Abstract
Cell culture studies of enterocytes are important in many fields. However, there are difficulties in obtaining cell lines from adult human intestine, such as microbial contamination of cultures from the tissue samples, short life span of enterocytes, overgrowth of mesenchymal cells, etc. Various model used to obtain adult intestinal cell lines are very complex requiring use of feeder layer or gel matrices. The aim of this study was to establish a novel method for the simple and reproducible isolation of human enterocytes. Enterocytes were isolated from SI samples (n = 5) obtained from cadaveric donors using a mechanical procedure, and separation with immunomagnetic beads coated with anti-EpCAM antibodies. Light and electron microscopy, flow cytometry and immunocytochemistry techniques were used to characterize the isolated cells. Immunohistochemical staining of normal SB biopsies confirmed that the cell cultures maintained an in vivo phenotype as reflected in cytokeratin expression CK18, CK20 and expression of intestine-specific markers such as sucrase isomaltase and maltase glucoamylase. Furthermore, the cells strongly expressed TLR-5, 6, 7, 8 and 10 and several molecules such as CD40, CD86, CD44, ICAM-1 and HLA-DR which are important in triggering cell-mediated immune responses. This novel technique provides a unique in vitro system to study the biology of enterocytes in normal conditions as well as to study inflammatory processes in various small bowel disorders.
Keywords: enterocytes, immune recognition, toll-like receptors, transplantation
Introduction
Enterocytes play a role not only in nutrient absorption but also perform a crucial function in immunophysiology [1]. They act as a barrier for the entry of microbes and toxins from lumen into the tissue [2–4]. Enterocytes can secrete cytokines as well as chemokines in response to inflammatory signals [5]. Through this secretion, enterocytes can activate and recruit inflammatory as well as immune cells [1]. They can also act as antigen presenting cells when stimulated [6,7]. Study of these cells is important in areas such as pharmacology, stem cells, gene therapy as well as immunology. They are also used for the study of many biological processes such as cell permeability and transport [8], differentiation [9], migration [10], cell-matrix interaction, apoptosis, etc. [11]. In spite of these important applications, cell culture models of fully differentiated adult human enterocytes are not well established [12].
Difficulties in obtaining cell lines of intestinal epithelial cells include microbial infections of the cultures, short in vivo life span of enterocytes [13,14], their highly differentiated state as well as their complex interaction with extracellular matrix [15,16]. Normal human intestinal lumen is inhabited by a number of microbial strains bringing with it a risk of microbial contamination to the culture. Second, intestinal epithelial lining is also a highly dynamic layer renewed every 4–5 days [17]. Because of their short life span there is a rapid turnover of cellular components. When enterocytes reach villus tip, they are in fully differentiated form gaining absorptive function with the loss of mitotic activity. The third important aspect of these epithelial cells is their dependency on the extracellular matrix. So culture model for enterocytes usually describe the use of feeder layer or in vitro constitution of extracellular matrix [18].
Because of the above-mentioned difficulties in culturing the intestinal epithelial cells, cell lines for these are not easily available in spite of its need in various fields [11]. Available cell lines are usually from rodent [19,20] fetal tissue or from intestinal cancer tissue [21]. All these models have their own limitations. It is becoming evident that observations performed with the experimental animals cannot be transposed to the human and there is difference in brush border enzyme expression and its regulation by hormones and growth factors [22]. Also there is a fundamental difference in the composition of the epithelial basement membrane along the crypt–villus axis [23,24]. Most commonly used cell lines are Caco-2 and HT29 cell line originally derived from human colon adenocarcinoma cells [25,26]. However, as transformed cells these models have their own limitations [27–29]; it is necessary to develop a simple and reproducible culture model for adult human enterocytes.
The aim of this study was to establish a simple and reproducible method for isolation and cultivation of human enterocytes from the small intestine (SI; ileum). The authors further characterized these cells with regards to expression of Toll-like receptors (TLRs) and various adhesion molecules involved in cell-mediated immune responses. They believed that these cells will also provide a unique in vitro system to study inflammatory processes in various SI disorders like Crohn's disease, ulcerative colitis, primary sclerosing cholangitis, etc.
Materials and methods
Reagents
The basal media used was a mixture of DMEM (Dulbecco's Modified Eagle Medium) and F12 in 1:1 proportion. For preparation of complete media, 5% heat inactivated FBS (fetal bovine serum), 1% l-glutamine and 1% penicillin–streptomycin (GIBCO, Paisley, UK) were added to the basal media mixture. The complete media was supplemented with HCM Single Quote kit (Lonza, Walkersville, MD, USA) containing ascorbic acid, BSA-FAF (bovine serum albumin-fatty acid free), hydrocortisone, transferrin, insulin, recombinant human epidermal growth factor and gentamicin sulfate. Culture vessels (BD Biosciences, San Diego, CA, USA) were coated with 1% gelatin. For enzymatic cell dissociation collagenase (Sigma, Gothenburg, Sweden) was used while trypsin-EDTA (ethylenediaminetetraacetic acid; Invitrogen, Gothenburg, Sweden) was used for passaging.
Human small bowel tissue specimen
Human small bowel specimens (approximately 20–30 cm) were obtained from cadaveric organ donors. Informed consent was obtained from the relatives of cadaveric donor and ethical approval from the local ethics committee. The tissue was placed in HTK (histidine tryptophan ketoglutarate) preservative solution and transported to the laboratory. The isolation of enterocytes was carried out within 4–6 h after organ retrieval. Obtained intestinal sample was cut into three pieces. One part was used for culture while remaining two parts were used for histological studies, one piece of which was fixed in liquid nitrogen while other part was fixed in formaldehyde. In total, five small bowel tissue specimens were obtained from different individuals.
Isolation and cultivation of human enterocytes
Initially all visible fat was removed and the intestinal piece was washed with PBS (phosphate buffered saline) containing 1% PEST and cut longitudinally. The lumen was flushed once again two to three times with PBS containing PEST. The piece was transferred to a 50 ml tube containing culture medium. The tissue sample was cut into small pieces (approximately 2–3 mm3) which were then transferred to pre-coated (1% gelatin) six-well plates containing 2 ml complete medium in each well. Approximately, two to three pieces were placed in individual wells and incubated with the complete medium (described earlier) under standard incubation conditions, that is, 90% humidity, 5% CO2 and 37°C temperature. Culture plates were monitored daily for microbial contamination and cell migration from the tissue pieces. After 5–6 days cells gradually migrated from the tissue. Tissue pieces were removed and cells were fed with fresh medium and allowed to grow until they reached about 90% confluence. Cultures were fed with fresh medium every third day. Culture plates were tested for yeast and bacterial contamination using cell culture contamination detection kit (Molecular Probe, Invitrogen, Paisley, UK).
Isolation of enterocytes from a mixed population of cells
Enterocytes were positively selected from the above heterogeneous cell population using a commercially available kit (STEMCELL Technologies SARL, Grenoble, France). In brief, selection cocktail was prepared as per manufacturers' protocol. This selection cocktail was used for isolation of EpCAM (epithelial cell adhesion molecule)-positive cells. Cell suspension was prepared after trypsinization and subsequent washing. Cells were suspended in HBSS (Hank's balanced salt solution) containing 1% BSA and 1 mM EDTA. Volume of the suspension medium was adjusted so that the cell concentration was 2 × 108. To this cell suspension, 100 µl/ml of the selection cocktail was added, mixed well and incubated for 15 min at room temperature. Magnetic nanoparticles at 50 µl/ml amount were added to the cell suspension and incubated for 10 min at room temperature. EpCAM-positive cells were separated by placing the tube in a magnetic block for 5 min. EpCAM expressing cells attached to the walls of the tube and were separated by decanting the cell suspension by inverting the whole magnetic block but still keeping tube in it. Lastly, the tube was removed from the magnetic block and washed with buffer to collect the cells attached to the wall. This step was repeated four times to get an EpCAM-positive enriched cell fraction. The EpCAM-positive fraction of cells was then seeded in gelatin coated 75 cm2 T flask. Medium was renewed twice a week. Subcultures were established through trypsin treatment and reseeding. Cells were passaged in 1:3 split ratio. All experiments were conducted on cells at 2–4 passages.
Phenotyping of human enterocytes by flow cytometry
Single-color fluorescence was used to phenotypically characterize the isolated enterocytes. Cells were first trypsinized, washed with PBS and the number of cells was counted using Neubauer counting chamber. About 5 × 105 cells/tube were distributed into various tubes for staining with different markers. Primary antibodies used for staining were: anti-CK18, -CK20, -maltase glucoamylase (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), -sucrase isomaltase (Raybiotech, Sweden), -CD80, -CD86, -CD44, -CD40, -ICAM-1, -HLA (human leukocyte antigen) class I and -HLA-DR, -CD31, -von Willebrand factor (vWF), -E-cadherin (all from BD Biosciences), -EpCAM (Miltenyi Biotec, Bergisch Glasbach, Germany), -fibroblast antigen and -alpha smooth muscle actin (from Sigma-Aldrich, Stockholm, Sweden), -TLR-1, -2, -3, -4, -5, -6, -7, -8, -9, -10. All TLR antibodies were purchased from eBiosciences Inc., San Diego, CA, USA, except for TLR-5 (Abnova, Heidelberg, Germany) and TLR-10 from (Abcam, Cambridge, UK) while FITC conjugated secondary antibodies were purchased from Molecular Probe, Invitrogen. Intracellular staining for CK18, CK20, α-actin and all TLR markers was performed after cell permeabilization using 5% saponin in PBS [30]. The stained cells were suspended in 200 µl PBS and further analyzed by a flow cytometer (Fluorescence-Activated Cell Sorter (FACS), Becton Dickinson, San José, CA, USA). Fluorescence signals from 10,000 cells were recorded and analyzed by the CellQuest software. Dead cells were excluded by using propidium iodide exclusion method (0.5 μg/ml; Sigma Chemicals Co., USA).
Immunocytochemistry
Cells were cultured on eight-well culture chamber slides (Lab Tek Nunc, Naperville, IL, USA) at a seeding density of 103 cells/chamber and cultured for 48–72 h. Cells were washed with PBS and fixed with two different fixatives, one for detection of cytoplasmic proteins and other for membrane-bound proteins. For detection of cytoplasmic protein, that is, CK8, CK18 and CK20, smooth muscle actin cells were fixed for 5 min in ice cold methanol containing 30% acetone at -20°C temperature. The fixative was removed and cells were washed with PBS. These culture slides were stored at -4°C and used for staining within 2–3 days. For detection of sucrase isomaltase and maltase glucoamylase, after washing cells were fixed in 2% paraformaldehyde for 8–10 min at room temperature. Cells were washed with PBS after fixation and then treated with 1 mM ammonium chloride for 5 min at room temperature. After washing cells were permeabilized with chilled methanol for 5 min at -20°C. Cells were washed with PBS and stored as stated above.
For immunocytochemical characterization of the cells, antibodies specific to epithelial cells and enterocytes were used. To identify their epithelial nature cytokeratin (CK) markers, CK18, CK20 were used while two markers and maltase glucoamylase (Santa Cruz Biotechnology), and sucrase isomaltase (Raybiotech) were used to confirm that the cells were enterocytes. Following staining protocol was employed. Cells were washed with PBS and blocked with appropriate blocking serum for 30 min at room temperature. Dilutions of primary antibodies were prepared (CK18, 1:100; CK20, 1:100, sucrase isomaltase, 1:100 and maltase glucoamylase, 1:100) in 1% BSA (prepared in PBS). Cells were incubated with primary antibodies for 3 h at room temperature. After washing with PBS, cells were treated with fluorochrome conjugated secondary antibodies. Secondary antibodies used were, Alexafluor 488 conjugated (Molecular Probe, Invitrogen), goat anti-mouse for CK18 and SI, rabbit anti-goat for CK20 and goat anti-rabbit for maltase glucoamylase. Dilutions of secondary antibodies were 1:100 with 1% BSA (prepared in PBS).
Presence of mucin-producing goblet cells was demonstrated by using the Alcian blue and PAS staining procedure. Briefly, EpCAM-positive cells were stained sequentially with Alcian blue (cat. no. 1.01647.0500, Merck, Germany), Periodic acid (cat. no. 1.00524.0100, Merck) and Schiff's reagent (3952016, Sigma-Aldrich, USA). The color development was as follows: acidic mucins: blue, neutral mucins: magenta, mixtures of above: blue/purple color.
Transmission electron microscopy
Cells were fixed in Karnovsky fixative (2% paraformaldehyde, 2,5% glutaraldehyde, 0,05 M Na-cacodylate, 0,02% Na-azid, pH 7.2), for 4 h at room temperature. Cells were washed with Na-cacodylate buffer (0.15 M, pH 7.2) and were stored in this buffer at 4°C till processed for transmission electron microscopy (TEM). Cells were postfixed in a fixative containing 1% OsO4, 1% 4Fe(CN)6 in 0.1 M Na-cacodylate for 2 h at +4°C temperature. After postfixation, cells were washed three times with distilled water. Cells were then treated with 0.5% uranyl acetate for 1 h at room temperature. Dehydration of the cells was performed using ethanol grades and cells were embedded in Agar 100 resin (AgarScientific Ltd., Esses, UK). About 60–70 nm thick sections were taken and mounted on Cu-grids in Leica UC6. Cells were treated with uranyl acetate followed by lead nitrate and examined at 120 kV in a 912 AB.
Immunohistochemistry
To check the in vivo expression pattern of the antibodies used for in vitro studies, intestinal sample was also stained with the same antibodies. For histochemical analysis, tissue pieces were fixed in 4% formalin for 24 h and processed for paraffin embedding. Four micron thick sections were cut and mounted on superfrost plus slides. Expression and localization of interested proteins were detected using indirect immunofluorescence or enzymatic staining. Briefly, tissue sections were deparaffinized and hydrated through alcohol grades. For unmasking the antigen heat-induced retrieval method was used. For this sections were kept in antigen retrieval solution (10 mM sodium citrate, pH 6.0, containing 0.05% Tween 20) and heated under pressure for 20 min. After cooling to room temperature, sections were incubated with blocking serum (5% in PBS) for 30 min. Prediluted primary antibodies were added to the sections and incubated overnight at 4°C. After washing the primary antibodies with PBS, fluorochrome conjugated secondary antibodies were added to detect the bound primary antibody. Sections were observed under fluorescence microscope (Olympus, Japan).
For the enzymatic assay, the diaminobenzidine tetrahydrochloride DAB-Nickel (stains brown/black color) substrate kit was used as color developer. Sections were counterstained with hematoxylin and mounted in mounting media (ImmunKemi, Stockholm, Sweden).
Results
Culture of isolated human enterocytes
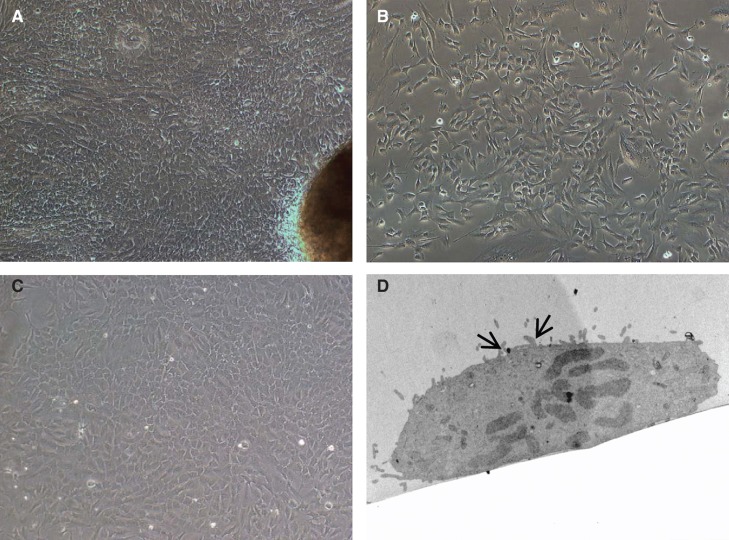
In general, the mechanical method for isolation of SI cells yielded a large number of mixed heterogenous populations of cells, with good viability. Following isolation, the number of magnetically isolated EpCAM-positive cells were counted and viability tested by trypan blue exclusion method. On an average, approximately 4–5 × 106 EpCAM-positive cells were isolated from 7 to 8 × 106 mixed cell population. The viability of the cells was approximately 90 ± 5%. The EpCAM-positive cells grew in several clusters and formed a monolayer within 10 days. They showed typical epitheloid morphology. The EpCAM-positive cells could be maintained with stable morphology for 5–6 passages, after which the cells ceased to divide. Figure 1 shows phase-contrast micrographs of the EpCAM-positive cells. Furthermore, TEM analysis of enterocytes revealed the presence of microvilli confirming the “enterocyte nature” of the isolated cells (Figure 1).
Figure 1.
Morphology of cells isolated from human small bowel. (A) Five to six days after placing tissue pieces in culture plates, cells started to migrate out from (B) a preconfluent culture of EpCAM-positive cells. These cells grew and showed epithelioid morphology and could be maintained for 6–7 passages. (C) Confluent monolayer of EpCAM-positive cells showing cobble-shaped morphology typical of epithelial cells. (D) Transmission electron micrographs of cultured primary enterocytes showing presence of microvilli (arrows). Magnification 20×.
Phenotypic characterization of enterocytes by flow cytometry
Enterocytes were characterized during growth by immunofluorescence labeling and flow cytometry. Flow cytometric analysis indicated that the cells were positive for CK18 and intestine-specific CK, CK20. In addition, the cells also expressed the two enterocyte-specific enzymes, sucrase isomaltase and maltase glucoamylase. On the other hand, the cells did not express the endothelial cell markers CD31 or vWF. In addition, the cells were negative for the fibroblast marker and smooth muscle cell actin (Table I).
Table I.
Markers expressed by human enterocytes isolated from small intestine.
| Antibodies to | Unstim. enterocytes (n = 4) (frequency of cells positive for marker) | TNF-α & IFN-γ stim. enterocytes (n = 4) (frequency of cells positive for marker) |
|---|---|---|
| HLA class I | ++ | +++ |
| HLA-DR | - | + |
| CD80 | - | - |
| CD86 | + | ++ |
| CD44 | + | +++ |
| CD40 | + | ++ |
| ICAM-1 | + | ++ |
| CD31 | - | - |
| vWF | - | - |
| Fibroblast Ag | - | - |
| α-Actin | - | - |
| EpCAM | ++ | +++ |
| E-cadherin | + | ++ |
| CK8 | ++ | +++ |
| CK18 | ++ | +++ |
| CK20 | + | ++ |
| Sucrose isomaltase | + | ++ |
| Maltase glucoamylase | + | ++ |
+ = >10–25, ++ = >26–50, +++ = >51–75 mean fluorescence channels as compared with negative control (only secondary antibodies).
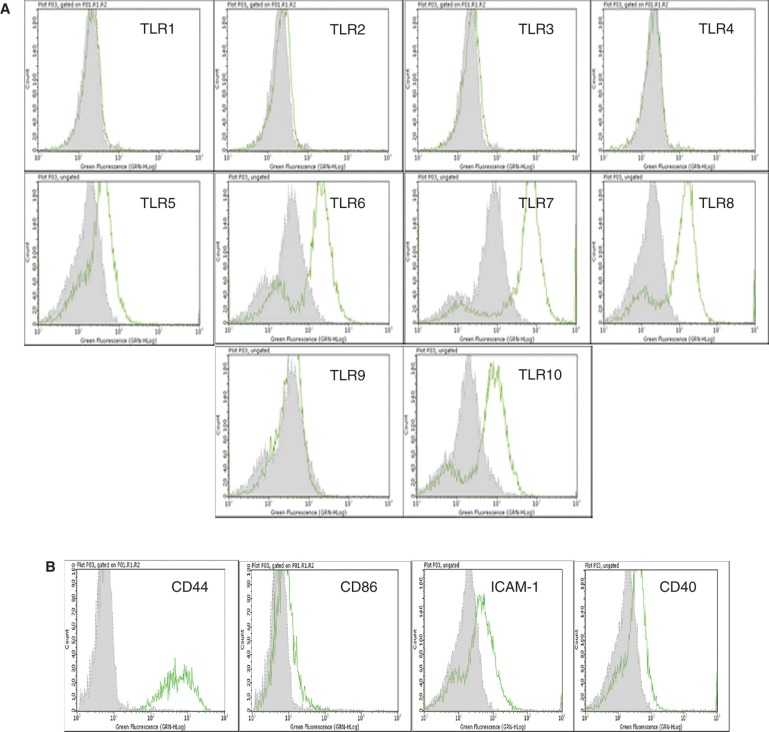
Interestingly, the isolated enterocytes expressed several TLRs and other surface molecules of importance in immunological reactions. They constitutively expressed weakly TLR-5, but demonstrated strong expression of TLR-6, -7, -8 and -10 (Figure 2) including the molecules CD44, CD86, ICAM-1 and CD40. On IFN-γ activation, these cells also expressed the histocompatibility antigen HLA-DR (Table I).
Figure 2.
Flow cytometric analysis of cultured human EpCAM-positive cells. (A) Flow cytometric results are presented in the form of histograms. Representative histograms for expression of TLRs in enterocytes are shown. Primary enterocytes did not express TLR-1, -2, -3, -4 and -9. They weakly expressed TLR-5, but demonstrated strong expression of TLR-6, -7, -8 and -10. The expression of TLRs was observed only in saponin-treated cells. (B) Isolated human enterocytes also stained positive for several immune recognition molecules such as CD44, CD86, ICAM-1 and CD40. Staining with only the secondary antibody served as negative control (grey filled histogram).
Immunocytochemistry
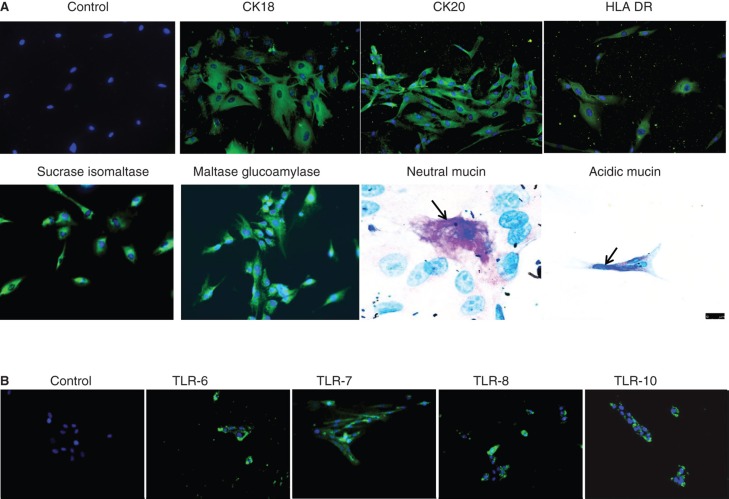
Immunofluorescence staining for CK18 and CK20 demonstrated positive reaction in the form of cytoplasmic strands while staining with anti-sucrase isomaltase and anti-maltase glucoamylase antibodies showed uniform positive staining (Figure 3A). In addition, activated enterocytes also expressed HLA-DR (Figure 3A). To confirm the FACS data, the enterocytes were stained with antibodies to TLR-6, -7, -8 and -10, using immunofluorescence. It was found that the cells stained positive for all the four tested TLRs (Figure 3B). Since the FACS analysis demonstrated that only 92 ± 5% (n = 4) of the isolated EpCAM cells were positive for the two enterocyte-specific markers sucrose isomaltase and maltase glucoamylase, the cells were further stained for expression of mucins and it was found that approximately 2–3% (n = 3) of the cells were positive for mucin indicating the presence of goblet cells. Both neutral (pink) and acidic (blue) mucins were detected (Figure3A, lower panel).
Figure 3.
Immunocytochemical staining of isolated human enterocytes for epithelial markers. (A)Top panel: isolated and cultured human EpCAM cells stained positive for cytokeratins 18, 20 and on activation with IFN-γ also expressed HLA-DR. Bottom panel: enterocyte-specific markers such as maltase glucoamylase and sucrase isomaltase were also found to be expressed by the cells. Staining for mucins showed the presence of few secretory epithelial cells – goblet cells which stained pink/magenta, or blue indicating the presence of neutral and acidic mucins. (B) Immunofluorescence staining of human enterocytes with antibodies to Toll-like receptors (TLRs) showed that these cells expressed TLR-6, -7, -8 and 10, thus confirming our FACS results. Magnification 20×, neutral mucin and acidic mucin 60×.
Immunohistochemistry
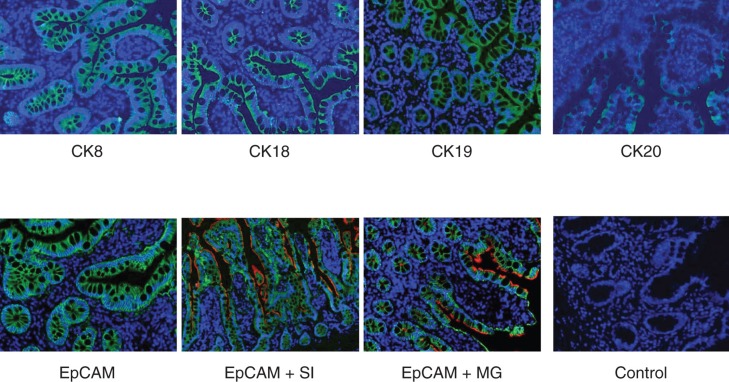
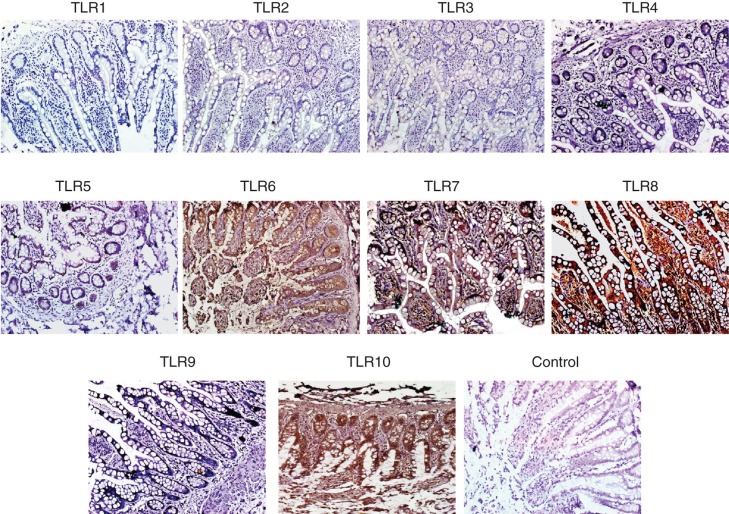
The authors also performed immunofluorescence staining for several enterocytes markers on frozen and paraffin sections of normal SI tissues (n = 4). Figure 4 demonstrates the staining pattern for several enterocyte markers. Positive staining for CK8, CK18 and CK19 was observed along the whole epithelial layer of crypt–villus axis while CK20-positive staining was observed only at the differentiated villus cells. Abundant expression of EpCAM was observed on all intestinal epithelial cells. Double staining by immunofluorescence for EpCAM and sucrase isomaltase as well as EpCAM and maltase glucoamylase was also performed (Figure 4). Positive reaction for anti-EpCAM antibody was seen on basolateral margins of epithelial cells while positive reaction for anti-sucrase isomaltase and anti-maltase glucoamylase antibodies was found to be only at the luminal margins of the cells. Positive reaction for anti-sucrase isomaltase antibody was observed in crypt as well as villus cells while positive reaction for anti-maltase glucoamylase antibody was seen only in villus cells (Figure 4). Staining for TLRs in biopsy sections of normal SI gave a similar pattern as observed in flow cytometric analysis (Figure 5). No expression of TLR-1, -2, -3, -4 and -9 was found, however intense staining for TLR-5, -6, -7, -8 and -10 was found in SI epithelial lining (Figure 5).
Figure 4.
Immunofluorescence staining of normal human small bowel tissue sections with antibodies to various epithelial markers. Indirect immunofluorescence single staining was performed for detection of CK8, CK18, CK19 and CK20. Positive staining for CK8, CK18 and CK19 was observed along the whole epithelial layer of crypt–villus axis while CK20-positive staining was observed only at the differentiated villus cells. Indirect immunofluorescence double staining for EpCAM (green) and enterocyte markers, sucrase isomaltase and maltase glucoamylase (both red) in small intestine was performed. EpCAM-positive reaction is found on basolateral and basal side of epithelial cells while sucrase isomaltase and maltase glucoamylase-positive reaction is observed on luminal side only. Positive reaction for sucrase isomaltase is present in crypt as well as villus cells but for maltase glucoamylase it is observed only in villus cells. Magnification 40×.
Figure 5.
Immunohistochemical detection of TLR on paraffin sections of human small intestine. Small intestine biopsies from healthy individuals were stained for various Toll-like receptors (TLRs). No expression of TLR-1, -2, -3, -4 and -9 were found. However, the following TLRs were found to be expressed in the SI sections. TLR-5: weak positive staining is observed along the entire epithelial layer. TLR-6: epithelial layer showed moderate staining while intense reaction observed in the lamina propria cells and granules of paneth cells. TLR-7: both villus and crypt epithelial cells showed granular-positive reaction. TLR-8: diffuse positive staining of moderate intensity is observed along the whole epithelial layer. Many cells in the lamina propria also showed positive reaction. In two samples, intense positive reaction for TLR-8 is observed at the luminal side of epithelial layer; in these samples, few lamina propria cells showed positive reaction. TLR-10: intense positive reaction is observed in epithelial cells along the entire crypt–villus axis. Intense brown reaction was observed in the granules of paneth cells and also many lamina propria cells are intensely stained. Magnification 40×.
Discussion
In the present study, the authors described a simple and novel method for the selective isolation of human enterocytes from the SI. Using magnetic beads coupled with antibodies to a pan epithelial cells marker EpCAM, they selectively isolated EpCAM-positive epithelial cells from a mixed primary culture of human small intestinal cells. EpCAM is a pan epithelial cell marker expressed abundantly in normal intestinal epithelial cells as detected in immunohistochemical staining of intestinal sample with anti-EpCAM antibody. Epithelial cells expressing EpCAM have also been isolated from other organs such as lungs [31] and livers [32]. Here, the authors demonstrated that most of the isolated EpCAM-positive cells expressed all the markers typical for enterocytes, that is, positivity for CK18, CK20 and the enterocyte-specific marker enzymes; sucrase isomaltase and maltase glucoamylase, indicating the presence of both undifferentiated crypt cells as well as differentiated villus cells in the culture. The purity of enterocytes in each isolate was approximately 93–95% as determined by the FACS analysis and expression of enterocyte-specific markers. In addition to enterocytes, approximately 2–3% of the EpCAM cells were also found to be positive for mucins indicating the presence of a small proportion of goblet cells. Further phenotyping of the EpCAM-positive cells showed that the cells expressed several TLRs and adhesion molecules involved in triggering immune reactions, implying an important role for these cells in inflammatory responses of SI during inflammation. The expression of these markers was further corroborated in SI biopsy sections.
The current method is reproducible and yielded enterocytes with high viability. The major advantages of the described protocol are the simplicity and avoidance of time-consuming steps such as density-gradient centrifugations or mechanical removal of contaminating cells [11]. Successful culturing of enterocytes depends on a variety of factors. One of the important factors is the harvesting technique. The authors used a mechanical method for tissue disruption. This approach entails a rapid isolation of the cells without subjecting them to the adverse effects of enzyme treatment which is normally used for isolation of this cell type. When using the mechanical method, the tissue is only minced to small pieces and kept in culture vessels keeping the epithelial layer attached to underlying mesenchymal tissue thus protecting the mature as well as stem cells from undergoing anoikis. According to the authors, this isolation method is reproducible and yields enterocytes with high viability. The major advantages of the described protocol are the simplicity and avoidance of time-consuming steps such as density-gradient centrifugations or mechanical removal of contaminating cells. Cultured cells maintained good morphology as well as expression of markers upto 6–7 passages. This feature is not affected by freezing the cells at earlier passage and then reculturing again (unpublished data).
An issue that is acute to intestinal cell culture is the avoidance of resident microbial contamination. Washing of the intestine sample with buffer containing antibiotic is necessary to avoid microbial contamination of cell culture. Minimum handling of the tissue sample is also important. If one employs the mucosa scraping method which is commonly used [11,17], there is increased risk of microbial contamination.
In conclusion, the authors describe a simple and novel protocol for the isolation and cultivation of human enterocytes which will be of advantage for in vitro research in gastrointestinal disorders such as Crohn's disease, ulcerative colitis as well as to elucidate the mechanisms of small bowel allograft rejections after transplantation. This procedure provides a guideline for a simple and reproducible culture method for enterocytes from adult human intestine.
Establishment of such cell lines will aid in elucidating the mechanisms underlying the pathogenesis of inflammatory reaction during rejections of SI allografts as well as interaction of immune cells with enterocytes in various small bowel disorders. Use of well-characterized enterocyte populations as targets in such studies may provide important clinical knowledge regarding the role of these cell types to respond during inflammation and various types of damage in gastrointestinal disorders.
Acknowledgments
The present study was financed by grants from The Swedish County Council, to Dr. Suchitra Sumitran-Holgersson.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- [1].Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)-13 and interferon gamma and inhibits tumor necrosis factor alpha-induced IL-8 release. J Exp Med. 1998;187:1285–94. doi: 10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Walker WA. Development of the intestinal mucosal barrier. J Pediatr Gastroenterol Nutr. 2002;34(Suppl 1):S33–9. doi: 10.1097/00005176-200205001-00009. [DOI] [PubMed] [Google Scholar]
- [3].Sansonetti P. Host-pathogen interactions: the seduction of molecular cross talk. Gut. 2002;(Suppl 3):50. III2–8. doi: 10.1136/gut.50.suppl_3.iii2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shen L, Turner JR. Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: tight junction dynamics exposed. Am J Physiol Gastrointest Liver Physiol. 2006;290:G577–82. doi: 10.1152/ajpgi.00439.2005. [DOI] [PubMed] [Google Scholar]
- [5].Strober W. Interactions between epithelial cells and immune cells in the intestine. Ann N Y Acad Sci. 1998;859:37–45. doi: 10.1111/j.1749-6632.1998.tb11109.x. [DOI] [PubMed] [Google Scholar]
- [6].Blumberg RS, Terhorst C, Bleicher P, et al. Expression of a nonpolymorphic MHC class I-like molecule, CD1D, by human intestinal epithelial cells. J Immunol. 1991;147:2518–24. [PubMed] [Google Scholar]
- [7].Hershberg RM, Framson PE, Cho DH, et al. Intestinal epithelial cells use two distinct pathways for HLA class II antigen processing. J Clin Invest. 1997;100:204–15. doi: 10.1172/JCI119514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Weng XH, Beyenbach KW, Quaroni A. Cultured monolayers of the dog jejunum with the structural and functional properties resembling the normal epithelium. Am J Physiol Gastrointest Liver Physiol. 2005;288:G705–17. doi: 10.1152/ajpgi.00518.2003. [DOI] [PubMed] [Google Scholar]
- [9].Rusu D, Loret S, Peulen O, Mainil J, Dandrifosse G. Immunochemical, biomolecular and biochemical characterization of bovine epithelial intestinal primocultures. BMC Cell Biol. 2005;6:42. doi: 10.1186/1471-2121-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stutzmann J, Bellissent-Waydelich A, Fontao L, Launay JF, Simon-Assmann P. Adhesion complexes implicated in intestinal epithelial cell-matrix interactions. Microsc Res Tech. 2000;51:179–90. doi: 10.1002/1097-0029(20001015)51:2<179::AID-JEMT9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- [11].Perreault N, Beaulieu JF. Primary cultures of fully differentiated and pure human intestinal epithelial cells. Exp Cell Res. 1998;245:34–42. doi: 10.1006/excr.1998.4221. [DOI] [PubMed] [Google Scholar]
- [12].Chopra DP, Dombkowski AA, Stemmer PM, Parker GC. Intestinal epithelial cells in vitro. Stem Cells Dev. 2010;19:131–42. doi: 10.1089/scd.2009.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schmidt R, Thews G. Physiologie des Menschen. Berlin, Heidelberg, New York: Springer Verlag; 1995. [Google Scholar]
- [14].Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol. 1979;80:248–65. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Podolsky DK. Regulation of intestinal epithelial proliferation: a few answers, many questions. Am J Physiol. 1993;264:G179–86. doi: 10.1152/ajpgi.1993.264.2.G179. [DOI] [PubMed] [Google Scholar]
- [16].Basson MD, Turowski G, Emenaker NJ. Regulation of human (Caco-2) intestinal epithelial cell differentiation by extracellular matrix proteins. Exp Cell Res. 1996;225:301–5. doi: 10.1006/excr.1996.0180. [DOI] [PubMed] [Google Scholar]
- [17].Bader A, Hansen T, Kirchner G, Allmeling C, Haverich A, Borlak JT. Primary porcine enterocyte and hepatocyte cultures to study drug oxidation reactions. Br J Pharmacol. 2000;129:331–42. doi: 10.1038/sj.bjp.0703062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hahn U, Cho A, Schuppan D, Hahn EG, Merker HJ, Riecken EO. Intestinal epithelial cells preferentially attach to a biomatrix derived from human intestinal mucosa. Gut. 1987;28(Suppl):153–8. doi: 10.1136/gut.28.suppl.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kedinger M, Haffen K, Simon-Assmann P. Intestinal tissue and cell cultures. Differentiation. 1987;36:71–85. doi: 10.1111/j.1432-0436.1987.tb00182.x. [DOI] [PubMed] [Google Scholar]
- [20].Evans GS, Flint N, Potten CS. Primary cultures for studies of cell regulation and physiology in intestinal epithelium. Annu Rev Physiol. 1994;56:399–417. doi: 10.1146/annurev.ph.56.030194.002151. [DOI] [PubMed] [Google Scholar]
- [21].Simon-Assmann P, Turck N, Sidhoum-Jenny M, Gradwohl G, Kedinger M. In vitro models of intestinal epithelial cell differentiation. Cell Biol Toxicol. 2007;23:241–56. doi: 10.1007/s10565-006-0175-0. [DOI] [PubMed] [Google Scholar]
- [22].Ménard D, Beaulieu JF. Human intestinal brush border membrane hydrolases. In: G B, editor. Membrane Physiopathology. Norwell: Kluwer Academic Publisher; 1994. pp. 319–41. [Google Scholar]
- [23].Beaulieu JF, Vachon PH. Reciprocal expression of laminin A-chain isoforms along the crypt-villus axis in the human small intestine. Gastroenterology. 1994;106:829–39. doi: 10.1016/0016-5085(94)90740-4. [DOI] [PubMed] [Google Scholar]
- [24].Simon-Assmann P, Duclos B, Orian-Rousseau V, et al. Differential expression of laminin isoforms and alpha 6-beta 4 integrin subunits in the developing human and mouse intestine. Dev Dyn. 1994;201:71–85. doi: 10.1002/aja.1002010108. [DOI] [PubMed] [Google Scholar]
- [25].Huang DB, DuPont HL, Jiang ZD, Carlin L, Okhuysen PC. Interleukin-8 response in an intestinal HCT-8 cell line infected with enteroaggregative and enterotoxigenic Escherichia coli. Clin Diagn Lab Immunol. 2004;11:548–51. doi: 10.1128/CDLI.11.3.548-551.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Parlesak A, Haller D, Brinz S, Baeuerlein A, Bode C. Modulation of cytokine release by differentiated CACO-2 cells in a compartmentalized coculture model with mononuclear leucocytes and nonpathogenic bacteria. Scand J Immunol. 2004;60:477–85. doi: 10.1111/j.0300-9475.2004.01495.x. [DOI] [PubMed] [Google Scholar]
- [27].Rousset M. The human colon carcinoma cell lines HT-29 and Caco-2: two in vitro models for the study of intestinal differentiation. Biochimie. 1986;68:1035–40. doi: 10.1016/s0300-9084(86)80177-8. [DOI] [PubMed] [Google Scholar]
- [28].Moyer MP, Dixon PS, Culpepper AL, Aust JB. Colon Cell Cancer. San Diego: Academic Press; 1990. [Google Scholar]
- [29].Carriere V, Lesuffleur T, Barbat A, et al. Expression of cytochrome P-450 3A in HT29-MTX cells and Caco-2 clone TC7. FEBS Lett. 1994;355:247–50. doi: 10.1016/0014-5793(94)01199-0. [DOI] [PubMed] [Google Scholar]
- [30].Bo X, Broome U, Remberger M, Sumitran-Holgersson S. Tumour necrosis factor alpha impairs function of liver derived T lymphocytes and natural killer cells in patients with primary sclerosing cholangitis. Gut. 2001;49:131–41. doi: 10.1136/gut.49.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fujino N, Kubo H, Ota C, et al. A novel method for isolating individual cellular components from the adult human distal lung. Am J Respir Cell Mol Biol. 2012;46:422–30. doi: 10.1165/rcmb.2011-0172OC. [DOI] [PubMed] [Google Scholar]
- [32].Tanaka M, Miyajima A. Identification and isolation of adult liver stem/progenitor cells. Methods Mol Biol. 2012;826:25–32. doi: 10.1007/978-1-61779-468-1_3. [DOI] [PubMed] [Google Scholar]