Abstract
Aim
To determine the diagnostic value of single symptoms and signs for coronary heart disease (CHD) in patients with chest pain.
Methods
Searches of two electronic databases (EMBASE 1980 to March 2008, PubMed 1970 to May 2009) and hand searching in seven journals were conducted. Eligible studies recruited patients presenting with acute or chronic chest pain. The target disease was CHD, with no restrictions regarding case definitions, eg, stable CHD, acute coronary syndrome (ACS), acute myocardial infarction (MI), or major cardiac event (MCE). Diagnostic tests of interest were items of medical history and physical examination. Bivariate random effects model was used to derive summary estimates of positive (pLR) and negative likelihood ratios (nLR).
Results
We included 172 studies providing data on the diagnostic value of 42 symptoms and signs. With respect to case definition of CHD, diagnostically most useful tests were history of CHD (pLR = 3.59), known MI (pLR = 3.21), typical angina (pLR = 2.35), history of diabetes mellitus (pLR = 2.16), exertional pain (pLR = 2.13), history of angina pectoris (nLR = 0.42), and male sex (nLR = 0.49) for diagnosing stable CHD; pain radiation to right arm/shoulder (pLR = 4.43) and palpitation (pLR = 0.47) for diagnosing MI; visceral pain (pLR = 2.05) for diagnosing ACS; and typical angina (pLR = 2.60) and pain reproducible by palpation (pLR = 0.13) for predicting MCE.
Conclusions
We comprehensively reported the accuracy of a broad spectrum of single symptoms and signs for diagnosing myocardial ischemia. Our results suggested that the accuracy of several symptoms and signs varied in the published studies according to the case definition of CHD.
Chest pain is a common complaint in all health care settings, with one of the relevant causes being coronary heart disease (CHD) (1). Despite advanced diagnostic technology being available to diagnose CHD, important first steps in the evaluation of patients with chest pain are history and physical examination. Since they allow to more appropriately identify patients in need of further investigations, they help to protect patients from harm caused by unnecessary testing and to save costs. The risk of an underlying CHD can be assessed by many symptoms, signs, and items of the medical history, each of which can be seen as a diagnostic test. Like in the case of laboratory or imaging tests, their accuracy should be assessed rigorously and corresponding recommendations should be based on the best available evidence.
The accuracy of medical history and physical examination for diagnosing CHD has been the subject of previous reviews. Mant et al (2) restricted their research question to the diagnostic value of signs and symptoms for acute coronary syndrome (ACS) and myocardial infarction (MI) in studies published until 1999. Bruyninckx et al (3) also limited the scope of their review to the outcomes ACS and MI. Furthermore, they narrowed down their review on the value of 10 pre-specified clinical symptoms and signs. Chun and McGee (4) did not restrict their research question to pre-specified symptoms, signs, or case definitions of CHD but searched only Medline. The search was conducted in 2003. Two of these reviews reported a substantial variance of results across studies but none addressed the question of potential sources of heterogeneity (2,3). None of these reviews used statistical methods currently being recommended for diagnostic accuracy reviews (5).
The aim of this study was to perform a comprehensive systematic review and quantitative meta-analysis to determine the diagnostic value of medical history and physical examination for CHD in patients with chest pain. Additionally, we explored the amount and potential sources of heterogeneity between studies.
Methods
Search strategy and study selection
Studies that were considered eligible were those recruiting patients presenting with acute or chronic chest pain. The target disease was CHD, with no restrictions regarding case definitions, eg, stable CHD, ACS, MI, or major cardiac event (MCE). Diagnostic tests of interest were any items of physical examination or medical history like pain characteristics, associated symptoms, cardiovascular risk factors, or history of cardiac conditions. In the following text, we refer to these items as index tests or tests. Initially, no restrictions with regard to the definition or wording of index tests were made. Studies to be included had to present data on the diagnostic value of at least one test. We considered only those tests where data for a 2 × 2 table were available in three or more studies. Otherwise, there were no restrictions in regard to the setting, study design, or study quality. However, the studies investigating solely patients with a diagnosis of CHD were excluded.
We conducted comprehensive searches of two electronic databases (EMBASE 1980 to March 2008, PubMed 1970 to August 2007). The PubMed search was updated in May 2009. Along with terms to identify chest pain and terms to identify CHD, diagnostic terms were used. Search strategies included subject headings (MeSH, Embtree) as well as free-text terms and were restricted to English and German. Detailed search strategies are available from the authors. Additionally, we searched seven relevant journals (American Heart Journal, American Journal of Cardiology, Journal of the American College of Cardiology, Circulation, European Heart Journal, Heart, Clinical Research in Cardiology) by hand from 1970 to June 2009 and screened the reference lists of review articles and eligible studies.
One out of three reviewers screened all identified titles and abstracts for inclusion. If uncertainty remained, full-text articles were retrieved. All potentially relevant full text articles were comprehensively assessed for eligibility. All eligible studies were reassessed by a second reviewer. Any disagreements were resolved by discussion.
Data extraction and quality assessment
One out of three reviewers extracted relevant data, eg, bibliographic information, study design, reference disease, reference standard, definition of the index test, number of true and false positives, and true and false negatives from each study. A random sample of 10% of the data records was checked by a fourth reviewer.
Two reviewers independently assessed the study quality using the Quality of Diagnostic Accuracy Studies (QUADAS) framework (6). The categories “not clear” and “no” were combined into one. Since some of the questions referred to the index test, the assessment was made for each single index test if a study presented data on more than one test. Because items of the medical history and physical examination were the diagnostic tests of interest, question 12 (presence of clinical information for interpretation of reference tests) was omitted. In case of disagreement, the study quality was reassessed by a third reviewer.
Analysis and data synthesis
The steps of the analysis described below were conducted separately for each index test. From the 2 × 2 tables we calculated sensitivity, specificity, and positive (pLR) and negative likelihood ratios (nLR). Additionally, we drew forest plots of sensitivity and specificity and plotted sensitivity against 1-specificity in the receiver operating characteristics (ROC) space.
We used several approaches to explore potential sources of heterogeneity and calculated the I2 statistic. It can be interpreted as the percentage of total variability that is due to true heterogeneity rather than sampling error (7). A special source of heterogeneity in diagnostic accuracy reviews is the so-called threshold effect, which occurs when different cut-off values are used to define test positives across primary studies. Because a negative correlation between sensitivities and specificities indicates a threshold effect, we calculated Spearman rank correlations between the logits of sensitivity and specificity (8). The R2 statistic was used to quantify the amount of variation that could be explained by differences in thresholds (9). If the number of studies was ≥10, we performed a meta-regression to examine whether study specific characteristics affected the results. The covariates considered in the meta-regression are listed in Table 1. Concrete definitions and categories for each covariate are provided in the supplementary Table 1.(web extra material 1)
Table 1.
Covariates used in the meta-regression*
| Criteria describing study quality: |
|---|
| Representative spectrum? |
| Acceptable reference standard? |
| Partial verification avoided? |
| Differential verification avoided? |
| Incorporation avoided? |
| Details execution index test? |
| Index test results blinded? |
| Reference standard results blinded? |
| Withdrawals explained? |
| Criteria describing clinical characteristics of patients and methodological characteristics of studies: |
|---|
| Reference diagnosis/ case definition of coronary heart disease |
| Reference standard |
| Setting |
| Patients selected? |
| Definite sick excluded? |
| Pain acute – intermediate? |
| Prevalence of reference disease |
*For details see supplementary Table 1.(web extra material 1)
In addition to the statistical analyses, two reviewers independently assessed the forest plots and ROC curves visually (eye-balling) (10). They rated the amount of heterogeneity, the presence of a threshold effect, and the effect of the respective covariates. Any disagreements were resolved by discussion. This procedure was performed separately for each index test.
Based on the findings of the analyses of heterogeneity, we calculated summary estimates of sensitivity, specificity, and likelihood ratios using a bivariate random effects model (BREM). This approach was used because it preserves the original two-dimensional nature of the data. Using pairs of sensitivity and specificity it accounts for any possible negative correlation between these two measures. In addition, study size and between-study heterogeneity using a random effects model are taken into account (5,10,11). However, as hierarchical models are complex they do not always produce stable estimates, especially if the number of studies is small. In such cases, we presented the results of the primary studies and qualitative syntheses instead of summary estimates.
We used likelihood ratios (LR) as measures of diagnostic accuracy since they are clinically more meaningful than sensitivities, specificities, or diagnostic odds ratios. They quantify how strongly the likelihood of a disease is changed by the presence (pLR) or absence (nLR) of a symptom or sign. A LR of 1 indicates a test result without any diagnostic value. LRs of 1 to 2 or 0.5 to 1 change the likelihood very little and they are rarely important (12). LRs ≤0.5 or ≥2 change it at least moderately and can be considered as clinically helpful in the context of medical history taking and physical examination.
I2 statistic was calculated using MetaDiSc software (13). Meta-regressions and pooling of estimates were performed using SAS 9.2 (14), particularly the SAS macro METADAS (15). All other calculations were performed using SPSS 17 (16).
Results
Characteristics of included studies
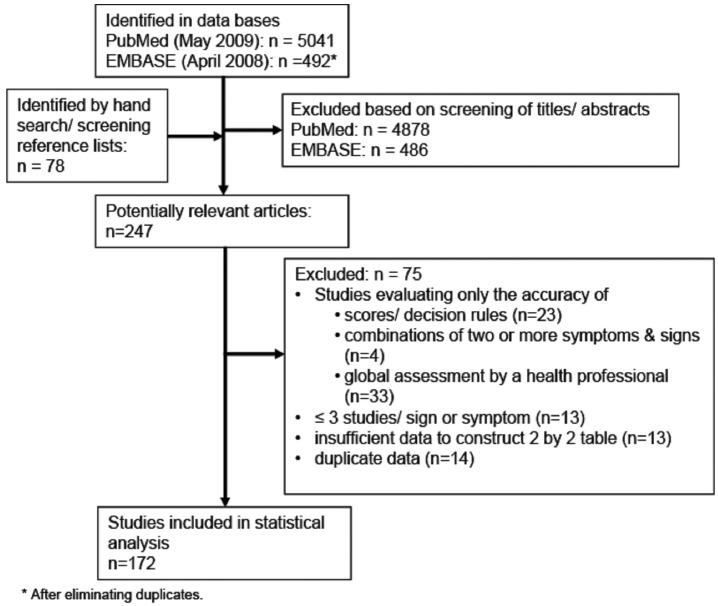
The electronic search identified 5533 records. After having screened for inclusion criteria, we comprehensively assessed 247 full text articles (Figure 1). The range of the tests described was very broad. We combined similar symptoms or signs into one index test if appropriate (details are provided in the supplementary Table 2(web extra material 2)). In the statistical analyses we considered 42 index tests, for which at least 3 studies provided sufficient data to construct 2 × 2 tables (Table 2).
Figure 1.
Flow of study selection.
Table 2.
Diagnostic criteria/index tests (n = 42) considered in the analyses
| Cardiovascular risk factors/pre-existing cardiac conditions: |
|---|
| Male sex |
| Higher age |
| History of diabetes mellitus |
| History of dyslipidemia |
| History of dyslipidemia |
| History of hypertension |
| History of coronary heart disease |
| History of myocardial infarction |
| History of myocardial infarction |
| History of angina pectoris |
| Family history of myocardial infarction |
| Smoking |
| Obesity |
| Menopause |
| Pain characteristics: |
|---|
| Central chest pain |
| Left-sided chest pain |
| Right-sided chest pain |
| Radiation to left arm/shoulder |
| Radiation to right arm/ shoulder |
| Radiation to back |
| Visceral pain |
| Stabbing pain |
| Burning pain |
| Frightening pain |
| Time since onset of pain more than about 6 h |
| Typical angina pectoris |
| Atypical angina |
| Pain relief by nitro-glycerine |
| Crescendo angina |
| Pain related to breathing |
| Pain related to effort |
| Associated symptoms: |
| Sweating |
| Dyspnea |
| Nausea/vomiting |
| Dizziness |
| Collapse |
| Palpitation |
| Weakness |
| Fear/ anxiety |
| Physicals: |
| High blood pressure |
| Tachycardia |
| Bradycardia |
| Rales |
| Pain reproducible by palpation |
The results of several single studies were reported in several papers. As the set of index tests sometimes varied across the papers, the final decision about duplicate data could only be made on the level of the individual index test. This resulted in 172 studies included in the analyses.
The studies were published between 1966 and 2009. The number of included patients ranged from 36 to 10 806 per study. Seventy-six percent of the included studies assessed the presence of the target disease in a consecutive series of chest pain patients and 85% were prospectively conducted (supplementary Table 3(web extra material 3)).
Heterogeneity and subgroup analyses
I2 ranged from 0 to 98.6% and was above 80% in 40 of the analyzed index tests, indicating a substantial amount of between-study heterogeneity (supplementary Table 4(web extra material 4)). In 22 tests, we found evidence of a threshold effect, indicated by a significant negative correlation between the logits of sensitivity and specificity. R2 ranged from 0.21 to 1.00, indicating the proportion of variance that can be explained by differences in thresholds (supplementary Table 5(web extra material 5)).
In the meta-regression, no covariate showed a consistent effect on the diagnostic accuracy. Among the covariates, reference diagnosis/case definition of CHD most frequently influenced the accuracy of an index test (supplementary Table 6(web extra material 6)). Therefore, we decided to provide pooled estimates across all studies and additionally within each subgroup determined by the case definition of CHD. The reference diagnosis or case definition of CHD used was ACS in 17%, MI in 43%, stable CHD in 35%, and MCE in 5% of the included studies. If ACS or MI was the target disease, the majority of patients presented with acute chest pain and the reference diagnosis was established by a combination of clinical presentation, biomarker elevations, and ECG changes (supplementary Table 3(web extra material 3)). ACS comprised ST elevation myocardial infarction, non-ST elevation myocardial infarction, and unstable angina. The first included study using this case definition as reference diagnosis was published in 1995 (17). If stable CHD was the target disease, the majority of patients presented with chronic/intermediate chest pain. In 74% of these 60 studies, the diagnosis was established using coronary angiography (supplementary Table 3(web extra material 3)). The outcome MCE comprised combinations of different outcomes such as MI, ventricular tachycardia, and revascularization. The prevalence of CHD with respect to the case definition of CHD ranged from 8.1 to 79.7% (ACS), from 3.8 to 88.0 (MI), from 16.7 to 89.9 (stable CHD), and from 2.8 to 34.1% (MCE).
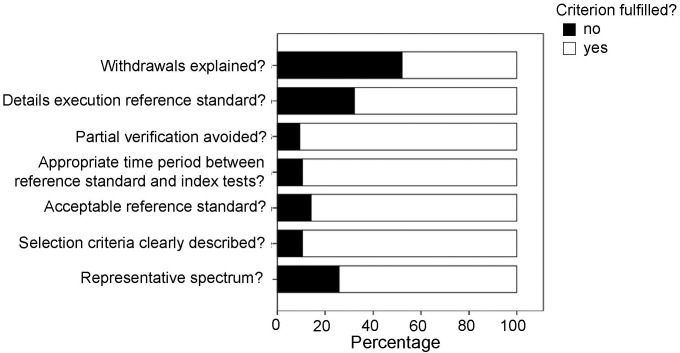
Out of the 13 QUADAS items, only 7 referred to the whole study. Considering these items, the quality of the included studies was fair (Figure 2). The other 6 items can only be applied to an individual index test. In 8 index tests, we found evidence that one or two of the quality criterions considered as covariates were significantly associated with the accuracy of an index test (supplementary Table 6(web extra material 6)). In that case, we calculated pooled estimates of pLR and nLR within the respective subgroups (supplementary Table 7(web extra material 7)).
Figure 2.
Methodological quality of included studies in regard to 7 quality criteria.
Diagnostic value of symptoms and signs
Cardiovascular risk factors and pre-existing cardiac conditions. Twelve index tests referred to the presence or absence of cardiovascular risk factors and pre-existing cardiac conditions. The number of studies per index test ranged from 5 (menopause) to 102 (male sex) (median: 49). For 11 of these index tests, a quantitative synthesis was possible across all studies and within subgroups. For the diagnosis of myocardial ischemia in general, pLRs ranged from 1.06 (obesity) to 1.67 (history of diabetes), and nLRs ranged from 0.70 (age, sex) to 0.97 (obesity). However, accuracy varied across subgroups determined by case definition of CHD. Within the stable CHD subgroup, the most helpful diagnostic criteria were history of diabetes (pLR = 2.16), history of CHD (pLR = 3.59), and history of MI (pLR = 3.21). In the MI subgroup, the confidence interval of the positive and negative LRs of 8 index tests included “1,” indicating that absence or presence of these findings did not significantly change the likelihood of CHD as an underlying cause (Table 3).
Table 3.
Diagnostic accuracy of cardiovascular risk factors and pre-existing cardiac conditions*
| Case definition of CHD |
Studies |
Patients |
LR (95% CI) if RF is |
|
|---|---|---|---|---|
| present | absent | |||
|
Male sex |
||||
| Any CHD |
102 |
99 959 |
1.21 (1.16-1.27) |
0.70 (0.65-0.75) |
| Stable CHD |
28 |
18 162 |
1.49 (1.35-1.63) |
0.49 (0.45-0.55) |
| MI |
44 |
33 776 |
1.12 (1.07-1.18) |
0.77 (0.71-0.85) |
| ACS |
22 |
33 125 |
1.17 (1.08-1.27) |
0.79 (0.71-0.88) |
| MCE |
8 |
14 896 |
1.13 (0.99-1.29) |
0.82 (0.67-1.00) |
|
Higher age |
||||
| Any CHD |
32 |
49 730 |
1.47 (1.34-1.60) |
0.70 (0.61-0.80) |
| Stable CHD |
7 |
10 804 |
1.49 (1.24-1.80) |
0.63 (0.47-0.85) |
| MI |
15 |
20 180 |
1.41 (1.25-1.59) |
0.76 (0.65-0.89) |
| ACS |
7 |
13 597 |
1.44 (1.19-1.73) |
0.69 (0.52-0.92) |
| MCE |
3 |
5149 |
1.75 (1.26-2.44) |
0.55 (0.33-0.93) |
|
History of diabetes mellitus |
||||
| Any CHD |
72 |
74 418 |
1.67 (1.47-1.90) |
0.91 (0.89-0.93) |
| Stable CHD |
30 |
16 371 |
2.16 (1.81-2.58) |
0.87 (0.84-0.90) |
| MI |
21 |
18 139 |
1.18 (0.97-1.43) |
0.97 (0.95-1.00) |
| ACS |
15 |
27 429 |
1.68 (1.35-2.09) |
0.89 (0.85-0.94) |
| MCE |
6 |
12 479 |
1.85 (1.30-2.64) |
0.87 (0.79-0.95) |
|
History of dyslipidemia |
||||
| Any CHD |
46 |
41 377 |
1.52 (1.34-1.73) |
0.73 (0.67-0.80) |
| Stable CHD |
23 |
15 908 |
1.53 (1.30-1.80) |
0.68 (0.60-0.76) |
| MI |
7 |
6331 |
1.20 (0.89-1.62) |
0.88 (0.73-1.06) |
| ACS |
12 |
15 634 |
1.62 (1.24-2.11) |
0.76 (0.66-0.87) |
| MCE |
4 |
3504 |
1.72 (1.07-2.75) |
0.75 (0.59-0.95) |
|
History of hypertension |
||||
| Any CHD |
70 |
61 858 |
1.30 (1.19-1.43) |
0.83 (0.78-0.89) |
| Stable CHD |
33 |
19 241 |
1.30 (1.16-1.46) |
0.80 (0.72-0.88) |
| MI |
19 |
16 077 |
1.10 (0.91-1.33) |
0.96 (0.88-1.04) |
| ACS |
14 |
17 185 |
1.55 (1.27-1.89) |
0.72 (0.62-0.84) |
| MCE |
4 |
9355 |
1.39 (0.98-1.98) |
0.78 (0.58-1.04) |
|
History of CHD |
||||
| Any CHD |
65 |
66 364 |
1.47 (1.25-1.74) |
0.83 (0.77-0.90) |
| Stable CHD |
13 |
4615 |
3.59 (2.63-4.90) |
0.59 (0.51-0.68) |
| MI |
32 |
28 077 |
0.97 (0.83-1.13) |
1.01 (0.94-1.09) |
| ACS |
15 |
29 616 |
1.70 (1.36-2.13) |
0.77 (0.68-0.86) |
| MCE |
5 |
4056 |
1.65 (1.09-2.50) |
0.80 (0.66-0.98) |
|
History of MI |
||||
| Any CHD |
52 |
47 056 |
1.37 (1.14-1.64) |
0.87 (0.80-0.95) |
| Stable CHD |
11 |
3234 |
3.21 (2.34-4.40) |
0.57 (0.49-0.67) |
| MI |
30 |
26 281 |
0.93 (0.80-1.09) |
1.03 (0.96-1.10) |
| ACS |
7 |
16 477 |
1.70 (1.24-2.33) |
0.80 (0.70-0.91) |
| MCE |
4 |
1064 |
1.42 (0.93-2.16) |
0.82 (0.65-1.04) |
|
History of angina pectoris |
||||
| Any CHD |
22 |
25 761 |
1.09 (0.92-1.30) |
0.93 (0.79-1.09) |
| Stable CHD |
2 |
490 |
1.40 (1.01-1.94) |
0.42 (0.23-0.77) |
| MI |
15 |
12 541 |
0.89 (0.77-1.03) |
1.08 (0.98-1.20) |
| ACS |
3 |
12 017 |
1.92 (1.37-2.68) |
0.57 (0.42-0.77) |
| MCE |
2 |
713 |
1.06 (0.80-1.42) |
0.91 (0.59-1.40) |
|
Family history of MI |
||||
| Any CHD |
34 |
28 060 |
1.19 (1.06-1.33) |
0.90 (0.85-0.96) |
| Stable CHD |
18 |
7403 |
1.07 (0.94-1.21) |
0.95 (0.86-1.04) |
| MI |
5 |
6397 |
1.20 (0.92-1.56) |
0.92 (0.81-1.04) |
| ACS |
8 |
13 748 |
1.44 (1.14-1.83) |
0.84 (0.76-0.94) |
| MCE |
3 |
512 |
1.44 (0.90-2.30) |
0.88 (0.73-1.05) |
|
Smoking |
||||
| Any CHD |
68 |
55 164 |
1.28 (1.20-1.37) |
0.87 (0.82-0.91) |
| Stable CHD |
34 |
19 512 |
1.38 (1.26-1.51) |
0.82 (0.77-0.88) |
| MI |
16 |
9900 |
1.24 (1.10-1.40) |
0.85 (0.76-0.95) |
| ACS |
13 |
16 265 |
1.15 (0.96-1.37) |
0.95 (0.88-1.02) |
| MCE |
5 |
9487 |
1.06 (0.82-1.37) |
0.96 (0.82-1.13) |
|
Obesity |
||||
| Any CHD |
12 |
9852 |
1.06 (0.88-1.26) |
0.97 (0.89-1.07) |
| Stable CHD |
7 |
8062 |
1.07 (0.89-1.30) |
0.95 (0.83-1.09) |
| MI |
2 |
451 |
0.95 (0.62-1.46) |
1.03 (0.81-1.32) |
| ACS |
2 |
1219 |
1.03 (0.59-1.80) |
0.99 (0.88-1.12) |
| MCE | 1 | 120 | 1.11 (0.36-3.38) | 0.98 (0.79-1.22) |
*Abbreviations: CHD – coronary heart disease; MI – myocardial infarction; ACS – acute coronary syndrome; MCE – major cardiac event; LR – likelihood ratio; RF – risk factor; CI – confidence interval.
Quantitative synthesis was not possible for one risk factor (menopause). Results of pLR and nLR in the five primary studies ranged from 1.11 to 1.18, and 0.62-0.75, respectively (supplementary Table 8(web extra material 8)).
Pain characteristics. Seventeen index tests referred to pain characteristics such as localization or radiation, pain quality, time of onset, and provoking or revealing factors. The number of studies per index test ranged from 3 (crescendo angina, pain related to breathing) to 17 (visceral pain) (median: 9).
In 14 index tests quantitative syntheses across all studies were possible, and in 10 index tests subgroup analyses in regard to case definition of CHD (Table 4). For the diagnosis of myocardial ischemia in general, most helpful pain characteristics were the presence of pain radiation to right arm/shoulder (pLR = 2.95) and typical angina (pLR = 2.36). In all other index tests, positive and negative LRs ranged between 0.5 and 2.0. In two index tests, the accuracy varied substantially across subgroups determined by the case definition of CHD. In pain radiation to right arm/shoulder, pLR was 4.43 if MI was the target disease and 1.42 if stable CHD was the target disease. The presence of stabbing pain showed a pLR of 3.65 if ACS was the target disease, 0.90 for stable CHD, and 0.69 for MI. However, only one study contributed to the diagnostic outcome ACS (18). The 95% confidence interval ranged from 0.45 to 29.94 and only 10 out of 248 patients presented with stabbing pain. In three index tests (left-sided chest pain, radiation to left arm/shoulder, frightening pain), quantitative syntheses across all studies were done but no subgroup analyses were possible. The qualitative subgroup analyses showed similar between- and within-subgroup variation (supplementary Table 9(web extra material 9)). In further three index tests (radiation to back, crescendo angina, and pain related to breathing), only qualitative syntheses were possible. Results showed that pain related to breathing might be helpful for ruling out myocardial ischemia, with pLR ranging from 0.20 to 0.36 in primary studies.
Table 4.
Diagnostic accuracy of pain characteristics
| Case definition of CHD |
Studies |
Patients |
LR (95% CI) if symptom is |
|
|---|---|---|---|---|
| present present | absent | |||
|
Central chest pain |
||||
| Any CHD |
14 |
11 673 |
1.26 (1.14-1.40) |
0.69 (0.53-0.92) |
| Stable CHD |
5 |
885 |
1.36 (1.09-1.69) |
0.65 (0.38-1.10) |
| MI |
9 |
10 788 |
1.23 (1.10-1.38) |
0.71 (0.50-0.99) |
|
Left-sided chest pain |
||||
| Any CHD† |
12 |
5608 |
0.85 (0.60-1.20) |
1.06 (0.96-1.18) |
|
Right-sided chest pain |
||||
| Any CHD |
5 |
2170 |
1.06 (0.55-2.05) |
0.99 (0.86-1.14) |
| Stable CHD |
2 |
535 |
0.76 (0.51-1.14) |
1.17 (0.81-1.68) |
| MI |
3 |
1635 |
1.39 (0.58-3.34) |
0.96 (0.87-1.06) |
|
Radiation to left arm/shoulder |
||||
| Any CHD† |
12 |
16 316 |
1.30 (1.12-1.52) |
0.86 (0.78-0.95) |
|
Radiation to right arm/shoulder |
||||
| Any CHD |
9 |
2717 |
2.95 (1.42-6.12) |
0.87 (0.80-0.96) |
| Stable CHD |
3 |
627 |
1.42 (0.66-3.03) |
0.93 (0.82-1.05) |
| MI |
6 |
2090 |
4.43 (1.77-11.10) |
0.87 (0.77-0.97) |
|
Visceral pain |
||||
| Any CHD |
17 |
15 059 |
1.34 (1.07-1.67) |
0.78 (0.60-1.02) |
| Stable CHD |
5 |
885 |
1.34 (1.01-1.78) |
0.68 (0.37-1.22) |
| MI |
10 |
13194 |
1.21 (0.89-1.63) |
0.87 (0.66-1.15) |
| ACS |
2 |
980 |
2.05 (1.14-3.68) |
0.63 (0.28-1.43) |
|
Stabbing pain |
||||
| Any CHD |
11 |
12 269 |
0.86 (0.48-1.56) |
1.02 (0.94-1.10) |
| Stable CHD |
4 |
939 |
0.90 (0.41-2.00) |
1.02 (0.87-1.19) |
| MI |
6 |
11082 |
0.69 (0.34-1.40) |
1.04 (0.94-1.15) |
| ACS |
1 |
248 |
3.65 (0.45-29.94) |
0.93 (0.83-1.04) |
|
Burning pain |
||||
| Any CHD |
7 |
2582 |
1.38 (0.93-2.04) |
0.97 (0.93-1.01) |
| Stable CHD |
2 |
535 |
1.51 (0.74-3.08) |
0.96 (0.90-1.03) |
| MI |
5 |
2047 |
1.35 (0.87-2.09) |
0.97 (0.93-1.02) |
|
Frightening pain |
||||
| Any CHD† |
4 |
1608 |
1.40 (0.61-3.21) |
0.92 (0.79-1.08) |
|
Time since onset of pain >6 h |
||||
| Any CHD |
9 |
14 499 |
0.85 (0.66-1.09) |
1.09 (0.95-1.26) |
| MI |
6 |
12 212 |
0.82 (0.59-1.14) |
1.10 (0.93-1.29) |
| ACS |
3 |
2287 |
0.90 (0.62-1.30) |
1.08 (0.82-1.44) |
|
Typical angina |
||||
| Any CHD |
14 |
28 482 |
2.36 (1.47-3.79) |
0.61 (0.44-0.86) |
| Stable CHD |
13 |
19 720 |
2.35 (1.44-3.83) |
0.61 (0.43-0.87) |
| MCE |
1 |
8762 |
2.60 (0.46-14.63) |
0.58 (0.17-2.06) |
|
Atypical angina |
||||
| Any CHD |
5 |
19 524 |
0.72 (0.53-0.97) |
1.28 (1.01-1.62) |
| Stable CHD |
4 |
10 762 |
0.74 (0.53-1.05) |
1.22 (0.96-1.57) |
| MCE |
1 |
8762 |
0.64 (0.37-1.11) |
1.53 (0.87-2.67) |
|
Pain relief by nitro-glycerine |
||||
| Stable CHD |
9 |
2813 |
1.25 (1.05-1.48) |
0.77 (0.65-0.92) |
|
Pain related to effort |
||||
| Any CHD |
8 |
2975 |
1.89 (1.23-2.92) |
0.74 (0.56-0.97) |
| Stable CHD |
6 |
1302 |
2.13 (1.36-3.33) |
0.67 (0.49-0.91) |
| MI | 2 | 1673 | 1.22 (0.50-2.96) | 0.94 (0.69-1.28) |
*Abbreviations: CHD – coronary heart disease; MI – myocardial infarction; ACS – acute coronary syndrome; MCE – major cardiac event; LR – likelihood ratio; CI – confidence interval.
†Results for different case definitions of CHD not provided, since the random effects meta-analysis with covariate “case definition” did not produce stable estimates.
Associated symptoms and signs. Eight index tests referred to the presence or absence of associated symptoms. The number of studies per index test ranged from 3 (fear/anxiety) to 20 (dyspnea) (median: 9.5). A quantitative synthesis across all studies to determine the accuracy of the diagnosis of myocardial ischemia in general was possible in 7 index tests (Table 5). The most helpful symptom was the presence of sweating (pLR = 2.05). In most index tests, variation between subgroups was similar to the variation within subgroups (supplementary Table 10(web extra material 10)). In the index test palpitations, the pLR was 0.47 for the diagnosis of MI, 0.61 for ACS, and 0.66 for stable CHD. In the index test collapse studies using the diagnostic outcome, ACS showed a pLR ranging from 0.25 to 0.42. In contrast, if MI was the diagnostic outcome pLRs ranged from 0.66 to 1.82.
Table 5.
Diagnostic accuracy of associated symptoms and physical examination
| Case definition of CHD |
Studies |
Patients |
LR (95% CI) if symptom or sign is |
|
|---|---|---|---|---|
| present | absent | |||
|
Sweating |
||||
| Any CHD† |
11 |
16 011 |
2.05 (1.73-2.42) |
0.73 (0.61-0.87) |
|
Dyspnea |
||||
| Any CHD |
20 |
27 322 |
0.86 (0.77-0.96) |
1.08 (1.01-1.14) |
| Stable CHD |
6 |
2914 |
0.95 (0.78-1.15) |
1.04 (0.91-1.18) |
| MI |
10 |
11 939 |
0.89 (0.76-1.03) |
1.06 (0.98-1.15) |
| ACS |
4 |
12 469 |
0.72 (0.56-0.93) |
1.14 (0.99-1.30) |
|
Nausea/vomiting |
||||
| Any CHD† |
13 |
16 082 |
1.54 (1.32-1.79) |
0.83 (0.75-0.92) |
|
Dizziness |
||||
| Any CHD† |
7 |
11 456 |
0.59 (0.52-0.67) |
1.13 (1.04-1.22) |
|
Collapse |
||||
| Any CHD† |
9 |
13 870 |
0.53 (0.35-0.81) |
1.06 (1.00-1.13) |
|
Palpitations |
||||
| Any CHD |
10 |
5187 |
0.55 (0.38-0.79) |
1.11 (1.01-1.22) |
| Stable CHD |
3 |
809 |
0.66 (0.37-1.18) |
1.04 (0.95-1.13) |
| MI |
5 |
2588 |
0.47 (0.28-0.81) |
1.12 (0.98-1.27) |
| ACS |
2 |
1790 |
0.61 (0.27-1.37) |
1.04 (0.95-1.13) |
|
Weakness |
||||
| Any CHD† |
6 |
4611 |
1.20 (0.97-1.48) |
0.90 (0.78-1.04) |
|
Rales |
||||
| Any CHD† | 8 | 19 700 | 1.81 (1.03-3.17) | 0.88 (0.81-0.95) |
*Abbreviations: CHD – coronary heart disease; MI – myocardial infarction; ACS – acute coronary syndrome; LR – likelihood ratio; CI – confidence interval.
†Results for different case definitions of CHD not provided, since the model with covariate “case definition” did not produce stable estimates.
Five index tests referred to results of the physical examination. The number of studies per index test ranged from 3 (high blood pressure, tachycardia, bradycardia) to 8 (rales, pain reproducible by palpation) (median: 3). A quantitative synthesis across all studies to determine the accuracy for the diagnosis of myocardial ischemia in general was only possible in one index test (Table 5). Results of the qualitative subgroup analyses showed that pain reproducible by palpation might be helpful for ruling out myocardial ischemia, with pLR ranging from 0.13 to 0.41 in primary studies (supplementary Table 11(web extra material 11)). Noticeably, one study showed extreme values for the presence of tachycardia (pLR: 20.50), bradycardia (pLR:13.04), and rales (pLR: 13.70) (19).
Discussion
In this study, we investigated the accuracy of single symptoms and signs for CHD in patients with chest pain. Overall, the presence of clinical findings was more informative than the absence. Most helpful for the diagnosis of myocardial ischemia in general was the presence of typical angina, radiation of pain to the right arm/shoulder, pain reproducible by palpation, and pain related to breathing. However, in several index tests we found that the accuracy varied across subgroups determined by case definition of CHD. In respect to the case definition, diagnostically most useful tests were history of CHD (pLR = 3.59), known MI (pLR = 3.21), typical angina (pLR = 2.35), history of diabetes mellitus (pLR = 2.16), exertional pain (pLR = 2.13), history of angina pectoris (nLR = 0.42), and male sex (nLR = 0.49) for diagnosing stable CHD; pain radiation to right arm/shoulder (pLR = 4.43), and palpitation (pLR = 0.47) for diagnosing MI; visceral pain (pLR = 2.05) for diagnosing ACS, and typical angina (pLR = 2.60) and pain reproducible by palpation (pLR = 0.13) for predicting MCE.
About 60 studies included in this review were published after Chun and McGee (4) had made their search, indicating that it was reasonable to conduct a new review. A recently published review on the diagnostic value of nitro-glycerine included 5 studies with 1978 patients (20). We identified 4 additional studies including 835 patients. This may be an indication that our more comprehensive search strategy without restriction on pre-specified symptoms and signs resulted in high sensitivity.
We used the BREM approach to calculate pooled estimates. Since this model is rather complex it sometimes fails to converge or produce stable estimates, especially if the number of studies is small. As a consequence we could not present pooled estimates for several index tests across all studies and/or within subgroups. However, it was mandatory to use the BREM since only hierarchical models like the BREM or the hierarchical summary ROC model account for the different sources of heterogeneity we identified in the analyses (5).
We separately determined the accuracy of each single symptom and sign. This approach did not account for dependence between the respective index tests and might have resulted in a biased estimation of the measures of accuracy. However, considering dependence between the index tests would require a multivariate approach using individual patient data (21,22).
Current guidelines recommend that the clinician should consider items of the medical history like age, sex, characteristics of pain like localization, radiation, and quality, associated symptoms, cardiovascular risk factors, and pre-existing cardiac conditions in the diagnosis of CHD in patients with chest pain (23,24). The symptoms and signs considered in our review represented all these categories. Not surprisingly, none of these clinical findings showed in isolation a sufficient diagnostic accuracy for safely ruling in or ruling out myocardial ischemia in patients presenting with chest pain.
Assessment of cardiovascular risk profile is supposed to be a corner stone in the clinical evaluation of patients with chest pain. However, in our study the diagnostic value of single cardiovascular risk factors was low to moderate. This is not surprising. Wald et al (25) pointed out that the association between a risk factor and a disease must be very strong before the risk factor could be considered as a worthwhile test. The accuracy of most single cardiovascular risk factors was higher when estimating the likelihood of a stable CHD compared to ACS, MI, or MCE. Most noticeably, information on the presence of most single cardiovascular risk factors was diagnostically useless when estimating the likelihood of MI. This seems to be plausible if one considers that cardiovascular risk factors are supposed to predict the development of a chronic disease rather than the acute manifestation of the disease.
The diagnostically most helpful symptom describing the localization and radiation of pain was the right-sided radiation of pain. The accuracy was highest for the diagnostic outcome MI. This finding was consistent with previous reviews (2,3). When estimating the likelihood of stable CHD, the most informative symptom was pain described as typical angina or pain related to effort. These classical features remain the most persuasive findings arguing for a diagnosis of stable CHD (26).
Among the associated symptoms, the presence of sweating and nausea/vomiting showed a consistent but small effect when estimating the likelihood of MI in the majority of studies. The most helpful finding arguing against myocardial ischemia in general was pain that was reproducible by palpation. However, these findings could only be based on the qualitative syntheses.
One study showed remarkable results for the accuracy of three physical findings (tachycardia, bradycardia, rales) (19). Among the studies investigating the accuracy of these index tests, this study was the only one conducted in primary care. The difference might be a hint toward a spectrum effect. The proportion of patients without an underlying coronary ischemia but with an underlying serious cardiac condition and correspondent findings might be higher in secondary than in primary care (27). Accordingly, the accuracy of findings might differ between settings. Because the number of primary care studies was extremely small we could not systematically address this issue.
Medical history taking and physical examination are crucial steps in the evaluation of patients with chest pain. In this study, we reported the accuracy of a broad spectrum of symptoms and signs for myocardial ischemia as the underlying reason. Our results also suggested that the accuracy of several symptoms and signs varied in the published papers according to the case definition of CHD.
Acknowledgments
Funding None.
Ethical approval Not required.
Declaration of authorship DS, CW, MAH, AS, JR, and NDB formulated the research question and designed the study. JH, DS, GW, and CW conducted the search, selected the studies, and extracted the data. JH, DS, GW, CW, MAH, AS, and NDB assessed study quality. JH, DS, GW, CW, and SB, analyzed and interpreted the data, supervised by JR and NDB. JH drafted the article; DS, GW, CW, MAH, AS, SB, JR, and NDB revised it critically. NDB is the guarantor. JH as corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Additional Material
Additional Material
Additional Material
Additional Material
Additional Material
Additional Material
Additional Material
Additional Material
Additional Material
Additional Material
Additional Material
References
- 1.Hurst JW, Morris DC. Chest pain. Armonk (NY): Futura Pub; 2001. [Google Scholar]
- 2.Mant J, McManus RJ, Oakes RA, Delaney BC, Barton PM, Deeks JJ, et al. Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care. Health Technol Assess. 2004;8:1–iii158. doi: 10.3310/hta8020. [DOI] [PubMed] [Google Scholar]
- 3.Bruyninckx R, Aertgeerts B, Bruyninckx P, Buntinx F. Signs and symptoms in diagnosing acute myocardial infarction and acute coronary syndrome: a diagnostic meta-analysis. Br J Gen Pract. 2008;58:105–11. doi: 10.3399/bjgp08X277014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chun AA, McGee SR. Bedside diagnosis of coronary artery disease: a systematic review. Am J Med. 2004;117:334–43. doi: 10.1016/j.amjmed.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Harbord RM, Whiting P, Sterne JA, Egger M, Deeks JJ, Shang A, et al. An empirical comparison of methods for meta-analysis of diagnostic accuracy showed hierarchical models are necessary. J Clin Epidemiol. 2008;61:1095–103. doi: 10.1016/j.jclinepi.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol. 2006;6:9. doi: 10.1186/1471-2288-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinnes J, Deeks J, Kirby J, Roderick P. A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy. Health Technol Assess. 2005;9:1–113. doi: 10.3310/hta9120. . iii. [DOI] [PubMed] [Google Scholar]
- 8.Deville WL, Buntinx F, Bouter LM, Montori VM, Vet HCW, van der Windt DA, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9. doi: 10.1186/1471-2288-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cnossen JS, Leeflang MMG, Haan EEM, Mol BWJ, van der Post JAM, Khan KS, et al. Accuracy of body mass index in predicting pre-eclampsia: bivariate meta-analysis. BJOG. 2007;114:1477–85. doi: 10.1111/j.1471-0528.2007.01483.x. [DOI] [PubMed] [Google Scholar]
- 10.Leeflang MMG, Deeks JJ, Gatsonis C, Bossuyt PMM. Systematic reviews of diagnostic test accuracy. Ann Intern Med. 2008;149:889–97. doi: 10.7326/0003-4819-149-12-200812160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–90. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa TA, Strauss S, Bucher HC, Guyatt G. Diagnostic tests. In: Guyatt G, Rennie D, Meade MO, Cook DJ, editors. Users' guides to the medical literature A manual for evidence-based clinical practice. 2nd ed. New York: McGraw Hill Medical; 2008.p. 419-38. [Google Scholar]
- 13.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SAS Institute Inc. Base SAS ® 9.2 Procedures Guide. Cary (NC): SAS Institute Inc.; 2009. [Google Scholar]
- 15.METADAS. A SAS macro for meta-analysis of diagnostic accuracy studies. Cochrane 15. Diagnostic Test Accuracy Working Group; 2010; Available from: http://srdta.cochrane.org/software-development Accessed: September 24, 2012.
- 16.SPSS Inc. SPSS statistics base 17.0 user’s guide. Chicago (IL): SPSS Inc.; 2007. [Google Scholar]
- 17.Grijseels EW, Deckers JW, Hoes AW, Hartman JA, Van der Does E, Van Loenen E, et al. Pre-hospital triage of patients with suspected myocardial infarction. Evaluation of previously developed algorithms and new proposals. Eur Heart J. 1995;16:325–32. doi: 10.1093/oxfordjournals.eurheartj.a060914. [DOI] [PubMed] [Google Scholar]
- 18.Jerlock M, Welin C, Rosengren A, Gaston-Johansson F. Pain characteristics in patients with unexplained chest pain and patients with ischemic heart disease. Eur J Cardiovasc Nurs. 2007;6:130–6. doi: 10.1016/j.ejcnurse.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 19.van der Does E, Lubsen J, Pool J. Acute myocardial infarction: an easy diagnosis in general practice? J R Coll Gen Pract. 1980;30:405–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Grailey K, Glasziou PP. Diagnostic accuracy of nitroglycerine as a 'test of treatment' for cardiac chest pain: a systematic review. Emerg Med J. 2012;29:173–6. doi: 10.1136/emj.2010.103994. [DOI] [PubMed] [Google Scholar]
- 21.Buntinx F, Aertgeerts B, Aerts M, Bruyninckx R, Knottnerus JA, Van den Bruel A, et al. Multivariable analysis in diagnostic accuracy studies: what are the possobilities? In: Knottnerus JA, Buntinx F, editors. The evidence base of clinical diagnosis. 2nd ed. Oxford, Hoboken (NJ): Wiley-Blackwell Pub./BMJ Books; 2009. p. 146-66. [Google Scholar]
- 22.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221–5. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 23.Fox K, Garcia MA, Ardissino D, Buszman P, Camici PG, Crea F, et al. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27:1341–81. doi: 10.1093/eurheartj/ehl001. [DOI] [PubMed] [Google Scholar]
- 24.Skinner JS, Smeeth L, Kendall JM, Adams PC, Timmis A. NICE guidance. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. Heart. 2010;96:974–8. doi: 10.1136/hrt.2009.190066. [DOI] [PubMed] [Google Scholar]
- 25.Wald NJ, Hackshaw AK, Frost CD. When can a risk factor be used as a worthwhile screening test? BMJ. 1999;319:1562–5. doi: 10.1136/bmj.319.7224.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350–8. doi: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 27.Buntinx F, Knockaert D, Bruyninckx R, de Blaey N, Aerts M, Knottnerus JA, et al. Chest pain in general practice or in the hospital emergency department: is it the same? Fam Pract. 2001;18:586–9. doi: 10.1093/fampra/18.6.586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.