Abstract
Objectives
Decreased blood hemoglobin (HbB) levels and anemia have been associated with abnormal brainstem auditory evoked responses (BAER). Lead (Pb) exposure has also been associated with anemia and aberrant BAER. This study investigated the relationship between HbB level and BAER wave latency and amplitude in Pb-exposed Andean children.
Design and methods
Sixty-six children aged 2 to 15 years (mean age: 9.1; SD: 3.3) living in Pb-contaminated villages were screened for HbB levels, blood Pb (PbB) levels and BAER latencies and amplitudes.
Results
The mean HbB level observed in the study group was 11.9 g/dL (SD: 1.4; range: 8.6–14.8 g/dL). The mean HbB level corrected for altitude was 10.3 g/dL (SD: 1.4; range: 6.9–13.1 g/dL), and suggestive of anemia. The mean PbB level was 49.3 μg/dL (SD: 30.1; range: 4.4–119.1 μg/dL) and indicative of Pb poisoning. Spearman Rho correlation analyses revealed significant associations between the BAER absolute latencies and HbB level, indicating that as the HbB level decreased, the BAER wave latency increased. Children with low HbB levels (≤11 g/dL) showed significantly prolonged absolute latencies of waves I, II, III, IV and V compared to the children with normal HbB levels. Although a significant relationship between HbB and BAER waves was observed, no significant associations between PbB level and BAER parameters were found.
Conclusion
Low hemoglobin levels may diminish auditory sensory-neural function, and is therefore an important variable to consider when assessing BAER in children with anemia and/or Pb exposure.
Keywords: Hemoglobin, Brainstem auditory evoked responses, Anemia, Lead exposure
Introduction
Decreased blood hemoglobin (HbB) levels and anemia have been associated with abnormal brainstem auditory evoked responses (BAER) in both animal and human studies [1–4]. Prolonged absolute and interpeak BAER latencies, suggesting anomalous auditory sensory-neural functioning, have been found to be inversely correlated with HbB levels. Hemoglobin level has been identified as a significant variable in altering BAER latency and conduction times in children with diagnosed iron deficiency anemia. The link between pediatric anemia, as indicated by low HbB levels, and lead (Pb) exposure has been shown in a number of studies [5–7]. Pb is a neurotoxin, and Pb exposure may cause a reduction in Hb levels in whole blood and plasma, and may induce anemia [8]. Some studies have reported abnormal BAER in Pb-exposed children and adults [9–11], whereas other studies of Pb-exposed children have found no association between Pb levels and BAER parameters [12,13].
In previous studies in the Andes Mountains of Ecuador, the authors found children and adults with severely elevated blood Pb (PbB) levels and low HbB levels [14,15]. Related studies have revealed neurocognitive impairment, and elevated zinc protoporphyrin levels, reflecting chronic Pb exposure in the children of the study area [16, 17]. However, no unequivocal findings regarding Pb-induced effects on the peripheral and central auditory systems were found [12, 13]. While we have observed low HbB levels in the presence of high PbB levels in both children and adults in the study area, the effects of Hb levels on BAER were not examined in this Pb-exposed population. Recent reports associating low Hb levels with abnormal BAER indicate that Hb level may be an important variable in assessing auditory brainstem function in a population of children with chronic Pb exposure. Therefore, the objective of the present study was to investigate the relationship between HbB levels and BAER in a group of Pb-exposed children to determine whether low HbB levels are associated with aberrant BAER parameters.
Materials and methods
Participants and location
The study group consisted of 66 Pb-exposed children (25 females and 41 males) living in villages in Ecuador at an altitude of approximately 2,800 m. The children ranged in age from 2–15 years (mean age: 9.1; SD: 3.3), and were of Quichua and Mestizo ethnocultural backgrounds. The sample of children tested in this study is considered representative of the population of children living in the study area because the participants were selected from several grades in the local school, and because no effort was made to select specific children. The primary source of Pb exposure in the study area is discarded Pb-acid automobile storage batteries from which children and adults extract Pb to use in the glazing of ceramics, particularly roof tiles, as part of a local Pb glazing cottage industry. The primary routes of Pb exposure are hand-to-mouth ingestion of Pb-contaminated food, dust and soil, and the inhalation of air-borne particulates from the heavily Pb-contaminated smoke produced by the glazing kilns [14,15].
Informed consent was obtained from the parents or guardians of the children prior to testing. This study was approved by the Human Studies Committee (Comité de Bioética) of the Universidad San Francisco de Quito Medical School. The study was conducted under the auspices of the Universidad San Francisco de Quito Medical School in Quito, Ecuador, and under the direction of Professor Fernando Ortega, MD, PhD.
This study was part of an ongoing medical and educational project for the prevention of Pb exposure in villages in the Andes Mountains of Ecuador, where Pb glazing is common. The results of this investigation were presented to the parents or guardians of the children who participated in the study. The parents/guardians were counseled regarding their children’s Pb exposure, and were referred to local health officials for medical intervention where appropriate. Since the current data were collected, the PbB levels of children in this study area have been reduced significantly through preventative measures and chelation therapy.
Blood collection and analysis
Hb and Pb concentrations in whole blood were determined by collecting 2–4 mL of blood from the antecubital vein following a thorough cleaning of the skin with swabs containing isopropanol. Blood was collected with a Vacutainer set, using 4-mL heparinized collection tubes. All whole blood samples were stored in a refrigerated container in the field, and laboratory analysis was performed approximately one week later. The concentration of Hb in blood in g/dL was measured by a hematology analyzer (Bayer H3 Hematology Analyzer, Tarrytown, N.Y.). The measured HbB levels were altitude-corrected according to an established formula for Ecuadorian Andean populations [18]. An HbB level of ≤ 11 g/dL was considered indicative of anemia. Pb concentrations in μg/dL were measured by graphite furnace atomic absorption spectroscopy (GFAAS). Blood aliquots of 1 mL from matched samples were analyzed by GFAAS at the Boston Children’s Hospital Clinical Laboratories. The instrumentation for GFAAS was a model Zeeman/3030 (Perkin-Elmer Corp., Norwalk, CT) spectrophotometer with graphite furnace and deuterium background correction. Additionally, some blood samples were measured for Pb concentration by inductively coupled plasma mass spectrometry (ICP-MS) at the Channing Laboratory of the Harvard School of Public Health. Samples were analyzed using ICP-MS (Sciex Elan 5000, Perkin-Elmer, Corp., Norwalk, CT) with standard instrument operating and data collection parameters using isotope dilution procedures [15]. A sample of empty tubes from the same batch used at the field test site was tested in the laboratory for metal contamination and found to be Pb free.
Brainstem auditory evoked responses
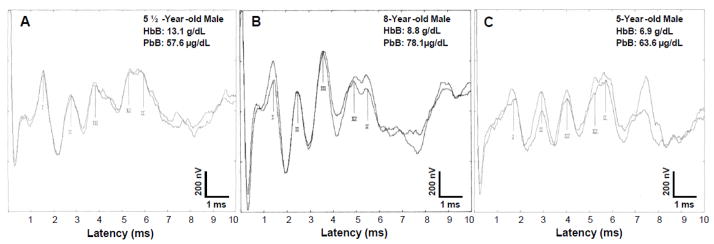
The BAER procedure was used to examine the functional integrity of the 8th nerve, and ascending auditory brainstem tracts and nuclei, including summated response amplitude, absolute latency and inter-nuclei neural transmission capacity. Prior to BAER testing, children in the study group were randomly chosen for audiometric examination. Any participant exhibiting a hearing loss was not included in BAER testing. Recordings of BAER were conducted in this study in a makeshift field clinic using the Medlec/GSI-50 (Grason-Stadler, Inc., USA) eletrophysiologic averaging system. Each participant was tested in the supine position on a small bed or flat matted surface. The patient’s scalp, forehead, and ear lobes were thoroughly cleaned with swabs containing isopropanol. Gold-plated surface electrodes were placed at Cz, (active), A1, A2 (reference), and Fpz (ground) in accordance with to the 10–20 International Electrode System. The electrode resistance was maintained at less than 5000 ohms. The electrodes (vertex positive) were connected to a physiologic pre-amplifier that amplified the ongoing electroencephalographic activity by 105. The BAER recordings were band-pass filtered between 100 and 3000 Hz and averaged. Broadband monaural rarefaction click stimuli of 80 dB normal hearing level (nHL) and 100-μsec duration were delivered to the ear/side under test through TDH 39 earphones at rates of 10 per second (1024 to 2048 sweep/trial) with contralateral masking. The absolute latencies (ms) of wave peaks I, II, III, IV, and V and the interpeak latencies of I–III, III–V, and I–V were measured. Additionally, the absolute amplitudes of waves I and V, and the V/I amplitude ratio were analyzed. Illustrations of BAER waveforms recorded from three children with varying HbB and PbB levels using standard scalp gold-plated electrodes are shown in Figure 1. This figure demonstrates the quality of the BAER recordings obtained in the field in the Andes Mountains, and show that electrical noise was not a factor in this field-testing environment because of meticulous electrode positioning for low scalp resistance, and proper equipment grounding.
Figure 1.
This figure shows replicated BAER recordings from three children in the study group with varying HbB levels and elevated PbB levels. The replicated recordings in Figure 1A illustrate the BAER amplitudes and latencies for a child with a normal HbB level of 13.1 g/dL, and a PbB level 57 μg/d/L. Figure 1B and Figure 1C show the BAER recordings for children with abnormally low HbB levels of 8.8 g/dL (PbB level: 78.1 μg/dL) and 6.9 g/dL (PbB level: 63.6 μg/dL), respectively. Figure 1B and Figure 1C show prolongations in absolute latencies of BAER waves peaks I–V.
Statistical analysis
Mean, median and range were determined for HbB and PbB levels. Differences between means were analyzed by Student’s t test and the Mann-Whitney U test. Correlation analyses were performed using the Pearson and Spearman correlation coefficients. In order to measure possible interactions among the variables of HbB, age and gender, and PbB, age and gender, the data were analyzed by multiple regression analyses (with HbB level as the dependent variable in one analysis, and with PbB level as the dependent variable in a second analysis). An alpha level ≤ .05 was accepted as indicative of statistical significance.
Results
HbB and PbB Levels
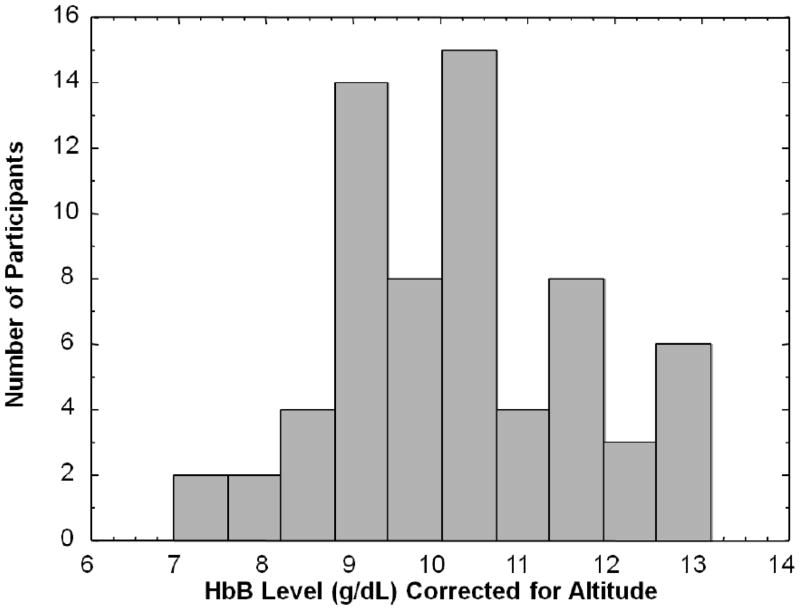
The measured mean HbB level of the study group was 11.9 g/dL (SD: 1.4; median: 11.8; range: 8.6–14.8 g/dL). The mean HbB level corrected for altitude (equivalent sea level value) was 10.2 g/dL (SD: 1.4; median: 10.1; range: 6.9–13.1 g/dL) and suggestive of anemia. The distribution of HbB levels corrected for altitude is presented in Figure 2. Of the 66 children in the study group, 72.7 % (n = 48) had corrected HbB levels ≤ 11 g/dL, and only 27.3% (n = 18) had normal HbB levels. The mean measured HbB level for the females (n = 25) was 11.7 g/dL (SD: 1.3; median: 11.8; range: 8.9–14.3 g/dL), but when corrected for altitude, the mean HbB level was10.0 g/dL (SD: 1.3; median: 10.1; range: 7.2–12.6 g/dL). The mean measured HbB level for the males (n = 41) was 11.9 g/dL (SD: 1.5; median: 11.8; range: 8.6–14.8 g/dL), and when corrected for altitude, the mean HbB level was 10.3 g/dL (SD: 1.5; median: 10.2; range: 6.9–13.1 g/dL). A Mann-Whitney U analysis revealed no statistically significant difference in HbB levels between females and males (tied Z = −.707, tied p = .479). A multiple regression analysis with HbB as the dependent variable showed no significant gender by age interaction (R2 = .018, F = .570, p = .568). A Spearman correlation analysis showed no statistically significant association between HbB level and Pb-B level in this study group (tied Z = −.400, tied p = .689).
Figure 2.
Distribution of blood hemoglobin (HbB) levels corrected for altitude in 66 children with chronic lead exposure living at high elevations in the Andes Mountains.
The mean PbB level for the total study group was 49.3 μg/dL (SD: 30.1; median: 56.1; range: 4.4–119.1 μg/dL). The mean PbB level for the 25 females was 43.8 μg/dL (SD: 27.4; median: 47.9; range: 4.4–100.2 μg/dL), and the mean PbB level for the 41 males was 52.7 μg/dL (SD: 31.5; median: 59.2; range: 5.7–119.1 μg/dL). A Mann-Whitney U analysis showed no statistically significant difference in PbB levels between females and males (tied Z = −1.229, tied p = .219). A multiple regression analysis with PbB level as the dependent variable showed no significant gender by age interaction (R2 = .058, F = 1.952, p = .151).
BAER and HbB level
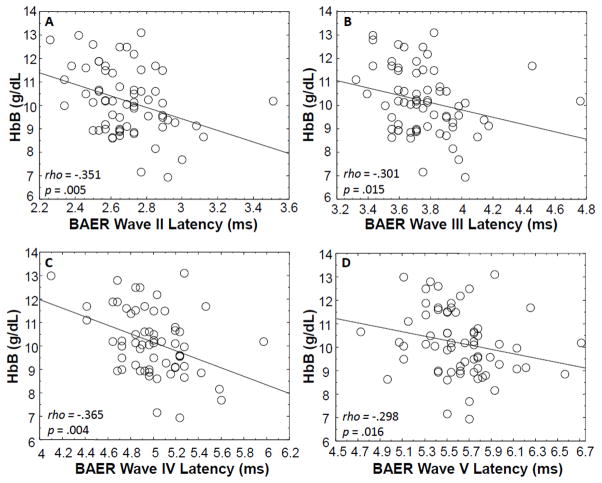
Figure 1 shows replicated BAER recordings from three children in the study group with varying HbB levels and elevated PbB levels. The replicated recordings in Figure 1A illustrate the BAER amplitudes and latencies for a child with a normal HbB level, while Figure 1B and Figure 1C show the BAER recordings for two children with abnormally low HbB levels. Figure 1B and Figure 1C show prolongations in absolute latencies of BAER waves I through V. Spearman rho correlation analyses revealed significant associations between the BAER absolute latencies of waves II (rho = −.351, p = .005), III (rho = −.301, p = .015), IV (rho = −.365, p = .004) and V (rho = −.298, p = .016 ) and HbB level, indicating that as the HbB level decreased, the BAER wave latency increased (see Figure 3). The absolute latency of Wave I was not significantly associated with HbB level (rho = −.213, p = .085). The interpeak latencies of I–III (rho = −.170, p = .171), III–V (rho = −.080, p = .516), and I–V (rho = −.208, p = .093) were not significantly correlated with HbB level. There was no significant relationship between the absolute amplitude of wave I and HbB level (rho = −.018, p = .887) or wave V and HbB level (rho = .080, p = .518). The amplitude ratio V/I also was not significantly associated with HbB level (rho = .077, p = .533).
Figure 3.
Correlation analyses showing statistically significant associations between blood hemoglobin (HbB) levels (corrected for altitude) and BAER absolute latencies of waves II (A), III (B), IV (C) and V (D) for 66 children with chronic lead exposure living at high altitudes in the Andes mountains.
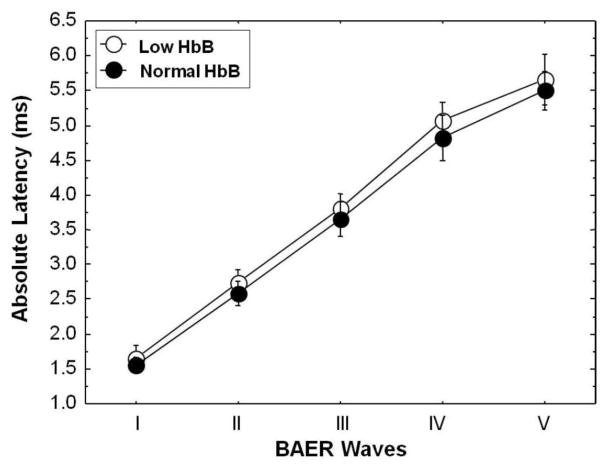
The data obtained in this investigation were further probed by examining BAER parameters in children in the study group with low (mean: 9.5 g/dL) and normal HbB (mean: 12.0 g/dL) levels. The differences in BAER latencies between the children with low HbB levels and those with normal HbB levels are shown in Figure 4. Mann Whitney U analyses showed that the absolute latencies of BAER waves I (tied Z = −2.306, tied p=.021), II (tied Z = −2.536, tied p=.011), III (tied Z = −2.535, tied p=.011), IV (tied Z = −2.790, tied p=.005) and V (tied Z = −2.409, tied p=.016) in children with low HbB levels were significantly delayed relative to the children with normal HbB levels. The interpeak latencies of waves I–III (tied Z = −.795, tied p=.426), III–V (tied Z = −.889, tied p=.374), and I–V (tied Z = −1.03, tied p=.302) were not significantly prolonged for the low and normal HbB groups. There were no significant differences between children with low HbB levels and children with normal HbB levels for BAER amplitudes of wave I (tied Z = −.649, tied p=.516) and wave V (tied Z = −.007, tied p=.994), or the amplitude ratio V/I (tied Z = −.547, tied p=.584).
Figure 4.
Comparison of BAER mean (with SD bars) absolute wave latencies for lead (Pb)-exposed children with normal blood hemoglobin (HbB) levels (> 11 g/dL) and Pb-exposed children with low HbB levels (≤ 11 g/dL). The data indicate statistically significant prolongations in the BAER absolute latencies for waves I, II, III, IV and V in the children with low HbB levels compared to the children with normal HbB levels.
BAER and PbB level
Analysis of BAER and PbB level showed that the absolute latencies of waves I (rho = −.042, p = .736), II (rho = −.037, p = .767), III (rho = .042, p = .737), IV (rho = .053, p = .676), and V (rho = .086, p = .489) were not significantly associated with PbB level. Similarly, the interpeak latencies I–III (rho = .086, p = .486), III–V (rho = .022, p = .861), and I–V (rho = −.150, p = .226) were not significantly correlated with PbB level. There was no statistically significant relationship between wave I absolute amplitude and PbB level (rho = .012, p = .922), or between wave V absolute amplitude and PbB level (rho = −.052, p = .677). In addition, the amplitude ratio V/I was not significantly associated with PbB level (rho = −.063, p = .612).
Discussion
This study investigated the effects of hemoglobin levels on BAER parameters in Pb-exposed children living in the Andes Mountains of Ecuador. It has been well established that Pb exposure may induce anemia, which may be exacerbated in children living in low oxygen, high altitude environments. In the present study, consisting of a sample of 66 Pb-exposed children, approximately 73 % were found to have abnormally low HbB levels. This finding suggests anemia in the study group, possibly related to Pb exposure. However, since no statistically significant association between HbB and PbB levels was found in this study group, the observed anemia may not be entirely Pb-related, but may be associated with some complex interaction between the low oxygen, high altitude environment and Pb exposure. The relationship between HbB and PbB is complex, and the fact that the present study found no significant association between HbB and PbB is not entirely unexpected, since the lack of an association between HbB and PbB also has been reported in other studies [19, 20].
Normal Hb levels are critical for neurodevelopment in children, and low Hb levels, indicative of anemia, have been associated with deficits in cognitive functioning [7, 21]. Anemia results from compromised Hb production as a result of inhibition of heme biosynthesis and the inhibition of the synthesis of α and β globin chains and hemolysis [22, 23]. The Hb levels observed in this study group are likely to be related to the high-altitude, low-oxygen environment in which the study group resides. Since higher altitudes may affect hematopoiesis, the development of high HbB levels as an adaptation to the low-oxygen environment enables normal physiological function and stability. The lower HbB levels observed in this group of children living at an elevated altitude may have an effect on neurophysiological functioning at the auditory brainstem level. Low Hb levels may reduce the oxygen supply to the auditory sensory neural elements that are responsible for generating the BAER. Thus, the abnormal BAER absolute latencies observed in the children in the present study may be associated with reduced oxygen supply to the peripheral and central auditory systems.
In the present study, BAER measurements revealed significant associations between HbB levels and the absolute latencies of waves II, III, IV and V, indicating that as the HbB levels decreased, the latencies of the BAER peaks increased. Further, when the children in the study group were divided into anemic and non-anemic groups, the anemic group had significantly prolonged absolute latencies for all BAER waves. These findings suggest that low HbB levels, particularly in the range of anemia, may affect the neural components that contribute to the summated responses seen in the temporal parameters of the BAER waves.
Wave I, which is generated by the bipolar neurons in the distal portion of the 8th cranial nerve was found to be significantly delayed in the anemic group, suggesting altered neural conduction. The prolongation in BAER wave II latency as the HbB level decreased suggests that low HbB levels have an effect on the neurophysiology of the proximal 8th cranial nerve fibers near the synaptic interface with the auditory brainstem, since wave II is believed to originate in the proximal portion of the 8th nerve, contiguous with the second order neurons of the cochlear nucleus. The putative auditory brainstem generators of summated waves III, IV, and V are the 2nd order neurons in the cochlear nucleus, superior olivary complex, and the lateral lemniscus/inferior colliculus interface, respectively [24]. It is possible that low Hb levels may adversely affect the summated neuronal potentials generated by each of these auditory brainstem nuclei. The observed transmission delays in the summated electrophysiological brain waves representing the auditory brainstem tracts and nuclei may suggest a reduction in functional neurons or altered status in the conducting elements and synapses due to reduced Hb. The prolongations in the absolute latencies observed for BAER are not considered to be related to conductive or cochlear (receptor level) hearing loss in this study group with normal hearing (≤20 dB HL). However, subclinical sensory auditory effects cannot be ruled out by this investigation.
A number of variables and their interactions may influence the neurological and sensory-neural status of Pb-exposed children, such as the participants in this study, including environment, genetic, and nutritional factors. Some studies have suggested a relationship between nutritional status and BAER abnormalities [3, 27, 28]. However, the findings of these studies were inconsistent and inconclusive with regard to an association between nutritional deficiency and BAER. Our previous work with children in the study area indicated no evidence poor nutrition, or iron deficiency as reflected in measurements of plasma ferritin, an indicator of body iron stores [26, 29].
In summary, our previous investigations of children in this study area, as well as the current study, showed no significant association between PbB level and the latency or amplitude of the BAER. The significant inverse association between HbB level and BAER latencies suggests that HbB may be a more suitable biomarker for assessing auditory brainstem involvement in Pb-exposed children residing at high elevations. The findings of the present study indicate that low HbB levels may have subtle effects on the sensory-neural auditory brainstem system that are manifested in alterations of BAER latencies. The results of this study are consistent with experimental studies in both newborn and adult sheep that demonstrated significant increases in BAER latencies as Hb levels were reduced to anemic levels [1]. However, before definitive conclusions can be reached regarding the relationship between Hb levels and auditory brainstem function in a Pb-exposed pediatric population, additional studies on a larger group of children should be undertaken.
Highlights.
The effect of hemoglobin levels on brainstem auditory evoked responses was studied.
Participants: children living in lead-contaminated villages in the Andes Mountains.
Low hemoglobin was associated with prolonged brainstem auditory response latencies.
Low hemoglobin may alter auditory sensory-neural function in lead-exposed children.
Acknowledgments
The authors thank Dr. Mauricio Espinel, Dean of Universidad San Francisco de Quito Medical School, Dr. José Izurieta, Director of Health, Cotopaxi Province, Dr. Galo Tapia, Director, Pujilí Hospital, Dr. Cecilia Zambrano, Director, La Victoria Subcentro de Salud, Ms. Gladys Pacheco, Nurse, Subcentro de Salud, La Victoria, and the Universidad San Francisco de Quito Medical School for continued support of this project. The authors are also grateful to Dr. Nader Rifai, Ms. Patricia Nolan Hoover and Mr. Gary Bradwin, Boston Children’s Hospital Medical Laboratories for laboratory support. Anthony B. Jacobs is thanked for excellent technical assistance. The authors are grateful to Dr. Merilee Grindle, Director of the David Rockefeller Center for Latin American Studies at Harvard, Dr. Jeremy Bloxham, Dean of Science, Harvard University; Harvard Biological Laboratories and Harvard University Health Services for support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quiñónez RE, Widness JA. Increased wave latency in auditory brainstem response to anemia in newborn and adult sheep. Biol Neonate. 2003;84:179–86. doi: 10.1159/000071954. [DOI] [PubMed] [Google Scholar]
- 2.Mahajan V, Gupta P, Tandon O, Aggarwal A. Brainstem auditory evoked responses in term small for gestational age newborn infants born to undernourished mothers. Eur J Paediatr Neurol. 2003;7:67–72. doi: 10.1016/s1090-3798(03)00015-1. [DOI] [PubMed] [Google Scholar]
- 3.Odabaş D, Caksen H, Sar S, Tombul T, Kisli M, Tuncer O, Yuca K, Yilmaz C. Auditory brainstem potentials in children with protein energy malnutrition. Int J Pediatr Otorhinolaryngol. 2005;69:923–8. doi: 10.1016/j.ijporl.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 4.Shanker N, Tandon OP, Bandhu R, et al. Brainstem auditory evoked potential responses in iron de cient anemic children. Indian J Physiol Pharmacol. 2000;44:297–303. [PubMed] [Google Scholar]
- 5.Adams WG, Geva J, Coffman J, Palfrey S, Bauchner H. Anemia and elevated lead levels in underimmunized inner-city children. Pediatrics. 1998;101:E6. doi: 10.1542/peds.101.3.e6. [DOI] [PubMed] [Google Scholar]
- 6.Shah F, Kazi TG, Afridi HI, Baig JA, Khan S, Kolachi NF, Wadhwa SK, Shah AQ. Environmental exposure of lead and iron deficit anemia in children age ranged 1–5 years: a cross sectional study. Sci Total Environ. 2010;408:5325–30. doi: 10.1016/j.scitotenv.2010.07.091. [DOI] [PubMed] [Google Scholar]
- 7.Roy A, Hu H, Bellinger DC, Mukherjee B, Modali R, Nasaruddin K, Schwartz J, Wright RO, Ettinger AS, Palaniapan K, Balakrishnan K. Hemoglobin, lead exposure, and intelligence quotient: effect modification by the DRD2 Taq IA polymorphism. Environ Health Perspect. 2011;119:144–9. doi: 10.1289/ehp.0901878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergdahl IA, Vahter M, Counter SA, Schutz A, Buchanan LH, Ortega F, Laurell G, Skerfving S. Lead in plasma and whole blood from lead-exposed children. Environ Res. 1999;80:25–33. doi: 10.1006/enrs.1998.3880. [DOI] [PubMed] [Google Scholar]
- 9.Holdstein Y, Pratt H, Goldsher M, et al. Auditory brainstem evoked potentials in asymptomatic lead-exposed subjects. J Laryngol Otol. 1986;100:1031–6. doi: 10.1017/s0022215100100519. [DOI] [PubMed] [Google Scholar]
- 10.Rothenberg SJ, Poblano A, Garza-Morales S. Prenatal and perinatal low-level lead exposure alters brainstem auditory evoked responses in infants. Neurotoxicology. 1994;15:695–700. [PubMed] [Google Scholar]
- 11.Bleecker ML, Ford DP, Lindgren KN, Scheetz K, Tiburzi MJ. Association of chronic and current measures of lead exposure with different components of brainstem auditory evoked potentials. Neurotoxicology. 2003;24:625–31. doi: 10.1016/s0161-813x(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 12.Counter SA, Buchanan LH, Ortega F, Laurell G. Normal auditory brainstem and cochlear function in extreme pediatric plumbism. J Neuro Sci. 1997;152:85–92. doi: 10.1016/s0022-510x(97)00149-4. [DOI] [PubMed] [Google Scholar]
- 13.Counter SA. Brainstem neural conduction biomarkers in lead-exposed children of Andean lead-glaze workers. J Occup Environ Med. 2002;44:855–64. doi: 10.1097/00043764-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Counter SA, Buchanan LH, Ortega F, Rifia N. Blood lead and hemoglobin levels in Andean children with chronic lead intoxication. Neurotoxicology. 2000;21:301–8. [PubMed] [Google Scholar]
- 15.Counter SA, Buchanan LH, Ortega F. Gender differences in blood lead and hemoglobin levels in Andean adults with chronic lead exposure. Int J Occup Environ Health. 2001;7:113–18. doi: 10.1179/107735201800339551. [DOI] [PubMed] [Google Scholar]
- 16.Counter SA, Buchanan LH, Ortega F, Rifai N, Shannon MW. Comparative analysis of zinc protoporphyrin and blood lead levels in lead-exposed Andean children. Clin Biochem. 2007;40:787–92. doi: 10.1016/j.clinbiochem.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Counter SA, Buchanan LH, Ortega F. Zinc protoporphyrin levels, blood lead levels and neurocognitive deficits in Andean children with chronic lead exposure. Clin Biochem. 2008;41:41–47. doi: 10.1016/j.clinbiochem.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Dirren H, Logman MH, Barclay DV, Freire WB. Altitude correction for hemoglobin. Eur J Clin Nutrition. 1994;48:625–32. [PubMed] [Google Scholar]
- 19.Solliway BM, Schaffer A, Pratt H, Yannai S. Effects of exposure to lead on selected biochemical and hematological variables. Pharmacol Toxicol. 1996;78:18–22. doi: 10.1111/j.1600-0773.1996.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 20.Froom P, Kristal-Boneh E, Benbassat J, Ashkanazi R, Ribak J. Lead exposure in battery- factory workers is not associated with anemia. J Occup Environ Med. 1999;41:120–23. doi: 10.1097/00043764-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Kordas K. Iron, lead, and children’s behavior and cognition. Annu Rev Nutr. 2010;30:123–48. doi: 10.1146/annurev.nutr.012809.104758. [DOI] [PubMed] [Google Scholar]
- 22.Hernberg S, Nikkanen J, Mellin G, Lilius H. G-aminolevulinic acid dehydrase as a measure of lead exposure. Arch Environ Health. 1970;21:140–46. doi: 10.1080/00039896.1970.10667211. [DOI] [PubMed] [Google Scholar]
- 23.National Research Council. Measuring lead exposure in infants, children, and other sensitive populations. National Academy Press; Washington, D.C. NRC: 1993. [PubMed] [Google Scholar]
- 24.Hall JW. New Handbook of Auditory Evoked Responses. New York: Pearson Education Inc; 2007. pp. 35–57. [Google Scholar]
- 26.Vahter M, Counter SA, Laurell G, Buchanan LH, Ortega F, Schütz A, Skerfving S. Extensive lead exposure in children living in an area with production of lead-glazed tiles in the Ecuadorian Andes. Int Arch Occup Environ Health. 1997;70:282–86. doi: 10.1007/s004200050220. [DOI] [PubMed] [Google Scholar]
- 27.Hassaan MR, Alghobashy AA, Abdel-Rahman HM. Auditory neural efficiency in protein energy malnourished toddlers with and without iron deficiency anemia. Egyptian J Ear Nose Throat Allied Health. 2011;12:105–14. [Google Scholar]
- 28.Vandana, Tandon OP. Auditory evoked potential responses in chronic malnourished children. Indian J Physiol Pharmacol. 2006;50:48–52. [PubMed] [Google Scholar]
- 29.Counter SA, Vahter M, Laurell G, Buchanan LH, Ortega F, Skerfving S. High lead exposure and auditory sensory-neural function in Andean children. Environ Health Perspect. 1997;105:522–26. doi: 10.1289/ehp.97105522. [DOI] [PMC free article] [PubMed] [Google Scholar]