Abstract
Conclusions:
With full insertion with a long electrode, hearing preservation can be achieved even in the presence of a long electrode covering the residual hearing region.
Objectives:
Advances in developing new atraumatic concepts of electrode design as well as surgical technique have enabled hearing preservation after cochlear implantation surgery, and EAS (electric acoustic stimulation) accompanied with hearing preservation is a new trend for patients with residual hearing at the lower frequencies. However, full insertion with a long/medium electrode and hearing preservation is still a challenging field that calls for discussion.
Method:
In this study, round window insertion, an atraumatic electrode, and dexamethasone administration were used and atraumaticity (hearing preservation and conservation of vestibular function) was evaluated with full insertion of the electrode.
Results:
Postoperative evaluation after full insertion of the electrodes showed that hearing at low frequencies was well preserved in all five cases. Combined postoperative imaging with the referential tonotopic map confirmed achievement of full insertion and indicated the corresponding frequencies and the depth of the electrode. Achievement of atraumaticity of round window insertion in the present cases was confirmed from the viewpoint of the minimal drilling time as well as the preserved vestibular function.
Keywords: EAS, electric acoustic stimulation, high frequency hearing loss, cochlear implantation, deep insertion, atraumaticity
Introduction
Advances in developing new atraumatic concepts of electrode design as well as surgical technique have enabled hearing preservation after cochlear implantation surgery, and EAS (electric acoustic stimulation) accompanied with hearing preservation is a new trend for patients with residual hearing at the lower frequencies.
However, a recent review collecting the data obtained by previous studies demonstrated that substantial acoustic hearing loss occurred in 24% of the patients, and among them 13% showed total loss [1]. Various techniques to preserve residual hearing at the lower frequencies have been attempted, including soft surgery technique when performing cochleostomy [2], round window insertion [3], use of atraumatic electrodes [4,5], and postoperative steroid administration.
Partial insertion up to 20 mm (where there is no residual hearing) is currently often performed [1], and full insertion with a long/medium electrode and hearing preservation is still a challenging field that calls for discussion. In this study, the method was based on atraumatic concepts and used round window insertion, an atraumatic electrode (in four of five cases), and dexamethasone administration. Hearing preservation and conservation of vestibular function were evaluated with full insertion of the electrode.
Material and methods
We performed cochlear implantation with full insertion of the electrode (MEDEL COMBI40+® with a 31.5 mm standard electrode in one case, PULSAR® with a 24 mm FLEXeas® in three cases, and PULSAR® with a 31.3 mm FLEXsoft® in one case). The patients were aged from 38 to 68 years; two male, three female. All cases had post-lingual hearing loss at higher frequencies, starting from 30 to 40 years old and slowly progressive. The round window approach was applied to reduce the insertion damage of the cochlea. All surgeries were performed by a single surgeon (S.U.). Intraoperative infusion of dexamethasone (8 mg) was applied before drilling of the bony edge of the round window niche. Also postoperative dexamethasone treatment was administered for 6 days (8, 8, 4, 4, 2, and 2 mg, respectively). Insertion depth of the electrode and the corresponding frequencies were estimated by using postoperative X-ray (the X-ray digital linear tomosynthesis [6]). For comparison between round window insertion and cochleostomy insertion, drilling time to reach the perilymphatic space was averaged based on the video recording of 21 cases (round window insertion, 12 cases including the present 5 cases; cochleostomy insertion, 9 cases).
In addition to postoperative assessment of audiological testing, vestibular evoked myogenic potential (VEMP) as well as caloric response were analyzed to monitor atraumaticity of the surgery using nine cases (either round window insertion or cochleostomy), including the present five cases. In VEMP testing, the electrographic signal from the stimulated side was amplified and averaged using a Neuropack evoked potential recorder (Nihon Kohden Co. Ltd, Tokyo, Japan). Clicks lasting for 0.1 ms at 105 dBnHL were presented through a headphone. The stimulation rate was 5 Hz, the bandpass filter intensity was 20–2000 Hz, and analysis time was 50 ms. The responses to 200 stimuli were averaged twice. In caloric testing, maximum slow eye velocity was measured by cold water irrigation (20°C, 5 ml, 20 s). Postoperative VEMP and caloric responses of the implanted ears and contralateral ears were compared.
Results
Postoperative evaluation after full insertion of the electrodes showed that hearing at low frequencies was well preserved in all 5 cases, and then a speech processor (DUET EAS®) was applied for electric acoustic stimulation (EAS). Combined postoperative imaging with the referential tonotopic map confirmed achievement of full insertion and indicated the corresponding frequencies and the depth of the electrode (Figures 1–5). Audiological testing showed preservation of residual hearing, especially for bone conduction hearing (Figures 1–5).
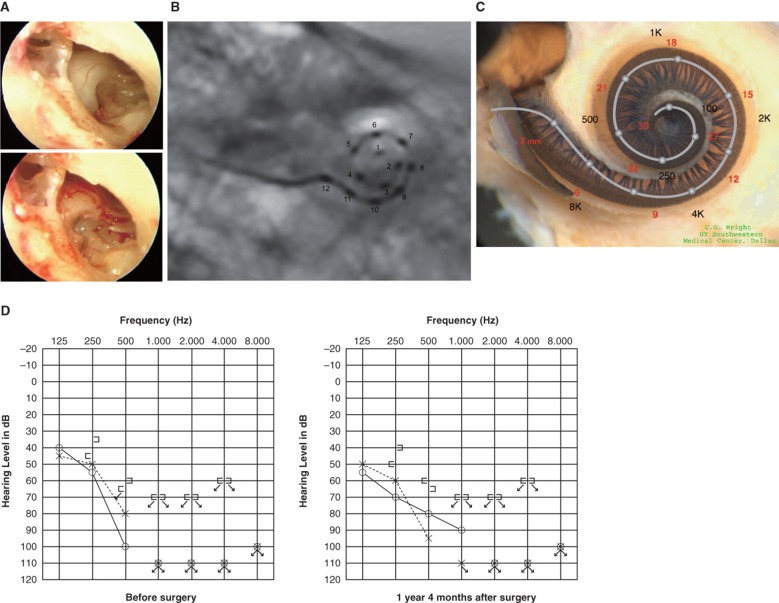
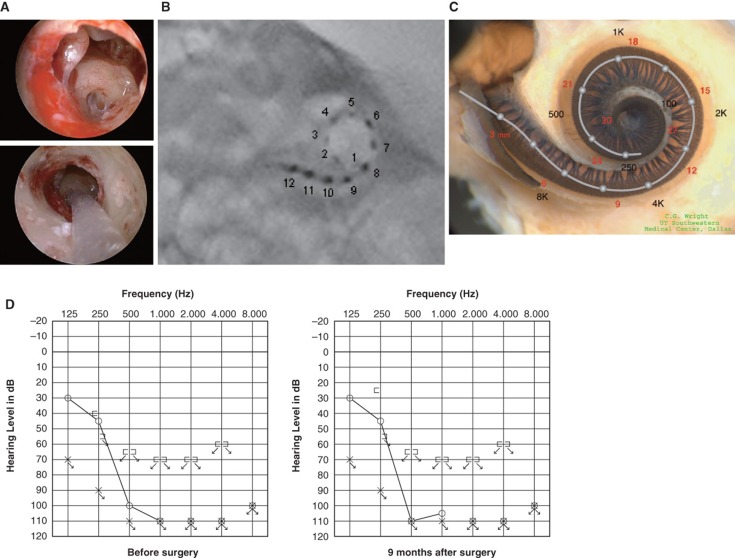
Figure 1.
Case 1. A 60-year-old woman presented with slowly progressive bilateral hearing loss from age 40. By age 50 she had only minimal gain from hearing aids and when we first saw her they were nearly useless in her daily life. COMBI40+ with regular electrode was used for this patient on Dec 10, 2008. For insertion, the round window approach was applied, and full insertion was achieved. Complete preservation of residual hearing was obtained. (A) Endoscopic view of round window insertion, (B) postoperative X-ray finding, (C) imaging with putative location of electrode and the referential tonotopic map, (D) preoperative and postoperative audiograms. The image of human cochlea neural tissues stained by osmium tetroxide used in Figures 1–5 was kindly provided by Dr C.G. Wright, USWT, Dallas, USA (red, mm from round window; black, corresponding frequency).
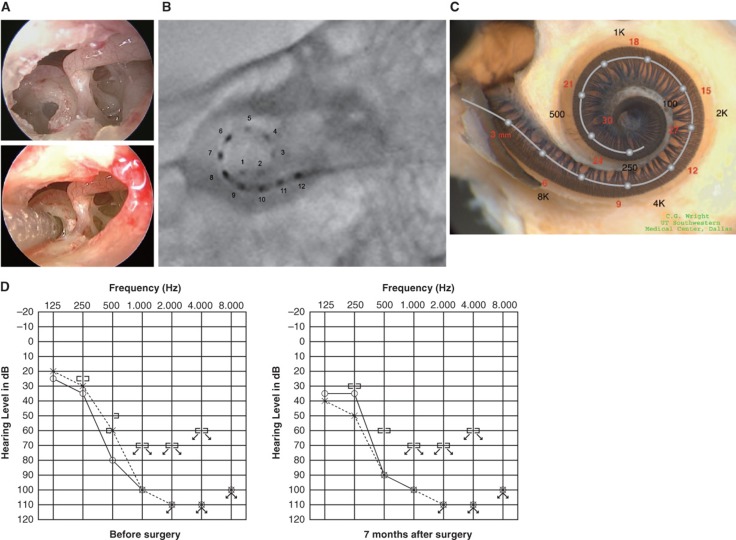
Figure 3.
Case 3. This 45-year-old woman became aware of bilateral hearing loss and tinnitus around age 25. When she presented to us it had been slowly progressing for 10 years. PULSAR FLEXeas/RW approach was applied on Nov 18, 2009. Preservation of residual hearing was obtained. (A) Endoscopic view of round window insertion, (B) postoperative X-ray finding, (C) imaging with putative location of electrode and the referential tonotopic map, (D) preoperative and postoperative audiograms.
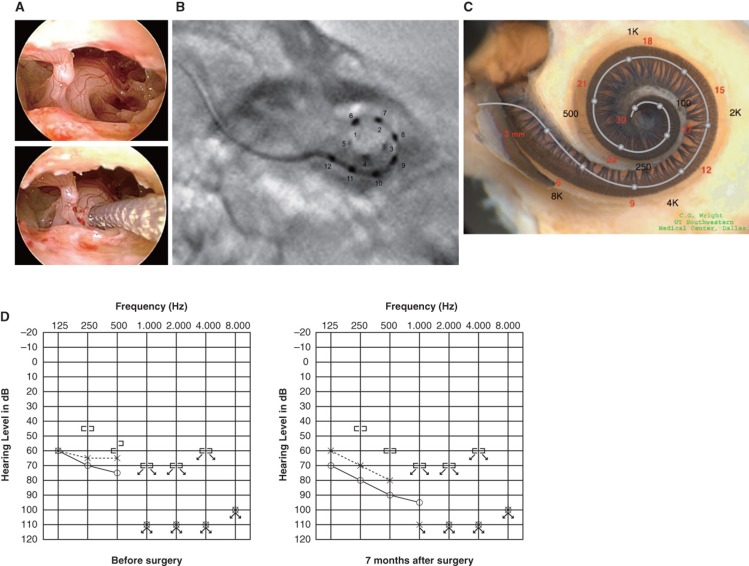
Figure 4.
Case 4. This 38-year-old woman had hearing loss detected by mass screening in primary school. It appeared to slowly progress as she grew up, and by age 25 she suffered inconvenience in hearing and communication, mainly using only her left ear. The PULSAR FLEXeas/RW approach was applied on Dec 21, 2009. Preservation of residual hearing was obtained. (A) Endoscopic view of round window insertion, (B) postoperative X-ray finding, (C) imaging with putative location of electrode and the referential tonotopic map, (D) preoperative and postoperative audiograms.
Figure 5.
Case 5. This 68-year-old man presented with slowly progressive bilateral hearing loss from around age 40. He had only minimal gain from hearing aids. The PULSAR FLEXsoft/RW approach was applied on May 17, 2010. Preservation of residual hearing was obtained. (A) Endoscopic view of round window insertion, (B) postoperative X-ray finding, (C) imaging with putative location of electrode and the referential tonotopic map, (D) preoperative and postoperative audiograms.
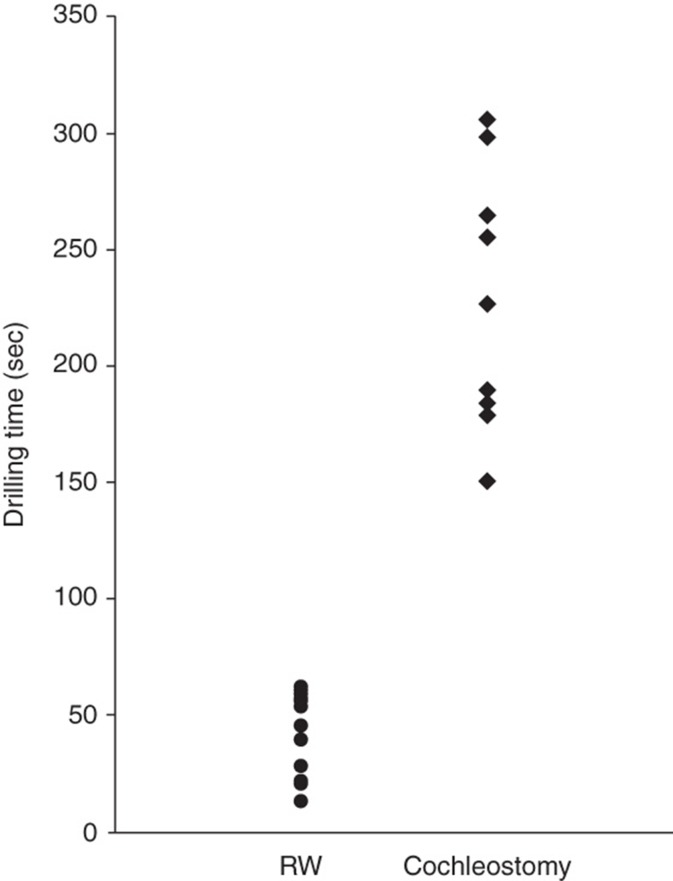
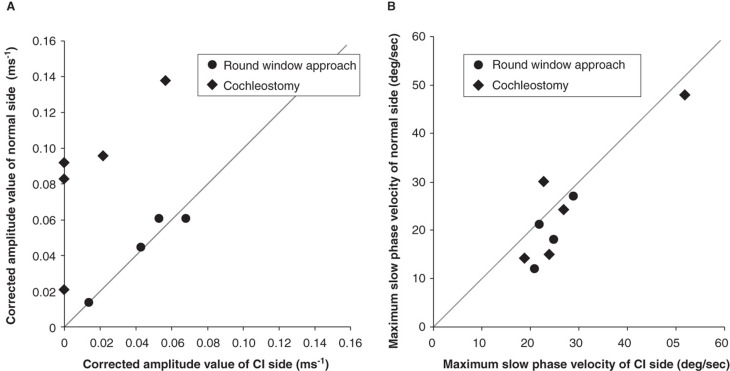
Drilling time to reach the perilymphatic space based on the video recording was significantly less in the cases with round window insertion compared with cochleostomy cases (Figure 6, p = 0.00001, t test). VEMP responses could be recorded in four of five cases and were well preserved postoperatively. VEMP responses were decreased postoperatively in the cases with cochleostomy, in contrast to the round window insertion cases where the responses were maintained (Figure 7A). The ratio of the corrected amplitude value of cochlear implantation side divided by the normal side value was significantly lower in the cochleostomy cases than in the round window insertion cases (p = 0.0001, t test). Caloric response was well preserved and no difference was found between the two groups (Figure 7B, p = 0.51, t test).
Figure 6.
Video recording showing that drilling time to reach the perilymphatic space is significantly shorter for the round window approach compared with cochleostomy insertion.
Figure 7.
(A) Vestibular evoked myogenic potential (VEMP) responses were recorded in four of five cases and were well preserved postoperatively. VEMP responses decreased postoperatively in the cochleostomy cases while they were maintained in the round window insertion cases. Corrected amplitude value Cp13-n23 (ms–1) = amplitude Cp13-n23 (micro V)/background electromyographic activities (micro V ms). (B) Caloric response was well preserved and there were no differences between the two groups. MSV, maximum slow eye velocity.
Discussion
Hearing loss in the majority of these patients is more or less progressive, although the speed of progression, i.e. rapid or rather stable, may be dependent on their etiology. An unresolved issue is the prediction of progressiveness based on the etiology of individual hearing loss, but we have recently reported at least four genes that are responsible for the candidates for EAS, and therefore there is not a single etiology but rather a great genetic heterogeneity involved in this particular type of hearing loss [7]. Since shallow insertion of short electrodes cannot recruit neurons in the apical region, deep insertion would be the best solution to prevent future hearing deterioration at the lower frequencies. Full insertion with a long/medium electrode for the patients with residual hearing at the low frequencies is still a controversial field because of possible loss of their residual hearing due to mechanical trauma of the corresponding area.
In the present series, combined postoperative imaging with the referential tonotopic map clearly indicated that hearing preservation is achievable even in the presence of a long electrode covering the residual hearing region. Due to individual variation in the length of the cochlear turn, it is not sufficient to describe the length of the inserted electrode for estimating the corresponding frequencies of the tip of the electrode. In the present study, the X-ray digital linear tomosynthesis, which is known to have less artifacts and provide better understanding of the morphological relationship with the cochlear turn, indicated tonotopic orientation.
With regard to the vibrations of the basilar membrane in the presence of the electrode, based on histological observations of morphologic changes in temporal bone studies, a close contact or even a slight lifting of the basilar membrane in the ascending basal and middle turns of the cochlea has been described [8]. However, in most cases, in adjacent regions, the basilar membrane was not in direct contact with the electrode, and lower frequencies were not affected by fixation in the basal and middle turn of the cochlea. Kiefer et al. [8] also reported the interesting phenomenon that audiological testing of the patients showed slightly better thresholds of the corresponding frequencies after implantation. Acoustic energy may increase perception in regions adjacent to the fixed regions, and basilar membrane behavior may be altered, i.e. some frequencies are redistributed and more amplified. In this series, some frequencies of the patients represented improvement after cochlear implantation (see Figure 1, air conduction hearing at 500 and 1000 Hz and bone conduction hearing at 500 Hz and Figure 2, bone conduction hearing at 250 Hz), supporting this phenomenon. On the other hand, in some cases, an air–bone gap was slightly recognized postoperatively (air conduction hearing was slightly elevated), perhaps due to a slight lifting of the basilar membrane in the middle turn observed in the temporal bone study [8].
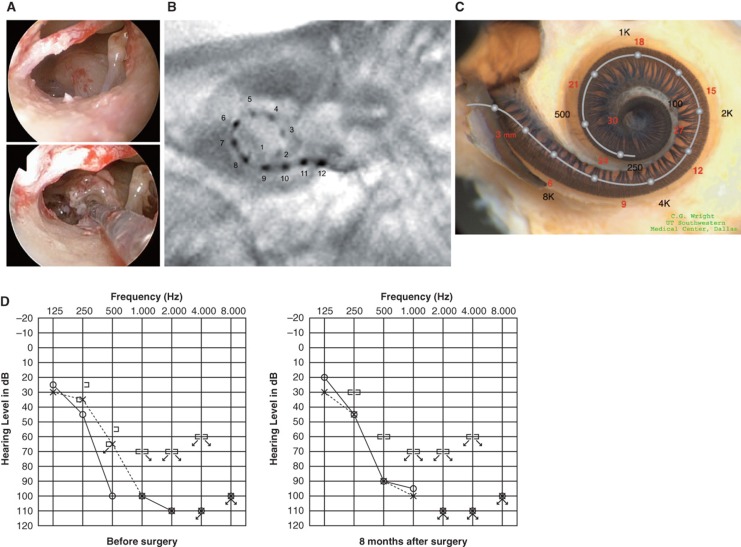
Figure 2.
Case 2. This 39-year-old man was congenitally deaf in the left ear. Mild hearing loss in his right ear was noticed in childhood, and he presented with progressive hearing loss of 10 years duration. FLEXeas/RW approach was applied on Nov 16, 2009. Preservation of residual hearing was obtained. (A) Endoscopic view of round window insertion, (B) postoperative X-ray finding, (C) imaging with putative location of electrode and the referential tonotopic map, (D) preoperative and postoperative audiograms.
These hearing improvement/deterioration results are not conclusive, because they could also be considered as within the margin of error. Serial testing as well as long follow-up observation period will resolve this issue, and we are currently working on this aspect.
Dexamethasone is known to have protective effects against insertion trauma as well as inflammatory process after implantation [9]. In this series, intraoperative infusion and postoperative dexamethasone treatment was administered systemically.
There have been a series of trials with the goal of minimizing intracochlear trauma, by both cochleostomy insertion and round window insertion. For cochleostomy insertion, to avoid trauma, much attention has been paid to the cochleostomy site with the aim of avoiding the critical structures of the inner ear [10,11]. According to Lane et al. [12], by using 64-slice multidetector computed tomography (CT), localization of the electrode in the scala vestibuli as well as migration of the electrode array from the scala tympani to the scala vestibuli, which may influence hearing preservation, was observed in the patients with cochleostomy. On that basis, round window insertion was chosen in the present series.
Detailed clinical evaluation has confirmed the atraumaticity of the surgical approach in the present cases from the point of drilling time as well as of vestibular function.
During cochleostomy, noise levels were reported ranging from 114 to 128 dB SPL, indicating that during inner ear surgery they reach levels that can cause noise-induced hearing loss [13].
Our measurements clearly showed that drilling time to reach the perilymphatic space is significantly less for the round window approach compared with cochleostomy insertion, suggesting reduced influence of noise-induced trauma that may cause sensorineural hearing loss.
The importance of conservation of vestibular function is recognized, especially for bilateral cochlear implantation. A recent study suggested that dysfunction of the saccular macula, an integral component of the otolith system, likely resulting from insertion trauma of the cochlear implant electrode, can cause chronic dizziness after cochlear implantation [14]. In the present series, postoperative assessment of VEMPs as well as caloric response also supported achievement of atraumatic surgery from the vestibular functional point of view. Comparison with the cochleostomy insertion cases showed symmetrical VEMP scores in round window cases. The cochleostomy cases showed poorer response postoperatively, indicating that saccular function may be affected by the cochleostomy. These data support the recent report that for the sacculus, which is known to be the most vulnerable vestibular organ, the round window approach is preferable from the viewpoint of vestibular function [15].
Conclusion
In our series of experiences with full insertion with a long electrode we were able to preserve residual hearing at low frequencies as well as the vestibular function. Combined postoperative imaging with the referential tonotopic map clearly indicated that hearing preservation can be achieved even in the presence of a long electrode covering the residual hearing region and indicated that development of atraumatic procedures, including fine flexible electrodes, surgical technique (round window insertion), and postoperative steroid application enabled successful hearing preservation.
Acknowledgments
We thank Ms A.C. Apple-Mathews for help in preparing the manuscript. This study was supported by a Health Sciences Research Grant (Research on Eye and Ear Science, Immunology, Allergy and Organ Transplantation) from the Ministry of Health and Welfare of Japan.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- [1].Talbot KN, Hartley DE. Combined electro-acoustic stimulation: a beneficial union? Clin Otolaryngol. 2008;33:536–45. doi: 10.1111/j.1749-4486.2008.01822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lehnhardt E, Laszig R. Specific surgical aspects of cochlear implant soft surgery. In: Hochmair-Desoyer IJ, Hochmair ES, editors. Advances in cochlear implants. Vienna: Manz; 1994. pp. 228–9. p. [Google Scholar]
- [3].Skarzynski H, Lorens A, Piotrowska A, Anderson I. Preservation of low frequency hearing in partial deafness cochlear implantation (PDCI) using the round window surgical approach. Acta Otolaryngol. 2007;127:41–8. doi: 10.1080/00016480500488917. [DOI] [PubMed] [Google Scholar]
- [4].Adunka O, Kiefer J, Unkelbach MH, Lehnert T, Gstoettner W. Development and evaluation of an improved cochlear implant electrode design for electric acoustic stimulation. Laryngoscope. 2004;114:1237–41. doi: 10.1097/00005537-200407000-00018. [DOI] [PubMed] [Google Scholar]
- [5].Baumgartner WD, Jappel A, Morera C, Gstöttner W, Müller J, Kiefer J, et al. Outcomes in adults implanted with the FLEXsoft electrode. Acta Otolaryngol. 2007;127:579–86. doi: 10.1080/00016480600987784. [DOI] [PubMed] [Google Scholar]
- [6].Gomi T, Hirano H, Umeda T. Evaluation of the X-ray digital linear tomosynthesis reconstruction processing method for metal artifact reduction. Comput Med Imaging Graph. 2009;33:267–74. doi: 10.1016/j.compmedimag.2009.01.004. [DOI] [PubMed] [Google Scholar]
- [7].Usami S, Miyagawa M, Suzuki N, Moteki H, Nishio S, Takumi Y, et al. Genetic background of candidates for EAS (Electric-Acoustic Stimulation) Audiol Med. 2010;8:28–32. [Google Scholar]
- [8].Kiefer J, Böhnke F, Adunka O, Arnold W. Representation of acoustic signals in the human cochlea in presence of a cochlear implant electrode. Hear Res. 2006;221:36–43. doi: 10.1016/j.heares.2006.07.013. [DOI] [PubMed] [Google Scholar]
- [9].van de Water TR, Dinh CT, Vivero R, Hoosien G, Eshraghi AA, Balkany TJ. Mechanisms of hearing loss from trauma and inflammation: otoprotective therapies from the laboratory to the clinic. Acta Otolaryngol. 2010;130:308–11. doi: 10.1080/00016480903124655. [DOI] [PubMed] [Google Scholar]
- [10].Briggs RJ, Tykocinski M, Stidham K, Roberson JB. Cochleostomy site: implications for electrode placement and hearing preservation. Acta Otolaryngol. 2005;125:870–6. doi: 10.1080/00016480510031489. [DOI] [PubMed] [Google Scholar]
- [11].Adunka OF, Pillsbury HC, Buchman CA. Minimizing intracochlear trauma during cochlear implantation. Adv Otorhinolaryngol. 2010;67:96–107. doi: 10.1159/000262601. [DOI] [PubMed] [Google Scholar]
- [12].Lane JI, Witte RJ, Driscoll CL, Shallop JK, Beatty CW, Primak AN. Scalar localization of the electrode array after cochlear implantation: clinical experience using 64-slice multidetector computed tomography. Otol Neurotol. 2007;28:658–62. doi: 10.1097/MAO.0b013e3180686e26. [DOI] [PubMed] [Google Scholar]
- [13].Strömberg AK, Yin X, Olofsson A, Duan M. Evaluation of the usefulness of a silicone tube connected to a microphone in monitoring noise levels induced by drilling during mastoidectomy and cochleostomy. Acta Otolaryngol. 2010;130:1163–8. doi: 10.3109/00016481003743050. [DOI] [PubMed] [Google Scholar]
- [14].Basta D, Todt I, Goepel F, Ernst A. Loss of saccular function after cochlear implantation: the diagnostic impact of intracochlear electrically elicited vestibular evoked myogenic potentials. Audiol Neurootol. 2008;13:187–92. doi: 10.1159/000113509. [DOI] [PubMed] [Google Scholar]
- [15].Todt I, Basta D, Ernst A. Does the surgical approach in cochlear implantation influence the occurrence of postoperative vertigo? Otolaryngol Head Neck Surg. 2008;138:8–12. doi: 10.1016/j.otohns.2007.09.003. [DOI] [PubMed] [Google Scholar]