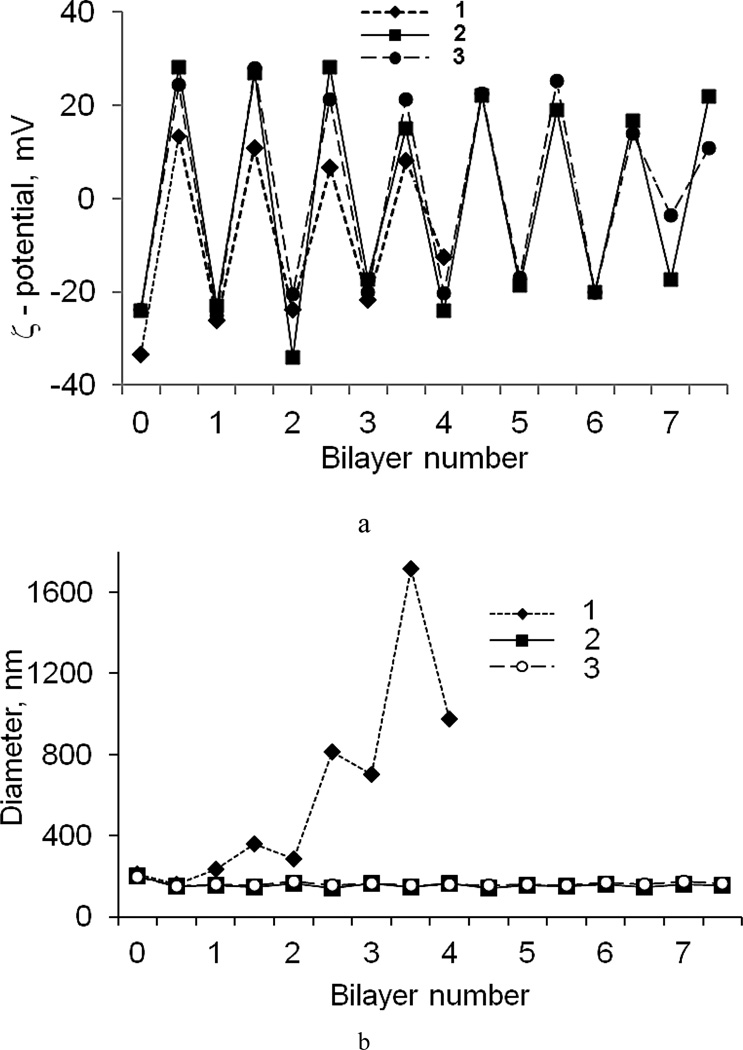
An alternation of ζ-potential value (Fig. 4a) confirms the polyelectrolyte attachment to the nanoparticles for PEGylated and non-PEGylated assembly (PLB16-5/Hep and PLL/Hep). The usage of PLB16-5 instead of PLL allows minimizing nanocapsule aggregation during coating process while working with concentrated dispersion (~2 mg/mL). With PLB16-5, the nanocapsules diameter slightly increases with each coating cycle but remains below 250 nm even for capsules containing 7.5 bilayers in the shell (Fig. 4b). For non-PEGylated polyelectrolytes, colloidal stability is based on repulsion of highly charged nanoparticles and as soon as the surface potential becomes lower, aggregation occurs (SI, Fig. F). In the case of PEGylated nanocapsules, electrostatic forces are supplemented with repulsion of highly hydrophilic PEG tails that prevent aggregation. It was important to use PLB in the first layer attached to AOT sulfate groups layer to preserve nanoparticles size and colloidal stability of a sample.
Fig. 4.
ζ-potential (a) and apparent hydrodynamic diameter (b) of PXT nanoparticles in the process of shell assembly. Shell: non-PEGylated (PLL/Hep)4 (1), and PEGylated (PLB16-5/Hep)7.5 (2), (PLB16-5/Hep)5/(PLB16-5/Hep)2.5 (3)
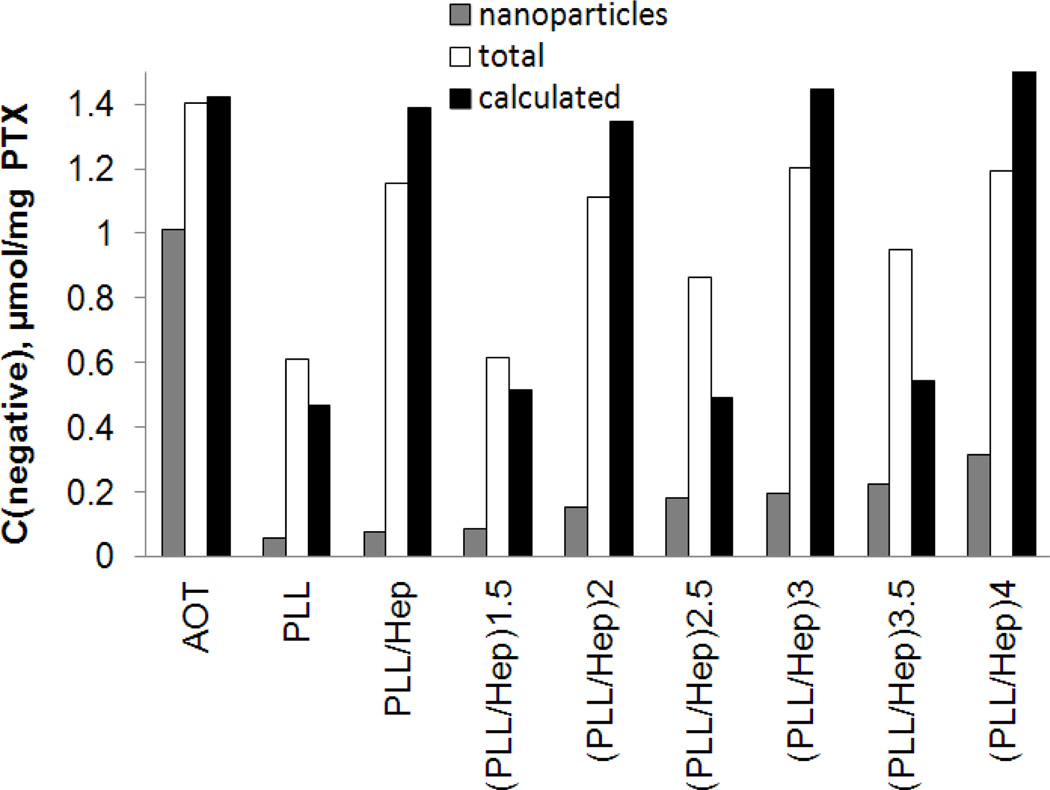
In building-up LbL shell on drug nanocores (Fig. 5), the total amount of accessible for pinacyanol negative sites in a dispersion (white) increases upon addition of Hep and decreases after adding a PLL solution, generally following the theoretical trend (black). The observed difference between experimental and theoretical, calculated with the assumption of 1:1 negative to positive sites binding and full ionization of both polyelectrolytes, values can be explained by a lower degree of ionization of polyelectrolytes and a different stoichiometry of PLL/Hep binding.17 The amount of negative groups accessible for pinacyanol in a nanocapsule shell increases as the shell grows (grey). If the negative groups are situated only on the nanocapsule outer surface, it may indicate shell external surface growth. If some unpaired to PLL negative Hep sites are accessible for pinacyanol in inner shell layers, it indicates an increase of shell thickness as LbL coating develops.
Fig. 5.
Changes of available on nanoparticles surface (grey bar), total (white bar), and calculated (black bar) amounts of negative sites in the coating of a PTX dispersion as estimated by the pinacyanol assay
On TEM images (Fig. 2), each paclitaxel nanocrystal is surrounded by a halo of less dense material, which can be recognized as a polyelectrolyte shell of 10–15 nm thickness. Besides 10–20 nm polyelectrolyte globular complexes also visible on the surface. A top-view SEM image (Fig. 2e) confirms amorphous coating on 50–250 nm paclitaxel particles.
On laser confocal images (Fig 2f), the paclitaxel nanocapsules prepared with fluorescein-labelled BSA look like well dispersed ca. 300 nm bright spots. This observed larger particles dimension is due to instrumental function determined by convolution of smaller real particle size and resolution of confocal microscope which is ca. 300 nm. A point of view exists that FITC can promote adsorption of FITC-labelled proteins.20 To spatially separate the core and FITC-bearing polymer the 3rd and 5th polycation layer in the shell of complex (PLB16-5/Hep)5/(PLB16-20/Hep)2.5 architecture were replaced with PLL-FITC. A high fluorescence of nanoparticles (corresponding to ~0.10 mg PLL-FITC per 1 mg PTX) confirms the attachment of the fluorescent polyaminoacid to the shell that covers the nanoparticle surface.
The drug loading in 200 nm LbL-coated nanoparticles separated from supernatant and lyophilized was 60–70 wt % for a (PLB16-5/Hep)3.5 coating and 30–45 wt % for longer PEG-chain (PLB16-20/BSA)2.5 coating.
Specific features of camptothecin encapsulation
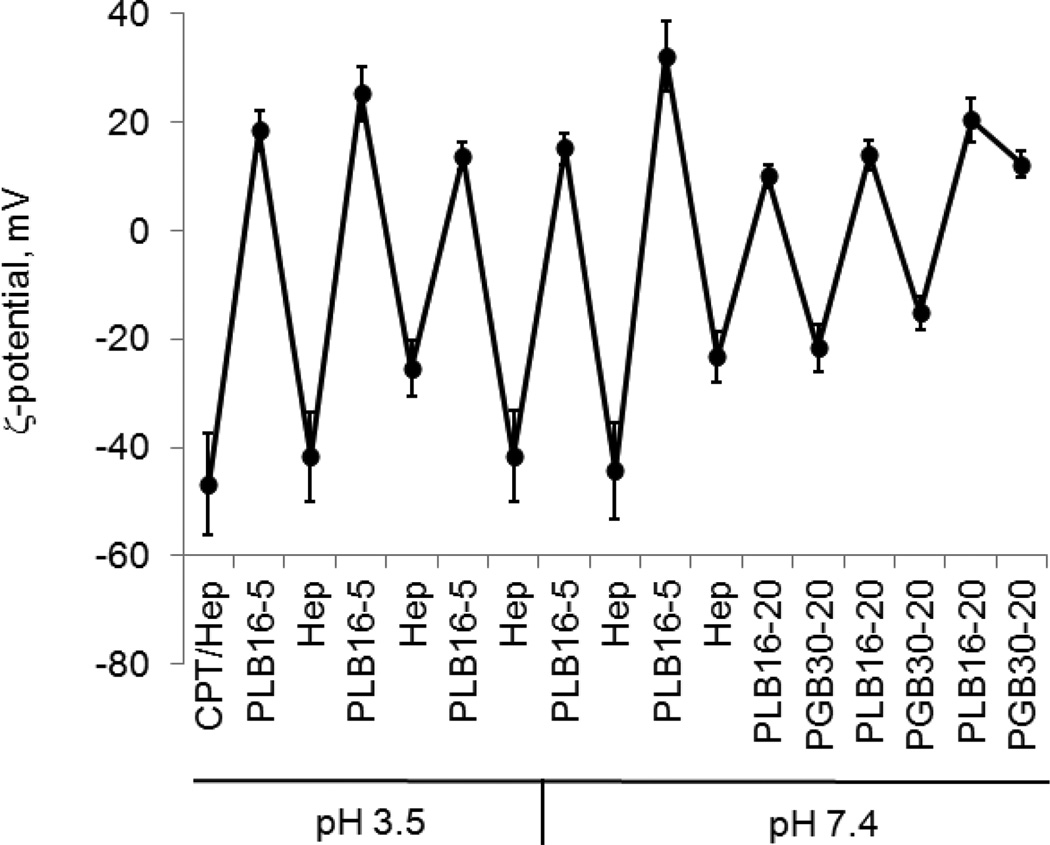
To achieve two different goals: 1) preserving the active, lactone form of camptothecin that is easily hydrolysed to its inactive carboxylate form at a slightly basic pH,10 and 2) obtaining drug nanocolloids stable in PBS at pH 7.4, the LbL coating on the drug nanocores was made in two steps. First, CPT nanocores were formed and stabilized with a Hep/(PLB16-5/Hep)3 shell at pH 3.5, and then the rest of the layers was assembled at pH 7.4 (Fig. 6; SI, Fig.G). 140-nm diameter capsules with a drug loading of 70 wt %, consisting mainly of the lactone camptothecin form, were obtained and their chemical and colloidal stability were preserved for long time even in the presence of albumin in the media (SI, Fig. H).
Fig. 6.
ζ-potential (a) and apparent hydrodynamic diameter (b) of CPT nanoparticles in the process of LbL shell assembly at different pH
2.5. Nanocapsule shell architecture and dispersion stability
One of main parameters in LbL design of drug nanocapsules for intravenous administration is small 200 nm diameter. Several polyelectrolytes of natural origin (hyaluronic acid, chondroitin sulfate, dextran sulfate) were excluded because of high molecular weight of the polymers that does not allow producing capsules of desired sizes. Polymer length has to allow approximately single loop around the particle to minimize neighbour particles bridging.11 A 72 h support of colloidal stability of a 3 mg/mL PTX dispersion in PBS buffer was the requirement for further in vivo testing.
LbL-shells consisting of a block copolymer of PLL with PEG of different length (PLB) as a cationic component of the shell and heparin or BSA (SI, Fig. J) as an anionic one were the most stable in PBS buffer among the multiple capsules wall architectures. The dispersions of paclitaxel coated with 3.5 or more PLB16-5/Heparin bilayers were stable in PBS buffer for at least 200 h (SI, Fig. I). The 170–190 nm colloids coated with a (PLB16-20/BSA)2.5 shell did not show any signs of aggregation for 240 h. Better colloidal stability of concentrated paclitaxel LbL-nanocapsules in PBS was obtained using in the assembly PLB16-5 copolymer and the shell architecture of (PLB/heparin)n, n= 3.5–7.5 rather than using the PEGylated polyelectrolyte only in the capsule outermost. Higher PEGylated polycation and polyanion, PLB16-20 and PGA30-20, further stabilize nanocapsules if applied in the outermost bilayers. Being assembled on the top of a (PLB16-5/heparin)5 shell (PLB16-20/PGA30-20)2.5 coating allows attachment lower amount of PGA30-20. ζ-potential of the nanoparticles is not reversed to a negative value, indicating the maximum possible PEGylation for the process (Fig. 6). PEGylated and non-PEGylated polylysine can be combined in the same shell. Without losing sample colloidal stability, one PLL layer can be introduced at any location in the shell wall, except the first one. More PLL layers can be included in a shell if they are separated by at least one PLB/Hep bilayer (SI, Fig. K).
A long-term stability of LbL shells with an outermost coating consisted of a grafted PLL-PEG, such as PLG65[4.5]5 or PLG20[3.3]2 was low (48–72 h). These less flexible highly PEGylated polyelectrolytes provide only limited number of charged groups per chain length and their cooperative binding to the nanoparticles surface is low.
To evaluate the complications arisen from possible nanoparticles aggregation in blood upon intravenous injection, colloidal stability of LbL-coated nanoparticles in fetal bovine serum was tested. In serum, paclitaxel nanoparticles coated with (PLB16-5/Heparin)3.5 shell or having one more negatively charged polyelectrolyte layer on the outermost retains the size below 250 nm for at least 24 h.
2.6. QCM evaluation of PEGylated and non-PEGylated film thickness, viscoelasticity and protein adsorption resistance
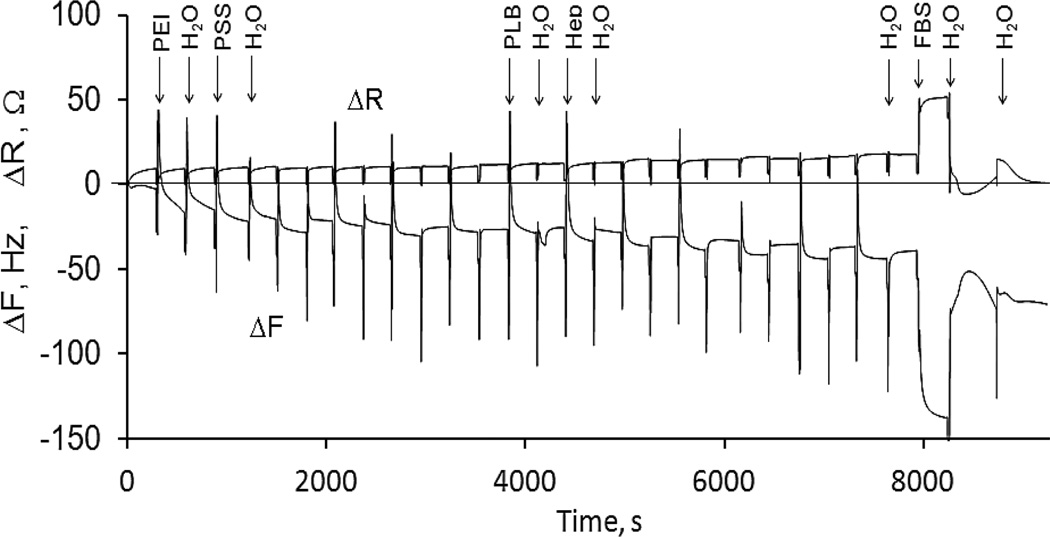
Additional data on LbL assembly of block-copolymers of PEG and polylysine with heparin were obtained with QCM-R technique. A decrease of resonance frequency ΔF and an increase of equivalent resistance ΔR during the LbL assembly on the resonator surface (Fig. 7) provide an evidence of a step-wise growth of the film. ΔR is an independent measure of viscous loading by the medium (liquid and soft-film) at the crystal’s surface, while ΔF depends upon the adsorbed rigid mass as well as the viscosity and density of the deposit and solution in the vicinity of the crystal-liquid interface.15 The equal increments of ΔF and ΔR for a bilayer adsorption from 0.1 and 0.5 mg/mL polyelectrolyte solutions indicate that both concentrations assure saturation of adsorbed polyelectrolyte layers at every deposition cycle (Table 2). On a single step of non-washing assembly, each polyelectrolyte is added in the amount that corresponds to its 0.1 mg/mL concentration in the nanoparticles dispersion. It is sufficient to form a saturated layer of the polyelectrolyte on the surface of nanoparticles.
Fig. 7.
ΔF and ΔR of a 5 MHz resonator in the process of adsorption of a (PLB16-20/Hep)3.5 film and FBS protein adhesion resistance testing
Table 2.
Average changes of resonance frequency, equivalent resistance, adsorbed mass, and thickness per a polycation/ heparin bilayer
Calculated assuming the formation of rigid films, the thicknesses of wet polycation/heparin layers formed with PLB and PLL are almost the same. A slightly higher value for PLB16-20/Hep bilayer can be related to the presence of longer upstanding PEG tails. The value of ΔR, that characterizes the energy dissipated during resonator vibration due to an inelastic film on its surface, is higher for a PLB16-20/Hep containing longer PEG than that for PLB16-5/Hep. The thickness and viscoelastic properties of a PLL-based bilayer are comparable to those for PEGylated polyelectrolytes. Presumably due to different chain conformation, PLL forms slightly thicker layers with rougher surface that impact film properties.18 For example, a larger amount of polyelectrolytes are needed to reverse surface charge of nanoparticles, if PLL is used for coating (Table 1).
Influence of PEGylation on serum protein adsorption
The ΔF and ΔR values of resonators modified with a LbL film and brought in contact with a 40 mg/mL BSA or serum are changing due to both higher viscosity of the media and protein adsorption on the film surface (Table 3). The LbL-films on the basis of PEGylated polylysine better resist to BSA adsorption than those of PLL, and a (PLB16-20/Hep)3.5 coating repels albumin better than the other compositions. It is in agreement with commonly accepted point of view on the role of PEG surface modification in increased protein adsorption resistance.21
Table 3.
ΔF and ΔR for a resonator with a (Polycation/Hep)3.5 film exposed to BSA and serum
| −ΔF, Hz; ΔR, Ω | |||||
|---|---|---|---|---|---|
| Polycation | C, mg/mL |
In contact with | Rinsed with water | ||
| BSA | FBS | BSA | FBS | ||
| PLL | 0.1 | 103; 30.9 | 103; 21.6 | 24; 0.6 | 60; −8.5 |
| PLB16-5 | 0.1 | 84; 27.9 | 110; 20.4 | 13; 0.0 | 100; −19.7 |
| 0.5 | 109; 24.8 | 40; −2.3 | |||
| PLB16-20 | 0.1 | 62; 25.3 | 132; 7.3 | 9; −0.5 | 132; −12.5 |
| 0.5 | 77; 33.0 | 28; −16.1 | |||
On the contrary, much larger ΔF and ΔR for a resonator immersed in serum and rinsed with water indicate a significant adsorption of serum proteins on all polycation/heparin films. Serum is rich in small globular proteins that can penetrate through a rather loose brush of PEG-tails on the film surface. Moreover, globulins have a certain affinity towards PEG moieties that can promote their interaction.22 There is a decrease in FBS protein adsorption on (PLB/Hep)3.5 films formed from more concentrated (0.5 mg/mL) polyelectrolyte solutions as compared with the lower concentration. The PEG surface density of such films can be higher although there is no difference between calculated characteristics of the films. A negative shift of ΔR in water after FBS protein adsorption can be explained by decreasing surface roughness or facilitating surface sliding friction by the adsorbed proteins.15
2.7. Influence of LbL shells on release of paclitaxel and camptothecin
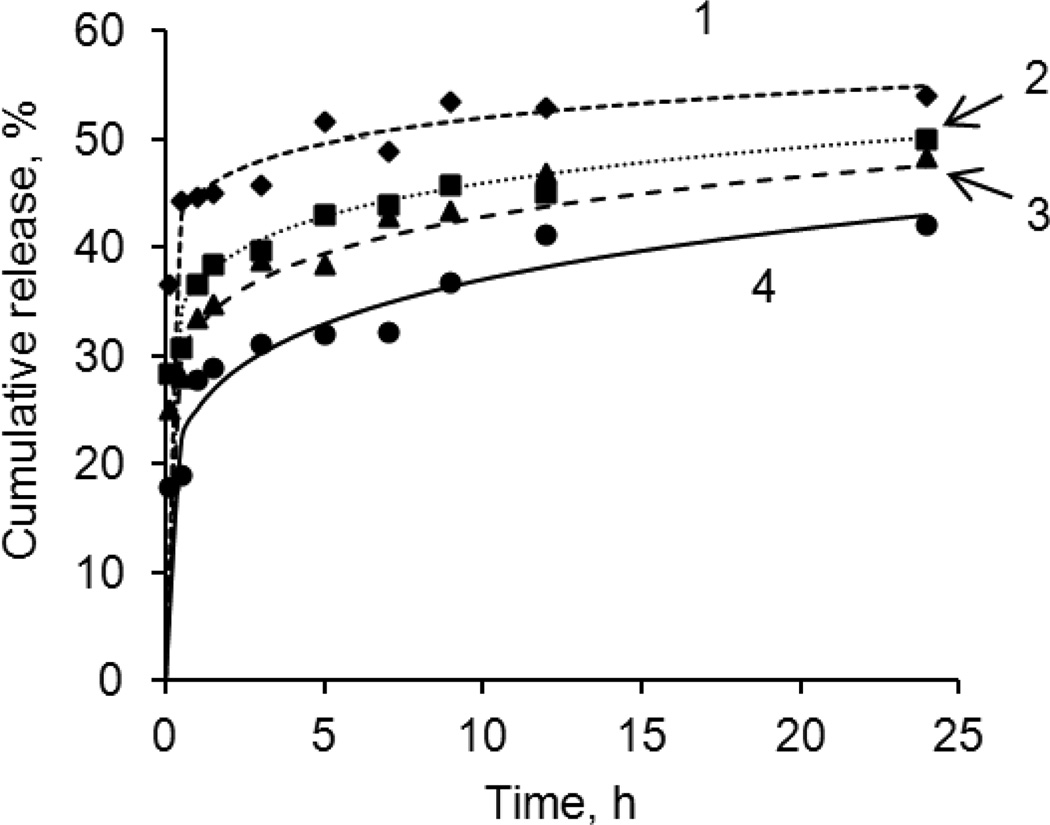
Paclitaxel and camptothecin possess an extremely low solubility in aqueous solutions (5.6 mg/L for PTX9 and <5.0 mg/L for CPT10) and without a solubility enhancer their released concentrations are very difficult to monitor.9–10 For all tested PTX colloids, a fast initial dissolution (more than 20 %) of the drug was observed within 15 min after addition of the nanoparticles to a 0.2 mg/mL Polysorbate 80 release medium. The rest of the drug was released very slowly; a complete dissolution of the nanoparticles was observed within 65–70 h (Fig. 8). No influence on release rate was found for shells thinner than 3.5 PLB16-5/Hep bilayers (SI, Fig. L). With larger number of polyelectrolyte layers (4, 8 and 12 bilayers of PLL and heparin), a slight decrease in the drug release rate with increasing number of layers in the shell was detected.
Fig. 8.
PTX release from 300 nm nanocolloids prepared using top-down sonication assisted LbL method8 and coated with a (PLL/Hep)n shell. Number of bilayer in shell, n: 1– 0.5, 2–4, 3–8, 4–12. 0.2% Polysorbate 80 in water at 37 °C
Camptothecin is less soluble than paclitaxel and its slow 20 hours release from 150-nm capsules with (Hep/PLB16-5)4.5 shells was recorded in PBS containing 2% Polysorbate 80 as a solubility enhancer (SI, Fig. M). Without solubility enhancers, we expect even slower drug release kinetics.
Conclusions
Combination of desolvation nanocores formation with LbL shell assembly is a logical development of efforts towards stable concentrated aqueous dispersions of poorly soluble substances, particularly low solubility drugs. Our approach is aimed at stabilization of 140–180 nm camptothecin and paclitaxel colloids by building strongly charged polyelectrolyte shells around the amphiphile-stabilized drug cores.
First, we developed washless LbL technique for multiple polyelectrolyte coating of drug nanocores, both to avoid common centrifugation-based LbL technique and so that entire assembly was performed in the solution containing stabilizers necessary for drug cores formation. Skipping washing-centrifugation steps allowed avoiding sample losses and achieving high concentration of nanoparticles in the dispersion. After a LbL polyelectrolyte shell was completed, the formed nanocapsules were separated from supernatant by centrifugation and concentrated.
Second, nanoparticles aggregation in the process of LbL assembly was prevented by applying low molecular weight flexible block-copolymers of poly-L-lysine with hydrophilic polyethylene glycol (PEG) sequentially adsorbed with anionic heparin and bovine serum albumin. By using all polyelectrolytes in minimal amounts (just to recharge nanoparticles), the quantity of polyelectrolytes remaining unreacted at each step was minimized. With this we avoided nanoparticles aggregation in bulk solution. An advantage of PEGylated polyelectrolytes consists in supplementing electrostatic repulsion of polyelectrolyte shells with interaction of hydrophilic PEG tails, which prevent nanoparticles aggregation under intermediate conditions when surface charge of nanocapsules is low. This preserves colloidal stability of concentrated dispersions during addition of oppositely charged polyelectrolyte.
PEGylated polyelectrolyte shells provide drug nanoparticles with high colloidal stability in PBS buffer for an extended period of time and moderate protein adhesion resistance; a moderate extension of the drug release time was detected for 4–8 bilayer capsule walls. Variable LbL shell architecture of such nanocapsules can be further developed for anchoring tumour targeting agents.
Supplementary Material
Acknowledgements
This work was supported by Award 1R01CA134951 from the National Cancer Institute (NCI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or National Institute of Health. The authors acknowledge Dr. T. Levchenko for preliminary studies of nanoparticles activity and tolerability in mice and G. Parekh for assistance with camptothecin colloids preparation.
Footnotes
Electronic Supplementary Information (ESI) available: [Colloidal stability of LbL- coated PTX nanoparticles (prepared in water) in different media; TEM images of CPT nanoparticles coated with a Hep/(PLB16-5/Hep)4 shell; content of surfactants remaining adsorbed on PTX colloids; DLS distribution of apparent hydrodynamic diameter of LbL coated PTX nanoparticles; changes of ζ-potential and amounts of negative sites estimated by the pinacyanol assay in the process of step-wise addition of PLL and Hep to PTX nanoparticles; influence of PVP concentration on amounts of PLB and Hep added to reverse ζ-potential of nanoparticles; changes of apparent hydrodynamic diameter of PXT nanoparticles in the process of step-wise addition of heparin to PTX nanoparticles coated with a PLB and a PLL layer; changes of hydrodynamic diameter of CPT nanoparticles upon adsorption of different polyelectrolytes on their surface; degradation of the lactone form of CPT in PBS buffer and a BSA solution for free CPT and LbL-coated nanoparticles; apparent diameter of selected samples of PTX nanoparticles coated with a (PLB/Hep)3.5 shell as a function of time; changes of ζ- potential and hydrodynamic diameter in the process of LbL assembly using BSA; changes of hydrodynamic diameter in the process of LbL assembly of shells with different architecture using non-PEGylated and PEGylated polylysines; PTX release from nanocolloids coated with a (PLB/Heparin)n shell; release of CPT from nanocapsules with a (Hep/PLB)4.5 shell architecture]. See DOI: 10.1039/b000000x/
Notes and references
- 1.Marriott J, Wilson K, Langley C, Belcher D. Pharmaceutical Compounding and Dispensing. Pharmaceutical Press; 2006. p. 277. [Google Scholar]; Tsuzuki T. Int. J. Nanotech. 2009;6:567. [Google Scholar]; Sivasankar M, Kumar B. Int. J. Reseach Pharmaceut. Biomed. Sci. 2010;1:41. [Google Scholar]
- 2.Chen H, Khemtong C, Yang X, Chang X, Gao J. Drug Discov. Today. 2011;16:354. doi: 10.1016/j.drudis.2010.02.009. [DOI] [PubMed] [Google Scholar]; Keck C, Kobierski S, Mauludin R, Muller R. Dosis. 2008;2:124. [Google Scholar]
- 3.Lee J, Choi JY, Park CH. Int. J. Parm. 2008;355:328. doi: 10.1016/j.ijpharm.2007.12.032. [DOI] [PubMed] [Google Scholar]; Kahlweit M. Adv. Colloid Interface Sci. 1975;5:1. [Google Scholar]; Parikh I. 5922355. US Pat. 1999; Muller RH, Jacobs C, Kayser O. In: Pharmaceutical Emulsions and Suspensions. Drugs and the Pharmaceutical Sciences. Nielloud F, Marti-Mestres G, editors. Vol. 105. Marcel Dekker: 2000. p. 383. [Google Scholar]
- 4.Kim S, Kim JY, Huh KM, Acharya G, Park K. J. Control. Release. 2008;132:222. doi: 10.1016/j.jconrel.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cho YW, Lee J, Lee SC, Huh KM, Park K. J. Control. Release. 2004;97:249. doi: 10.1016/j.jconrel.2004.03.013. [DOI] [PubMed] [Google Scholar]; Wei XH, Nui YP, Xu YY, Du YZ, Hu FQ, Yuan H. Journal of Bioactive and Compatible Polymers. 2010;25:319. [Google Scholar]
- 5.Praetorius N, Mandal T. Recent Patents on Drug Delivery @Formulation. 2007;1:37. doi: 10.2174/187221107779814104. [DOI] [PubMed] [Google Scholar]; Zahr AS, Pishko MV. Biomacromolecules. 2007;8:2004. doi: 10.1021/bm070177m. [DOI] [PubMed] [Google Scholar]; Yu X, Pishko MV. Biomacromolecules. 2011;2:3205. doi: 10.1021/bm200681m. [DOI] [PubMed] [Google Scholar]; Luxenhofer R, Schulz A, Roques C, Li S, Bronich T, Batrakova E, Jordan R, Kabanov A. Biomaterials. 2010;31:4972. doi: 10.1016/j.biomaterials.2010.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lvov Y. In: Protein Architecture: Interfacial Molecular Assembly and Immobilization Biotechnology. Lvov Y, Möhwald H, editors. NY: Marcel Dekker Publ.; 2000. p. 1. [Google Scholar]; Sukhorukov . In: Dentrimers. Arshady R, Guyot A, editors. Vol. 5. NY: Citrus Books; 2002. p. 111. MML Series. [Google Scholar]; De Geest B, Sukhorukov G, Möhwald H. Expert Opinions Drug Deliv. 2009;6:613. doi: 10.1517/17425240902980162. [DOI] [PubMed] [Google Scholar]
- 7.Qiu X, Leporatti S, Donath E, Möhwald H. Langmuir. 2001;17:5375. [Google Scholar]; Ye SQ, Wang CY, Liu XX, Tong Z. J. Control. Release. 2005;106:319. doi: 10.1016/j.jconrel.2005.05.006. [DOI] [PubMed] [Google Scholar]; Antipov A, Sukhorukov G. Adv. Colloid Interface Sci. 2004;111:49. doi: 10.1016/j.cis.2004.07.006. [DOI] [PubMed] [Google Scholar]; Caruso F. Adv. Materials. 2001;13:11. [Google Scholar]; Antipov A, Sukhorukov G, Donath E, Möhwald H. J.Phys.Chem. B. 2001;105:2281. [Google Scholar]; Ai H, Jones S, de Villiers M, Lvov Y. J. Controlled Release. 2003;86:59. doi: 10.1016/s0168-3659(02)00322-x. [DOI] [PubMed] [Google Scholar]; Palankar R, Skirtach A, Kreft O, Bedard M, Garstka M, Gould K, Möhwald H, Sukhorukov G, Winterhalter M, Springer S. Small. 2009;5:2168. doi: 10.1002/smll.200900809. [DOI] [PubMed] [Google Scholar]; Pargaonkar N, Lvov Y, Li N, Steenekamp J, de Villiers M. Pharm. Res. 2005;22:826. doi: 10.1007/s11095-005-2600-0. [DOI] [PubMed] [Google Scholar]; Sheney D, Sukhorukov G. Eur. J. Phar. Biopharm. 2004;58:521. doi: 10.1016/j.ejpb.2004.05.008. [DOI] [PubMed] [Google Scholar]; Bédard M, De Geest B, Skirtach A, Möhwald H, Sukhorukov G. Adv. Colloid Interface Sci. 2010;158:2. doi: 10.1016/j.cis.2009.07.007. [DOI] [PubMed] [Google Scholar]; De Cock L, De Koke S, De Geest B, Grooten J, Vervaet C, Je. Remon, Sukhorukov G, Antipina M. Angew. Chem. 2010;122:9820. doi: 10.1002/anie.200906266. [DOI] [PubMed] [Google Scholar]; Yuan J, Zhou S, You B, Wu L. Chem. Mater. 2005;17:3587. [Google Scholar]
- 8.Agarwal A, Lvov Y, Sawant R, Torchilin V. J. Control. Release. 2008;128:255. doi: 10.1016/j.jconrel.2008.03.017. [DOI] [PubMed] [Google Scholar]; Lvov Y, Agarwal A, Sawant R, Torchilin V. Pharma Focus Asia. 2008;7:36. [Google Scholar]; Zheng Z, Zhang X, Carbo D, Clark C, Nathan C-A, Lvov Y. Langmuir. 2010;26:7679. doi: 10.1021/la101246a. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pattekari P, Zheng Z, Zhang X, Levchenko T, Torchilin V, Lvov Y. Phys. Chem. Chem. Phys. 2011;13:9014. doi: 10.1039/c0cp02549f. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lvov Y, Pattekari P, Zhang X, Torchilin V. Langmuir. 2011;27:1212. doi: 10.1021/la1041635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrente K, Winograd B, Canetta R. Cancer Chemother. Pharmacol. 1999;43:S61. doi: 10.1007/s002800051100. [DOI] [PubMed] [Google Scholar]; Feng S, Huang G. J. Control. Release. 2001;71:53. doi: 10.1016/s0168-3659(00)00364-3. [DOI] [PubMed] [Google Scholar]; Li F, Li J, Wen X, Zhou S, Tong X, Su P, Li H, Shi D. Material Science and Engineering: C. 2009;29:2392. [Google Scholar]; Liu Y, Huang L, Liu F. Molecular Pharmaceutics. 2010;7:863. doi: 10.1021/mp100012s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatefi A, Amsden B. Pharmaceutical Recearch. 2002;19:1389. doi: 10.1023/a:1020427227285. [DOI] [PubMed] [Google Scholar]; McCarron P, Marouf W, Quinn D, Fay F, Burden R, Olwill S, Scott C. Bioconjugate Chemistry. 2008;19:1561. doi: 10.1021/bc800057g. [DOI] [PubMed] [Google Scholar]; Sanna N, Chillemi G, Gontrani L, Grandi A, Mancini G, Castelli S, Zagotto G, Zazza C, Barone V, Desideri A. J. Phys. Chem. B. 2009;113:5369. doi: 10.1021/jp809801y. [DOI] [PubMed] [Google Scholar]; Dora C, Alvarez-Silva M, Trentin A, de Faria T, Fernandes D, da Costa R, Stimamiglio M, Lemos-Senna E. J. Pharm. Pharmaceut. Sci. 2006;9:22. [PubMed] [Google Scholar]; Hausheer F, Haridas K, Murali D, Reddy D. 5726181. US Pat. 1998 [PubMed]
- 11.Schneider G, Decher G. Nano Letters. 2004;4:1833. [Google Scholar]; Chodanowski P, Stoll S. J.Chem.Physics. 2001;115:4951. [Google Scholar]; Li Y, Xia J, Dubin PL. Macromolecules. 1994;27:7049. [Google Scholar]; Vertegel AV, Siegel RW, Dordick JS. Langmuir. 2004;20:6800. doi: 10.1021/la0497200. [DOI] [PubMed] [Google Scholar]
- 12.Guo Y, Sun Y, Gu J, Xu Y. Analytical Biochemistry. 2007;363:204. doi: 10.1016/j.ab.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Banchev G, Lu Z, Lvov Y. J. Nanosci. Nanotech. 2009;9:396. doi: 10.1166/jnn.2009.j055. [DOI] [PubMed] [Google Scholar]
- 14.Kugel R. In: Structure-Property Ralations in Polymer. Spectroscopy and Performance, ACS. Urban M, Craver C, editors. Vol. 236. 1993. p. 507. [Google Scholar]; Myers R, Fink J. 5032526. US Pat. 1991; Nandini R, Vishalakshi B. Orbital. 2009;1:255. [Google Scholar]
- 15.QCM 200. Quartz Crystal Microbalance Digital Controller. Operation and Service Manual. Stanford Research Systems, Inc.; 2005. p. 106. Revision 2.1. [Google Scholar]; Marx K. Biomacromolecules. 2003;4:1099. doi: 10.1021/bm020116i. [DOI] [PubMed] [Google Scholar]; Voros J. Biophysical J. 2004;87:553. doi: 10.1529/biophysj.103.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boudou T, Kharkar P, Jing J, Guillot R, Pignot-Paintrand I, Auzely-Velty R, Picart C. J. Control. Release. 2012 doi: 10.1016/j.jconrel.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]; You J, Hu F-Q, Du Y, Yuan H, Ye B. Nanotechnology. 2007;18:495101. doi: 10.1088/0957-4484/18/49/495101. [DOI] [PubMed] [Google Scholar]; Shi B, Shen Z, Bi J, Dai S. Biomacromolecules. 2012;13:146. doi: 10.1021/bm201380e. [DOI] [PubMed] [Google Scholar]; Chae S, Son S, Lee M, Jang M, Nah J. J.Control Release. 2005;109:330. doi: 10.1016/j.jconrel.2005.09.040. [DOI] [PubMed] [Google Scholar]; Nah J, Jung T, Jang M, Jeong Y. 0013885. US Pat. Application. 2006; Liu W, Zhang X, Sun S, Sun G, Yao K. Bioconjugate Chem. 2003;14:782. doi: 10.1021/bc020051g. [DOI] [PubMed] [Google Scholar]
- 17.Assuming the polymers are fully charged, 1 mg of PLB16-5 corresponds to 4.6 µmol of amine groups. 1 mg of Heparin is equal to 6.2 µmol (or 4.8 µmol) of charged groups, if one heparin polymer unit contains four (or three) charged groups. It gives 1.38 mol/mol (or 1.05 mol/mol) negative to positive charge binding ratio for Hep on the top of a PLB16-5-coated colloid and 0.72 mol/mol (or 0.93 mol/mol) of PLB16-5 amine to Heparin negative charges.
- 18.Furusawa K, Kanesaka M, Yamashita S. J.Colloid Interface Sci. 1984;99:341. [Google Scholar]; Burke S, Barrett C. Biomacromolecules. 2003;4:1773. doi: 10.1021/bm034184w. [DOI] [PubMed] [Google Scholar]; Haynie D, Balkundi S, Palath N, Chakravarthula K, Dave K. Langmuir. 2004;20:4540. doi: 10.1021/la036330p. [DOI] [PubMed] [Google Scholar]
- 19.Rashba–Step J, Darvari R, Lin Q, Kelly J, Shutava T, Lvov Y, Scott T. 33rd Annual Meeting and Exposition of the Controlled Release Society; 2006. p. 75/477. [Google Scholar]; Rashba-Step J, Scott T, Darvari R, Lvov Y, Shutava T. 0260777. US Pat. 2006
- 20.Castro J, Trzaskowski B, Deymier P, Bucay J, Adamowicz L, Hoying J. Material Science and Engineering: C. 2009;29:1609. [Google Scholar]
- 21.Harris J, Zalipsky S, editors. Poly(ethylene glycol). Chemistry and Biological Applications; ACS Symposium Series; 1997. p. 490. [Google Scholar]
- 22.Moghimi S, Szebeni J. Progress in Lipid Research. 2003;42:463. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.