Abstract
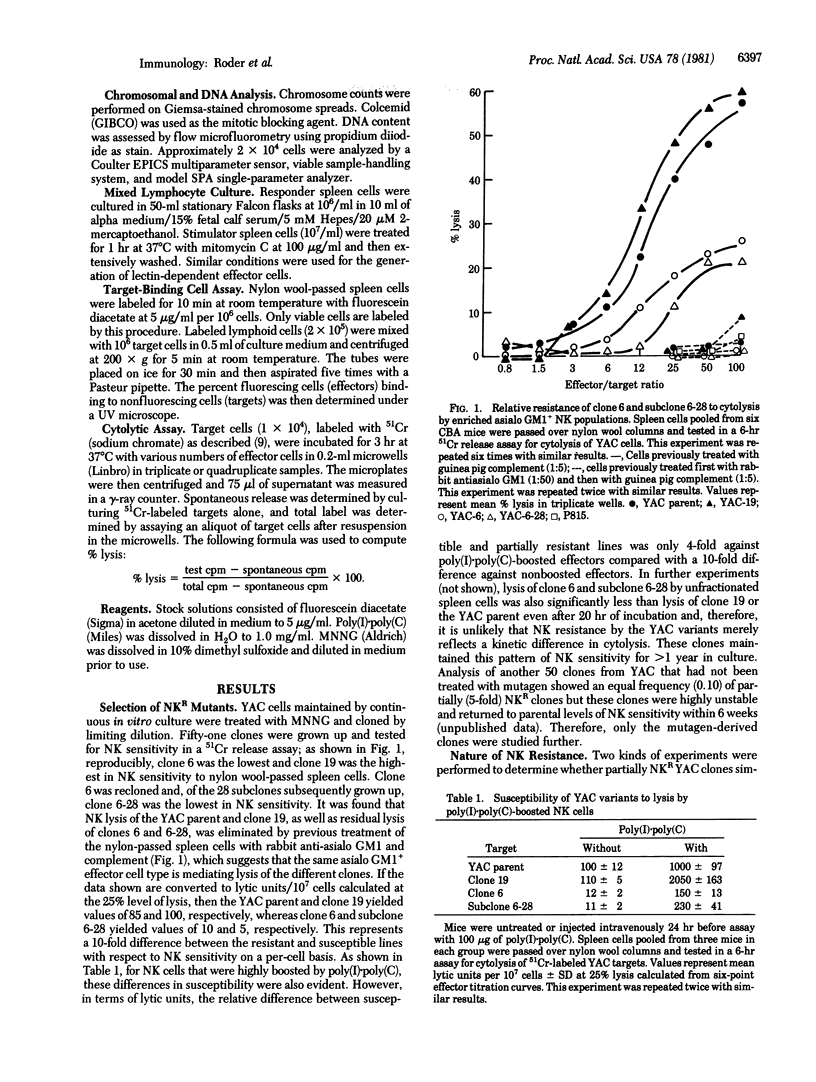
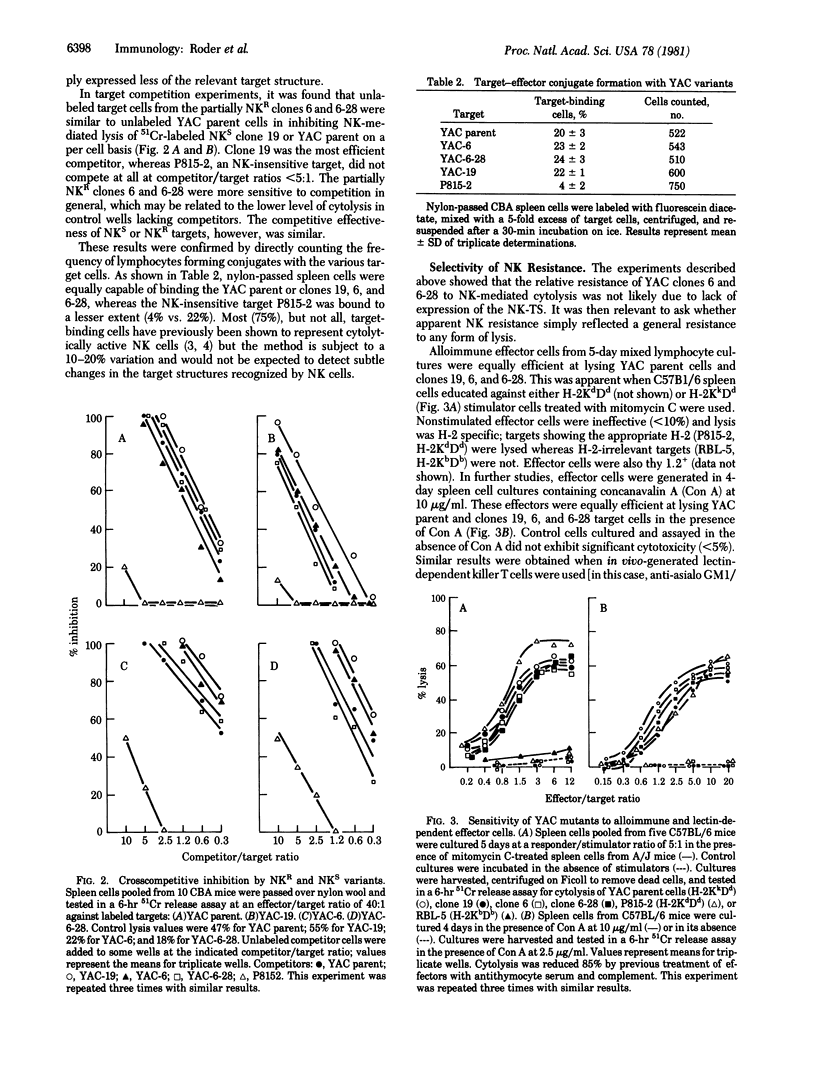
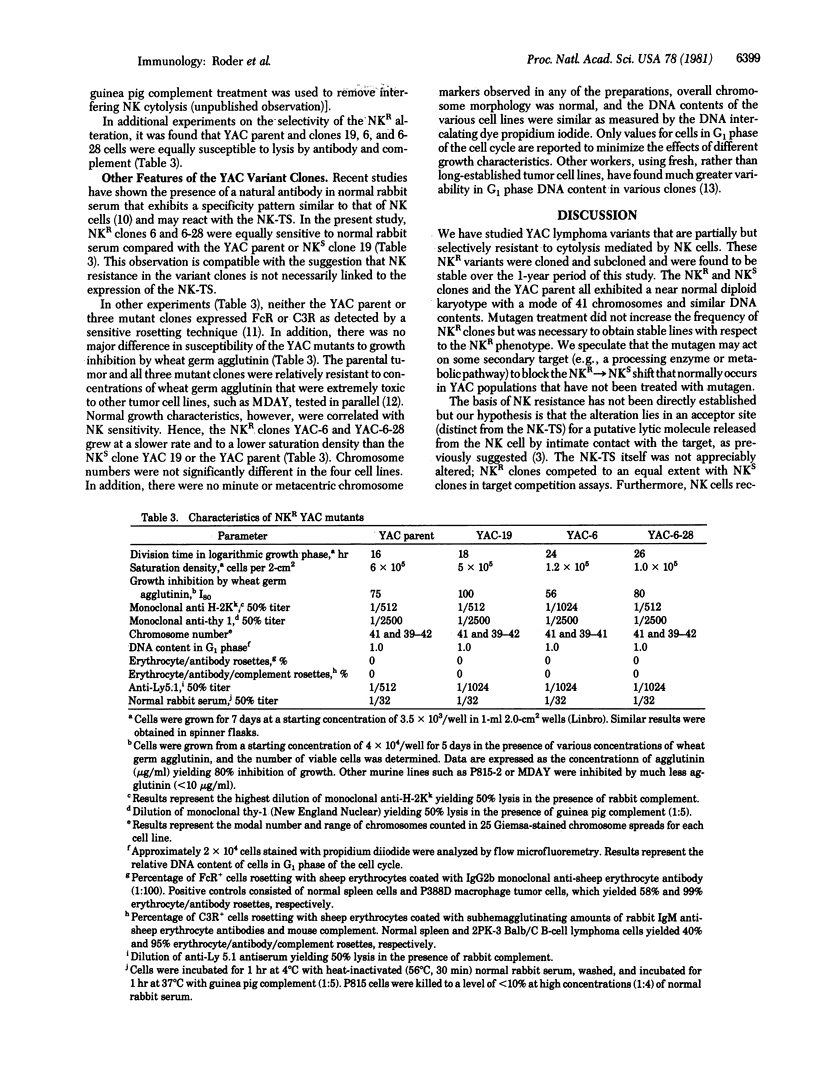
YAC lymphoma cells were treated with the mutagen N-methyl-N'-nitro-N-nitrosoguanidine and then cloned and subcloned. Of 51 clones, 3 were selected for further study. Ten-fold more natural killer (NK) effector cells were required to lyse YAC clone 6 and subclone 6-28 cells compared with clone 19 cells or the YAC parent cell line. The maximum plateau level of cytolysis of the NK-resistant (NKR) variants (20%) never approached that of the NK-sensitive (NKS) variants or YAC parental cells (60%) even after prolonged incubation (20 hr). NKR variants appeared with equal frequency (0.10) on cloning YAC cells that had not been treated with mutagen but these variants were highly unstable with respect to NK sensitivity and were not studied further. Cytolysis of both NKR and NKS lines was mediated by nylon-nonadherent asialo-GM1+ effector cells, and effectors from poly(I) . poly(C)-boosted mice preferentially lysed the NKS lines. The NKR alteration did not appear to change the NK target structure (NK-TS): (i) unlabeled NKR cells competed equally with NKS cells in reciprocal unlabeled-target competition assays; (ii) the frequency of target--effector conjugates was identical with NKR or NKS lines; and (iii) normal rabbit serum, which contains antibodies thought to react with the NK-TS, reacted equally against both NKR and NKS targets. The NKR alteration was selective for NK cells and did not result in a resistance to lysis in general; NKR and NKS variants were equally susceptible to (i) cytolysis mediated by alloimmune or lectin-dependent effector T cells and (ii) antibody- and complement-mediated lysis. These results are compatible with the hypothesis that the NKR variants have an altered acceptor site on the target cell membrane that normally binds the "lytic moiety" delivered by the effector cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrlund-Richter L., Klein E., Masucci G. Somatic hybrids between a high NK-sensitive lymphoid (YACIR) and several low sensitive sarcoma or L-cell-derived mouse lines exhibit low sensitivity. Somatic Cell Genet. 1980 Jan;6(1):89–99. doi: 10.1007/BF01538698. [DOI] [PubMed] [Google Scholar]
- Becker S., Stendahl O., Magnusson K. E. Physico-chemical characteristics of tumour cells susceptible to lysis by natural killer (NK) cells. Immunol Commun. 1979;8(1):73–83. doi: 10.3109/08820137909044708. [DOI] [PubMed] [Google Scholar]
- Collins J. L., Patek P. Q., Cohn M. Tumorigenicity and lysis by natural killers. J Exp Med. 1981 Jan 1;153(1):89–106. doi: 10.1084/jem.153.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durdik J. M., Beck B. N., Clark E. A., Henney C. S. Characterization of a lymphoma cell variant selectively resistant to natural killer cells. J Immunol. 1980 Aug;125(2):683–688. [PubMed] [Google Scholar]
- Grönberg A., Hansson M., Kiessling R., Andersson B., Kärre K., Roder J. Demonstration of natural antibodies in normal rabbit serum with similar specificity pattern as mouse natural killer cells. J Natl Cancer Inst. 1980 May;64(5):1113–1119. [PubMed] [Google Scholar]
- Kasai M., Iwamori M., Nagai Y., Okumura K., Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980 Mar;10(3):175–180. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S. Immunologic studies of membrane mutants of a highly metastatic murine tumor. Am J Pathol. 1979 Dec;97(3):609–622. [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S. Increased sensitivity of rosetting assay for Fc receptors obtained by using non-hemagglutinating monoclonal antibodies. J Immunol Methods. 1980;34(1):1–10. doi: 10.1016/0022-1759(80)90218-5. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Klein G., Zeuthen J., Eriksson I., Terasaki P., Bernoco M., Rosén A., Masucci G., Povey S., Ber R. Hybridization of a myeloid leukemia-derived human cell line (K562) with a human Burkitt's lymphoma line (P3HR-1). J Natl Cancer Inst. 1980 Apr;64(4):725–738. [PubMed] [Google Scholar]
- Lavrovsky V. A., Viksler V. K. Immunological properties of malignant and nonmalignant sublines of L-cells. Cancer Res. 1980 Sep;40(9):3252–3258. [PubMed] [Google Scholar]
- Roder J. C., Ahrlund-Richter L., Jondal M. Target-effector interaction in the human and murine natural killer system: specificity and xenogeneic reactivity of the solubilized natural killer-target structure complex and its loss in a somatic cell hybrid. J Exp Med. 1979 Sep 19;150(3):471–481. doi: 10.1084/jem.150.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder J. C., Kiessling R., Biberfeld P., Andersson B. Target-effector interaction in the natural killer (NK) cell system. II. The isolation of NK cells and studies on the mechanism of killing. J Immunol. 1978 Dec;121(6):2509–2517. [PubMed] [Google Scholar]
- Roder J. C., Kiessling R. Target--effector interaction in the natural killer cell system. I. Covariance and genetic control of cytolytic and target-cell-binding subpopulations in the mouse. Scand J Immunol. 1978;8(2):135–144. doi: 10.1111/j.1365-3083.1978.tb00505.x. [DOI] [PubMed] [Google Scholar]
- Roder J. C., Rosén A., Fenyö E. M., Troy F. A. Target-effector interaction in the natural killer cell system: isolation of target structures. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1405–1409. doi: 10.1073/pnas.76.3.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder J., Duwe A. The beige mutation in the mouse selectively impairs natural killer cell function. Nature. 1979 Mar 29;278(5703):451–453. doi: 10.1038/278451a0. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Withers H. R., Lee L. Y. Variability of DNA content of murine fibrosarcoma cells. Nature. 1977 Oct 6;269(5628):531–532. doi: 10.1038/269531a0. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978 May 1;147(5):1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]



