Abstract
Conclusion
Results suggested mercury-induced anomalies in the brainstem mediated acoustic stapedius muscle reflex in children.
Objectives
Mercury (Hg) exposure has been associated with hearing impairment and brainstem anomalies. Acoustic stapedius reflex (ASR) thresholds, growth functions, decay/adaptation times, and behavioral auditory thresholds were used to screen Andean children and adults for mercury-induced auditory brainstem and facial nerve impairment.
Methods
Fifty-one participants, which included 22 children (aged 6 -17 years) and 29 adults (aged 19 - 83 years) living in gold mining areas of Ecuador where Hg is widely used in amalgamation, were screened using ASR immittance procedures.
Results
Mean blood mercury (HgB) level in the children was 15.6 μg/L (SD: 21.3; median: 7 μg/L; range: 2.0-89 μg/L), and in the adults 8.5 μg/L (SD: 7.1; median: 6 μg/L range: 2.0-32 μg/L). Mean contralateral ASR thresholds (ASRT) for the screening frequency of 2000 Hz in the children (39 ears) was 92.9 dB HL (SD: 6.1; range: 80-105 dB HL), and in the adults (53 ears) 90.0 dB HL (SD: 6.4; range: 65-105 dB HL). The ASRT in the children increased significantly with HgB level (rho = 0.433; p =0.008).
Keywords: middle ear muscles, brainstem, facial nerve, methylmercury, elemental mercury, neurotoxicity, environmental
Introduction
Mercury (Hg) is neurotoxic, and exposure to both inorganic Hg vapors (Hg°) and methylmercury (MeHg) has been linked to neurological and neurocognitive impairment in children and adults [1,2]. MeHg crosses the blood-brain barrier and accumulates substantially in the central nervous system (CNS) [3]. At the cellular-molecular level, MeHg also inhibits DNA synthesis and reduces the size and levels of cyclins in the hippocampus [4]. Further, Hg has been found to accumulate in a number of organs, including the lungs, intestine, kidneys, liver, as well as in the placenta [5].
Hg exposure has also been associated with cochlear pathology and hearing impairment [6,7]. Further, a number of investigations have linked Hg exposure to auditory brainstem damage [8-10]. Using brainstem auditory evoked responses (ABR) in evaluating Hg-exposed subjects, some of these studies have revealed abnormal neural conduction latencies, suggesting neuronal damage in the brainstem auditory tracts and nuclei. Anomalies in ABR latency and morphology have also been found in Hg-exposed indigenous Andean children living in Ecuadorian gold mining settlements where elemental Hg is widely used in the gold amalgamation process [11]. Similarly, behavioral auditory tests have suggested an association between blood mercury level (HgB) and hearing thresholds in this population of Hg-exposed Andean children [12]. Biomarker studies in the same Andean population revealed elevated levels of Hg in hair and urine samples, as well as in blood [13].
The present study investigated the integrity of the brainstem auditory tracts and nuclei in Hg-exposed Andean children and adults using the brainstem mediated acoustic stapedius muscle reflex (ASR). Similar to the ABR procedure, the ASR is a noninvasive physiological technique that is used to examine auditory nerve and auditory brainstem function. The ASR and ABR are mediated by segments of the same ascending auditory pathways, including the 8th cranial nerve, and within the brainstem, the cochlear nucleus and superior olivary complex. However, the ASR involves a multi-synaptic brainstem arc that includes the afferent bipolar neurons of the 8th nerve, the cochlear nucleus, the ipsilateral (uncrossed) and contralateral (crossed) superior olives, axonal projections to neurons in and around the nucleus of the 7th cranial nerve, and efferent motoneurons from the facial nerve branch to the stapedius muscle [14]. The ASR may offer some additional diagnostic information beyond the ABR procedure in that it evaluates both afferent and efferent auditory brainstem and neuromuscular function [15]. Further, the ASR procedure requires less time to administer in a field-testing situation, and serves as a reliable and objective screening measure of the integrity of the auditory brainstem. To our knowledge, the ASR procedure has not been used previously in auditory neuro-sensory studies of children or adults with Hg exposure.
Methods
Participants and locations
Fifty-one participants, which included 22 children (aged 6-17 years) and 29 adults aged (19 - 83 years), from the gold mining areas of Nambija and Portovelo, Ecuador were administered ASR and hearing tests. Both the children and adults in the study areas were reported to be exposed to elemental Hg vapors and methylmercury. The Nambija gold mining settlement is located about 650 km from Quito, Ecuador at an altitude of approximately 1850 m, in the southeastern rain forest in Zamora Province, Ecuador, near the Peruvian border. The Nambija gold mining settlement is served by the rivers Nambija, Zamora, and Yacuambi, from which the local population collects fish as part of their diet. The Nambija settlement maintains a population of about 2000 persons of Mestizo background, and traditional Saraguro Amer-Indians. Most of the inhabitants of Nambija are involved with the year-round gold mining industry in the area. Quicksilver (liquid Hg) has been used for amalgamation for decades in the gold mining operations in the Nambija gold mines. Medical testing equipment and materials were transported to the Nambija community by foot over a rugged mountain path to an area near the top of the mountain settlement. The second study area, Portovelo is a small Andean gold mining town of mostly indigent Mestizo families in the Zaruma area of El Oro Province approximately 250 km northwest of Nambija in Southwestern Ecuador. Portovelo has been a major center of gold mining in Ecuador since the time of the Incas. Both Hg and sodium cyanide are widely used in the gold extraction process in the Portovelo gold mines.
Informed consent was requested and received from the adult participants and the parents or guardians of the children participants before testing was initiated. The children in the study area were accompanied by their parents, guardians, teachers or officials at the local schools or health clinics for testing. This study was approved by the Human Studies Committee ( Comité de Bioética) of Universidad San Francisco de Quito, under whose auspices the investigation was conducted. The project was conducted under the direction of Dr. Fernando Ortega, Professor of Medicine at Universidad San Francisco de Quito in Quito, Ecuador. The adult participants and the parents/guardians of the children who participated were advised of current HgB levels, and given instructions on ways to avoid or minimize Hg exposure. Children and adults who were found to have elevated HgB levels were referred to Ecuadorian physicians for medical treatment.
Measurement of blood Hg levels
Samples of whole blood were used to determine Hg levels in the 22 children and the 29 adults in the study area who received ASR tests. Blood samples of 4 ml were collected using Vacutainer evacuated blood collecting tubes with Li-Heparin. All whole blood samples were immediately stored and transported in refrigerated containers at approximately 4° C, and later analyzed for Hg concentration by ICP-MS at the Mayo Medical Laboratories in Rochester, Minnesota. The Hg exposure level was assessed by determining the total Hg concentration in whole blood using procedures describe elsewhere [16].
Auditory test procedures
Each participant was given a conventional audiological examination, including otoscopy, tympanometry, and pure-tone threshold measurements at the frequencies 250, 500, 1000, 2000, 3000, 4000, 6000 and 8000 Hz. All ASR test data were obtained concurrently with the collection of blood samples from the study participants, thus precluding any inadvertent investigator bias. The ASR was recorded with the Grason-Stadler GSI 33 immittance system using a probe tone frequency of 226 Hz. The probe was sealed in the external ear canal and tympanometry was performed to determine the status of the tympanic membrane and the middle ear system. In this field investigation, for the purpose of screening the study group, contralateral ASR measurements were made at one stimulus activator frequency, 2000Hz. The 2000 Hz activator stimulus was selected for screening purposes because it is less likely to be affected by ambient noise, and it may be more sensitive to ASR adaptation than the lower frequencies. The ASR was elicited with the probe in one ear, and the stimulus activator delivered by an insert earphone to the contralateral ear in order to measure the crossed brainstem reflex arc. The contralateral ASR was chosen as the screening procedure because it represents the crossed brain reflex arc that involves a series of neuronal synapses, including the cholinergic neuromuscular synapse with the stapedial branch of the facial nerve at the stapedius muscle, and thus serves as a more comprehensive neurological index of auditory brainstem integrity.
The acoustic stapedius reflex threshold (ASRT), ASR amplitude growth, and ASR decay (ASRD) were analyzed for the 2000 Hz activator stimulus. The ASRT was defined as the lowest stimulus intensity level (measured in clinical test units as hearing level [HL] in dB) at which a reproducible ASR deflection (representing a minimum of 0.1 cm3 change in immittance) from the recording baseline could be detected. Stimulus presentations of increasing intensity increments of 5 dB were used to evoke and confirm ASRT responses, and to measure amplitude growth over a 10-15-dB range above threshold (sensation level) in order to evaluate the dynamic range or magnitude of the stapedius muscle contraction.
The acoustic stapedius reflex decay (ASRD) was defined as the time in seconds at which the stapedius muscles relaxes or decays to at least 50% of its initial peak contraction (as indicated by the amplitude of the recorded deflection) over a 10-second stimulus period. Thus, a reduction in response amplitude of 50% or greater at any point during a 10-second period of acoustic stimulation (i.e., ASR half-life of < 10 seconds) was considered abnormal decay or abnormal muscle adaptation.
Unlike in a controlled clinical environment, in field investigations there are inherent uncontrollable variables and constrains, such as participants’ availability, work and school schedules, and time limitations. Because of some of these constraints, complete ASR measures could not be performed on every participant in the study group. For comparative observations, some participants were examined for ASR at the additional frequencies of 1000 and 4000Hz.
Data analysis
The arithmetic mean, standard deviation, standard error, range and percentile values were determined for HgB, ASRT and ASRD. Because several variables had skewed distributions, nonparametric statistical tests, as well as parametric tests, were used for data analysis. Student's t-test and the Mann-Whitney U test were used to analyze differences between means. Spearman correlation analyses were performed to examine relationships between HgB level and ASRT, HgB level and ASRD, and between HgB level and hearing thresholds. An ASRD value of 10 seconds was used in the data analysis for those participants who showed no ASR decay. An alpha level of ≤ 0.05 was accepted as an indication of statistical significance.
Results
Blood Mercury Concentration
The HgB levels for the total study group of 51 participants ranged from 2.0 to 89 μg/L (median: 6.0 μ/L). The mean HgB level for the 22 children in the study group was 15.6 μg/L (SD: 21.3; median: 7.0; range: 2.0-89 μg/L), and higher than the median HgB level of 0.26 μg/L reported for children in the U.S. [17]. The mean HgB level for the 29 adults in the study group was 8.5 μg/L (SD: 7.1; median: 6.0; range: 2.0-32.0 μg/L). The median HgB concentration for the adults was higher than the median of 0.6 μg/L found in a German survey of 25-69-year-old adults [18]. The difference in HgB levels between the children and adults was not statistically significant (unpaired t= -1.611; p = 0.114).
Hearing Thresholds
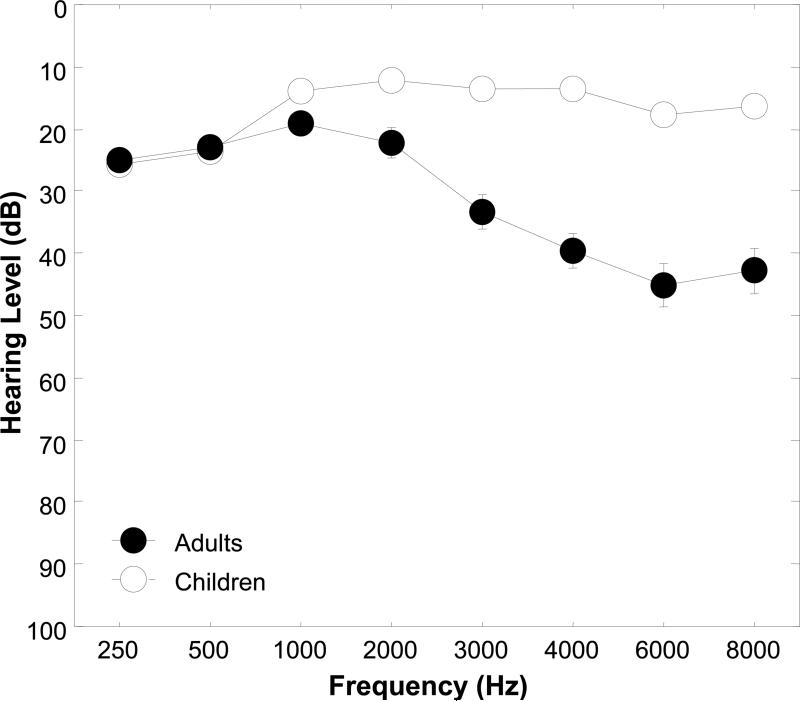
Figure 1 shows mean pure tone hearing thresholds for the Andean children and adults plotted in a conventional audiogram format for the frequencies 250, 500, 1000, 2000, 3000, 4000, 6000 and 8000 Hz. The hearing thresholds for the children were within the normal range (≤ 20 dB HL) at each test frequency above 500 Hz. The slightly depressed audiometric thresholds for the children at 250 and 500 Hz in the audiogram of Figure 1 reflect slight threshold shifts due to ambient noise levels in the field-testing environment. The averaged results for the adults revealed a high frequency hearing loss at 3000 Hz and above. A Mann-Whitney U analysis revealed significant differences in mean auditory thresholds between the children and adults at 1000, 2000, 3000, 4000, 6000, and 8000 Hz (tied Z ranged from -2.64 to -6.87, and tied p ranged from 0.008 to < 0.0001). Spearman correlation analyses revealed no significant associations between HgB and hearing thresholds for the pure tone test frequencies of 1000, 2000, 3000, 4000, 6000 and 8000 Hz for the children or adults. Because hearing thresholds for the lower frequencies, 250 and 500 Hz, were influenced by ambient noise, it was deemed inappropriate to perform correlation analyses at these frequencies.
Figure 1.
Mean pure tone hearing thresholds with standard error bars for children and adults for the frequencies 250, 500, 1000, 2000, 3000, 4000, 6000 and 8000 Hz. The standard errors for the children were not greater than 1.3 dB, and consequently are not visible in the threshold curve of Figure 1.
Acoustic stapedius reflex thresholds
Since there was no significant difference between ears for the hearing thresholds at the screening activator frequency of 2000 Hz in the children, and because there was no significant differences between ears in the ASRT at 2000 Hz, the ASRT results of the right and left ears were combined to increase the power of the data statistical analysis. The mean contralateral ASRT for 39 ears of the children at the screening stimulus activator frequency of 2000 Hz was 92.9 dB HL (SD: 6.1; range: 80-105 dB HL). A correlation analysis using the Spearman rho indicated a significant association between the ASRT (dB HL) and HgB level (rho = 0.433; p = 0.008) in the children, showing increases in ASRT with increases in HgB. The mean contralateral ASRT for 53 adult ears was 90.0 dB HL (SD: 6.4; range: 65-105 dB HL). In contrast to the children, the Spearman correlation coefficient showed no significant association between HgB and ASRT for the adults (rho = - 0.030; p = 0.827). The difference in the ASRT between the children and adults was statistically significant (unpaired t = -2.230; p = 0.028) with the children showing a 2.9 dB higher mean ASRT.
Table 1 shows the means and percentiles for ASRT in dB HL and SL for the 2000 Hz stimulus activator in the Hg-exposed Andean children (n = 39 ears) and adults (n = 53 ears). Standard deviation values for the means are shown in parentheses. It may be observed from Table 1 that the ASR sensation level (SL) is lower for the adults than the children, suggesting a more rapid growth in loudness in the adults, i.e., loudness recruitment or hyperacusis.
Table 1.
Mean and percentiles for contralateral acoustic stapedius reflex thresholds (ASRT) in dB hearing level (HL) and sensation level (SL) [level above the hearing threshold] for a 2000 Hz stimulus activator in mercury-exposed Andean children (n = 39 ears) and adults (n = 53 ears) Standard deviation values are shown in parentheses.
| ASRT (dB HL) | ASR SL (dB) | |||
|---|---|---|---|---|
| Children | Adults | Children | Adults | |
| Mean | 92.9 (6.1) | 90.0 (6.4) | 80.6 (8.1) | 67.3 (16.8) |
| 10th Percentile | 85.0 | 85.0 | 70.0 | 37.0 |
| 25th Percentile | 90.0 | 85.0 | 76.2 | 60.0 |
| 50th Percentile | 95.0 | 90.0 | 80.0 | 70.0 |
| 75th Percentile | 95.0 | 95.0 | 85.0 | 80.0 |
| 90th Percentile | 100.0 | 95.0 | 90.0 | 85.0 |
Acoustic stapedius reflex growth
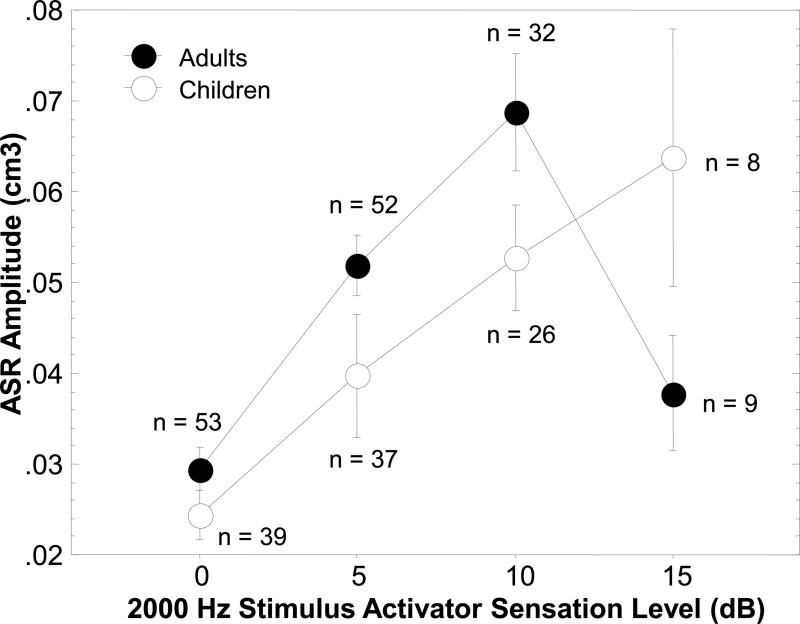
Figure 2 illustrates the ASR growth in amplitude over a range of 15 dB SL for the children and adults. The graph shows that the ASR growth curve for the children increases in a linear fashion with increases in the intensity of the 2000 Hz activator stimulus over a 15 dB range, with no evidence of saturation in growth. The adults, on the other hand, exhibited a steeper growth function and apparent saturation at the highest SL. To further quantify the ASR growth amplitude difference between the adults and children, an adults/children amplitude ratio was calculated at 5 and 10 dB SL. The ratio at 5 dB SL was 1.95, and at 10 dB SL was 1.75, further demonstrating that the growth was steeper for the adult ears.
Figure 2.
Contralateral acoustic stapedius reflex (ASR) amplitude growth functions for a 2000 Hz stimulus activator as a function of stimulus activator sensation levels (SL) of 0, 5, 10, and 15 dB for Hg-exposed Ecuadorian Andean children. The 0 dB SL indicates the ASR amplitude at threshold, and the 5, 10 and 15 dB SLs show the ASR growth at corresponding levels above the reflex threshold. The reflex growth amplitude as plotted on the y-axis is recorded in acoustic immittance-compliance units (cm3) illustrating the magnitude of the ASR contraction.
Acoustic stapedius reflex decay
The mean contralateral ASRD at the screening frequency of 2000 Hz and at the conventional clinical 10 dB SL for the children was 8.1 seconds (SD: 2.7 seconds; median 10 seconds). The mean contralateral ASRD in the adults was 8.1 seconds (SD: 2.6 seconds, median 10 seconds). There was no significant association between HgB and ASRD times in the children (rho =-0.047; p = 0.786). The adults also showed no significant association between HgB and ASRD times (rho =0.165; p = 0.284).
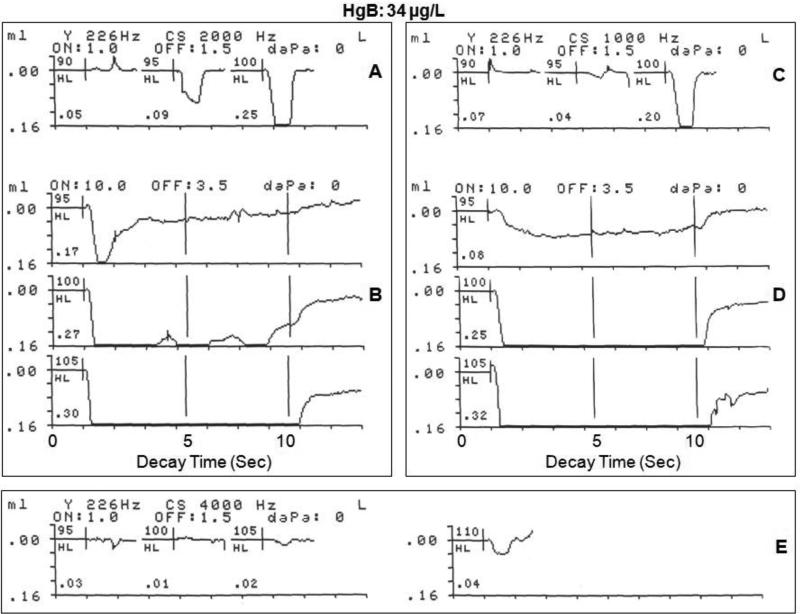
Several illustrations from the data in the study groups are presented to show ASR responses in the presence of elevated HgB levels, and to demonstrate the feasibility of employing the ASR procedure in the field, using stimulus activators of 1000, 2000, and 4000 Hz. Figure 3 shows the contralateral ASRT, amplitude growth and ASRD for a 2000 Hz stimulus activator in and a 12-year-old male with an elevated HgB level of 34 μg/L. The participant's audiometric threshold in the stimulus ear for the activator frequency of 2000 Hz was 15 dB HL and within the normal range. Figure 3A shows the contralateral ASRT response for the 2000 Hz activator stimulus. The recording shows a robust ASRT deflection within the normal range at 95 HL (0.09 mL or cm3 change in acoustic immittance), with a three-fold increase in amplitude for a 5 dB increase in intensity to 100 dB HL (0.25 cm3). Figure 3B shows the contralateral ASRD recordings at threshold (95 dB HL), and at supra threshold levels of 5 dB SL (100 dB HL) and at 10 dB SL (105 dB HL) for the 2000 Hz stimulus activator. The upper ASRD recording in Figure 3B shows a vigorous response at stimulus onset (0.17 cm3) at the ASRT (95 dB HL), indicated by a large deflection from baseline, followed by a rapid decay in response amplitude or muscle contraction at 2 seconds. The response could not be sustained for the clinically normal 10 second period. Rapid ASRD at threshold level is commonly seen in normal subjects with no exposure to neurotoxins or history of neurological impairment. Therefore, the rapid ASRD at threshold may not be indicative of auditory neural impairment. The middle recording in Figure 3B at 100 dB HL (5 dB SL) revealed a vigorous response at stimulus onset to a peak amplitude of 0.27 cm,3 followed by a sustained stapedius muscle contractions at peak amplitude with no significant adaptation or decay until stimulus offset at 10 seconds. Similarly, the lower recording in Figure 3B evoked at 105 dB HL (10 dB SL), shows a dynamic response at stimulus onset to a peak amplitude of 0.30 cm3, followed by a sustained stapedius muscle contraction at peak amplitude with no adaptation or decay until stimulus offset at 10 seconds. For comparison, Figure 3C,D shows the contralateral ASRT, amplitude growth and the ASRD in the same participant for a 1000 Hz stimulus activator. The audiometric threshold for this participant in the stimulus ear for 1000 Hz was 15 dB HL. The 1000 Hz activator stimulus elicited a contralateral ASRT response (Figure 3C) within the normal range at 95 HL (0.04 cm3), with a five-fold increase in amplitude for a 5 dB increase in intensity to 100 dB HL (0.20 cm3). The upper recording in Figure 3D shows the ASRD at the threshold level of 95 dB HL. The recording revealed a moderate change in acoustic immittance at stimulus onset (0.08 cm3), followed by sustained stapedius muscle contraction at the peak amplitude of 0.08 cm3 with no significant adaptation or decay until stimulus offset at 10 seconds. At 100 dB HL (5 dB SL), the middle recording in Figure 3D shows a robust response at stimulus onset (0.25 cm3), followed by sustained stapedius muscle contractions at peak amplitude with no discernable adaptation or decay until stimulus offset at 10 seconds. At the conventional clinical 10 dB SL (105 dB HL), the lower recording in Figure 3D shows an ASR of greater amplitude at stimulus onset (0.32 cm3), with sustained stapedius muscle contractions at peak amplitude with no adaptation or decay until stimulus offset at 10 seconds. The contralateral ASRT for the stimulus activator of 4000 Hz is shown in the recordings of Figure 3E. The audiometric threshold for this participant in the stimulus ear for 4000 Hz was 35 dB HL, indicating a mild hearing loss at this frequency. The audiometric thresholds at all other frequencies were within the normal range. The contralateral ASRT was reached at 105 dB (0.02 cm3) at 4000 Hz, which is within the normal range, in spite of the hearing loss. At 110 dB HL (5 dB SL) the ASR amplitude doubled in magnitude, increasing to 0.04 cm3. In summary, this 12 year old Hg-exposed male with a high HgB level of 34 μg/L showed normal ASR functioning, implying that the 8th cranial nerve and the auditory brainstem neurons and synapses involved in the ASR are unimpaired by the elevated HgB level.
Figure 3.
Physiological recordings of contralateral acoustic stapedius reflex threshold and acoustic reflex decay in response to a 2000 Hz stimulus activator (A,B) and a 1000 Hz stimulus activator (C,D), and the acoustic stapedius reflex threshold at 4000 Hz (E) for a 12 -year-old male with a blood mercury level of 34 μg/L living in gold mining areas of Ecuador where Hg is widely used in amalgamation.
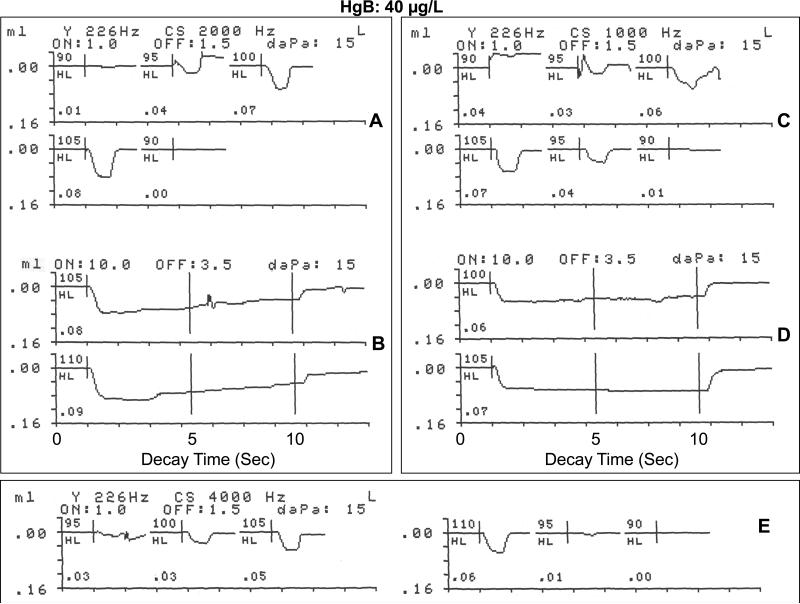
Figure 4 shows the ASR recordings from an 8-year-old female with an elevated HgB level of 40 μg/L. The audiometric threshold in the stimulus ear (right) for the activator frequency of 2000 Hz was normal at 10 dB HL. Her tympanogram indicated normal middle ear air pressure and tympanic membrane compliance. As shown in Figure 4 A, the 2000 Hz screening activator stimulus elicited an unequivocal contralateral ASRT response in the normal range at 95 dB HL (0.04 cm3 change in acoustic immittance), with increases in ASR amplitude as a function of intensity level up to 105 dB HL (10 dB SL), a 0.08 cm3 change in acoustic immittance. Figure 4B (upper recording) shows the contralateral ASRD recordings at 105 dB HL (10 dB SL) for the 2000 Hz stimulus activator. Consistent with the ASRT response seen in Figure 4A at 10 dB SL, the initial deflection in the ASRD response reached a 0.08 cm3 change in acoustic immittance, but revealed some adaptation or decay in response amplitude to less than 50% of peak value at approximately 6 seconds, before returning to the pre-stimulus baseline. The lower recording of Figure 4B shows the ASRD recording at 110 dB HL (15 dB SL) for the 2000 Hz stimulus activator. The deflection from baseline indicated a comparatively large change in acoustic immittance at stimulus onset (0.09 cm3), followed by sustained stapedius muscle contractions at peak amplitude with a slight, but not clinically significant adaptation or decay. Figure 4C shows that at a stimulus activator frequency of 1000 Hz (audiometric threshold: 15 dB HL), an ASRT of 95 dB was recorded, as indicated by a change in acoustic immittance at stimulus onset of 0.03 cm,3 and at 5 dB SL (100 dB HL), 0.06 cm.3 At 105 dB HL (10 dB SL) the change in acoustic immittance increased to 0.07 cm.3 The ASR amplitude or magnitude of stapedius muscle contraction followed a linear growth pattern from threshold to 10 dB above threshold.
Figure 4.
Physiological recordings of contralateral acoustic stapedius reflex threshold and acoustic reflex decay in response to a 2000 Hz stimulus activator (A,B) and a 1000 Hz stimulus activator (C,D), and the acoustic stapedius reflex threshold at 4000 Hz (E) for an 8-year-old female with a blood mercury level of 40 μg/L living in gold mining areas of Ecuador where Hg is widely used in amalgamation.
The ASRD recording in the upper curve of Figure 4D shows a substantial stapedius muscle reflex, as indicated by the initial deflection from baseline to a peak value of 0.06 cm3 at 100 dB HL (5 SL), and a sustained muscle contraction for the normal 10 seconds with no adaptation or decay. At 10 dB SL (105 dB HL) the ASR recording in the lower curve of Figure 4D shows a larger amplitude change (0.07 cm3), and normal, sustained stapedius muscle contraction without any amplitude reduction or decay up to stimulus offset at 10 seconds. Figure 4E illustrates the contralateral ASRT for a stimulus activator of 4000 Hz for this participant. The audiometric threshold for this participant in the stimulus ear for 4000 Hz was 10 dB HL. The recordings show some evidence of a contralateral ASR response at 95 dB HL (0.01 cm3), but an unequivocal response at 100 dB HL (0.03 cm3). There was a linear growth in ASR amplitude from 95 dB HL (0.01 cm3) to 110 dB HL (0.06 cm3change in acoustic immittance). In summary, this 8-year-old Hg-exposed female with an elevated HgB level of 40 μg/L showed normal ASRTs at each stimulus activator frequency, but some degree of adaptation or decay at 10 dB SL for the 2000 stimulus activator. The decay or stapedius muscle adaptation observed at the 10 dB SL for the 2000 Hz stimulus in this Hg-exposed participant may reflect the mild decay seen in some normal, non-Hg exposed children, or it may suggest some subtle retrocochlear impairment in the ASR brainstem pathways.
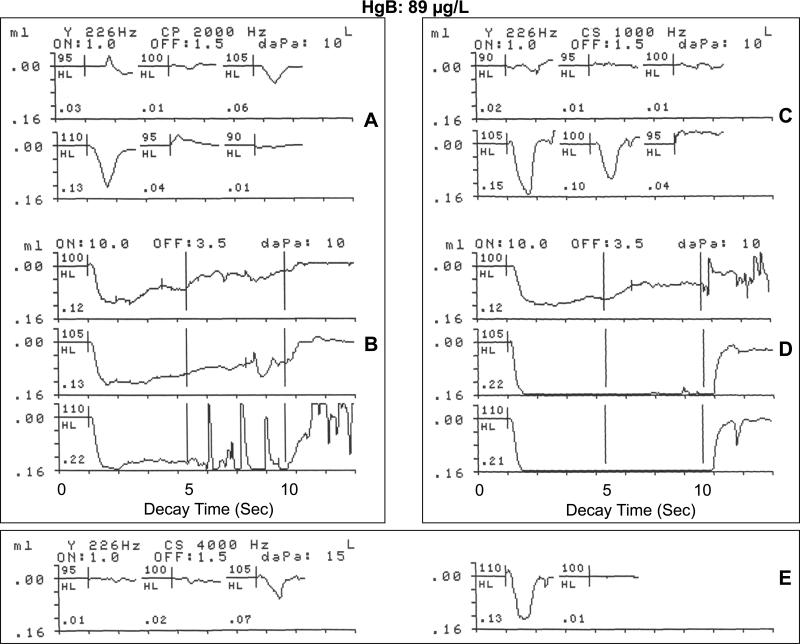
The ASR recordings in Figure 5A, B, C, D and E illustrate the contralateral or crossed brain ASRT, ASR growth, and the ASRD morphology in response to 3 pure-tone stimulus activators (1000 Hz, 2000 Hz and 4000 Hz) for a 14-year-old male gold miner with a highly elevated HgB level of 89 μg/L. The audiometric threshold in the stimulus ear at the activator frequency of 2000 Hz was 10 dB HL. As shown in Figure 5A, the 2000 Hz stimulus elicited a robust contralateral ASRT response within the normal range at 95 dB HL (0.03 mL or cm3 change in acoustic immittance), with amplitude growth up to 110 dB HL (0.13 cm3).
Figure 5.
Physiological recordings of contralateral acoustic stapedius reflex threshold and acoustic reflex decay in response to a 2000 Hz stimulus activator (A,B) and a 1000 Hz stimulus activator (C,D), and the acoustic stapedius reflex threshold at 4000 Hz (E) for 14-year-old male with an HgB exposure level of 89 μg/L living in gold mining areas of Ecuador where Hg is widely used in amalgamation.
The contralateral ASRD recording at 5 dB SL (100 dB HL) in Figure 5B (upper curve) revealed sustained stapedius muscle contractions at peak amplitude for only 4 seconds, followed by a gradual adaptation or decay to greater than 50 % of peak amplitude, which is normal at a low SL. The ASRD recording in Figure 5B (middle curve) at the conventional 10 SL (105 dB HL) shows that the stapedius muscle reflex was sustained for 8 seconds, suggesting minimal decay. At 15 dB SL (110 dB HL), the contralateral ASRD recording (lower curve of Figure 5B) revealed sustained stapedius muscle contractions at peak amplitude without significant amplitude reduction up to approximately 6 seconds. The noisy recording after around 6 seconds may suggest aberrant stapedius muscle twitches, patient noise or environmental electrical interference with the test instrumentation. At the stimulus activator frequency of 1000 Hz (audiometric threshold: 20 dB HL) the recordings in Figure 5C illustrate a strong ASRT at 100 dB HL (0.10 cm3), which is within the normal range.
The ASRD responses at 1000 Hz shown in figure 5D. The upper curve of Figure 5D shows the contralateral ASRD recording at threshold (100 dB HL). This reveals a stapedius muscle contractions at peak amplitude (0.12 cm3) for only 6 seconds, followed by a gradual adaptation or decay to approximately 50% of peak level before returning to baseline at stimulus offset at 10 seconds. The ASRD recordings in Figure 5D at 5 dB SL (105 dB HL) and at the conventional clinical 10 dB SL (110 dB HL) show vigorous stapedius muscle reflex that were sustained for the normal 10 seconds, with no adaptation or decay. Both the middle and lower ASRD recordings of Figure 5D showed large amplitude growth (0.22 and a 0.21 cm3 respectively) that reached the graphic display limits of the instrumentation. At 4000 Hz, the participant's audiometric threshold was 20 dB HL and within the normal limits. The ASR recordings in Figure 5E illustrate the contralateral ASRT in response to a 4000 Hz stimulus activator. The recordings show that the stimulus activator at 4000 Hz elicited an unequivocal ASRT response at 105 dB HL (0.07 cm3 change in acoustic immittance), with essentially a doubling of the muscle contraction amplitude at 110 dB HL (0.13 cm3), demonstrating substantial ASR amplitude growth. The ASRD was not measured at 4000 Hz because of the normal rapid adaptation or decay at this frequency. In summary, this 14-year-old Hg-exposed male with a high HgB level of 89 μg/L showed ASRTs at the upper end of the normal range at each stimulus activator frequency. However the ASRD responses showed minimal decay/adaptation at 5 dB SL and dysmorphic recordings at 10 dB SL for the 2000 stimulus activator. The decay or stapedius muscle adaptation observed at the 5 dB and 10 dB SL for the 2000 Hz stimulus may have no clinical significance, or it may suggest some mild retrocochlear impairment in the ASR brainstem pathways.
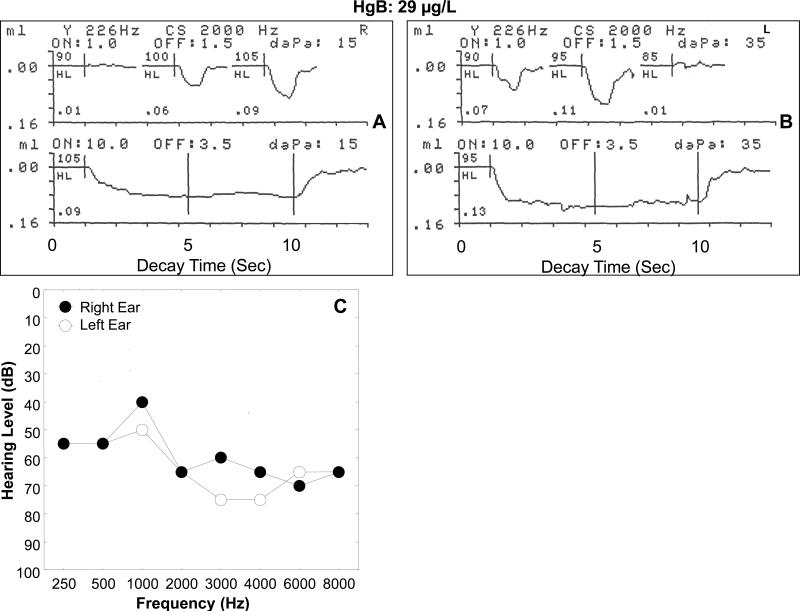
Figure 6 illustrates the ASR recordings from an adult (52-year-old) male gold miner with an elevated HgB level of 29 μg/L and a bilateral, moderate to severe sensorineural hearing loss. The electro-acoustic recordings in Figure 6A (upper curve) show that the 2000 Hz screening activator stimulus elicited a vigorous contralateral (probe right ear, stimulus left ear) ASRT response at 100 dB HL (0.06 mL or cm3 change in acoustic immittance). The ASR amplitude increased approximately 67% to 0.09 cm.3 when the intensity of the activator stimulus was increased by 5 dB (105 dB HL). The participant's left ear (stimulus ear) hearing threshold was 65 dB HL at 2000 Hz. The ASR at 100 HL indicates only a 30 dB difference between hearing sensitivity threshold and the ASRT. This narrow range suggests an abnormal increase in the sensation of loudness (i.e., loudness recruitment) in the left ear, which is pathognomonic of sensory hearing loss, and not retro-cochlear pathology. Figure 6A (lower curve) shows a robust contralateral ASRD recording at 105 dB HL, which is only 5 dB SL for the 2000 Hz stimulus activator. The deflection to peak amplitude in the ASRD response reached a peak of 0.09 cm,3 with no adaptation or decay in response amplitude before returning to the pre-stimulus baseline after 10 seconds of stimulation. Figure 6B reveals that the 2000 Hz screening activator stimulus delivered to the right ear elicited a clear contralateral (probe in left ear, stimulus right ear) ASRT response at 90 dB HL (0.07 cm,3). Repeated testing did not show a consistent response at 85 dB HL). There was an approximately 64% increase in ASR amplitude for an intensity increase of 5 dB (to 95 dB HL) equaling 0.11 cm.3 Since the participant's audiometric threshold in the right ear was 65 dB HL at 2000 Hz, the ASRT was elicited at only a 25 dB SL. This narrow range of 25 dB SL between audiometric threshold and ASRT deviates from the average 85 dB SL observed for the ASRT in persons with normal hearing. This low SL for the ASRT supports the implication of loudness recruitment attendant to a cochlear hearing loss.
Figure 6.
Physiological recordings of contralateral acoustic stapedius muscle reflex threshold (A) and acoustic reflex decay (B) in response to a 2000 Hz stimulus activator for a 52-year-old male with a blood mercury level of 29 μg/L living in gold mining areas of Ecuador where Hg is widely used in amalgamation. The associated audiogram is shown in Figure 6 C).
The recording in Figure 6B (lower curve) shows the contralateral (probe in left ear, stimulus right ear) ASRD recording at 95 dB HL (5 dB SL) in response to a 2000 Hz stimulus activator. The deflection indicated a large change in acoustic immittance at stimulus onset (0.13 cm3) followed by a sustained stapedius muscle contraction at peak amplitude with no clinically significant adaptation or decay until stimulus offset at 10 seconds. In summary, this 52-year-old Hg-exposed male with a moderate to severe sensorineural hearing loss and an elevated HgB level showed an uncommonly low ASRT. This finding suggests cochlear pathology, which may the result of Hg exposure, noise exposure, or a combination of the two. The normal ASRD responses are consistent with cochlear pathology or the absence of retrocochlear pathology.
Discussion
Mercury exposure has been reported to induce auditory sensory-neural impairment, with one target being the brainstem tracts and nuclei. However, the level of Hg exposure required to induce auditory impairment, and the extent of Hg-induced cochlear, auditory nerve and brainstem damage has not been fully established. Children and adults in the current study were found to have HgB levels that exceeded comparable groups in industrialized nations, such as the United States [17] and Germany [18], that may place them at risk for hearing impairment and neurocognitive disabilities or neurological deficits. In the U.S. for example, the median HgB level of children reported in the 1999-2000 NHANES survey [17] was 0.26 μg/L, or more than 25 times lower than the median of 7.0 μg/L found in the Andean children in this study. The adults in the present study were found to have a median HgB concentration 10 times higher than the levels reported for adults in a German survey [18]. Since Hg has no known biological benefit to humans, exposure at any level may lead to adverse neurodevelopmental and neurological outcomes.
A sample of the Hg-exposed children and adults in the Nambija and Portovelo gold mining study areas of Ecuador were previously screened for auditory brainstem Hg-related impairment using ABR measures in an earlier study [11]. The ABR findings revealed evidence of abnormal wave latencies and aberrant morphology in some Hg-exposed children. In the present study, brainstem mediated contralateral ASRTs, ASR amplitude growth and ASR decay/adaptation (ASRD) were used to assess the integrity of the cross-brain tracts, nuclei, synapses, and facial motoneurons innervating the stapedius muscle.
While the data show the mean acoustic reflex threshold (ASRT) for the 22 children to be in the normal range, i.e, between 80 and 105 dB HL, there was still a mild statistical correlation between HgB level and the ASRT. This finding may suggest a compromised brainstem ASR arc in the Hg poisoned children, but it may not reflect clinical significance. It is unclear why a similar correlation between HgB level and the ASRT was not found in the mature central auditory system of adults. The adults tended to have lower HgB levels than the children, but the mean difference was not statistically significant. The developing nervous system of children, including the central auditory system may be more susceptible to heavy metal poisoning than that of adults. A larger number of Hg-exposed children and adults in the study area must be examined in order to determine with confidence the deleterious effects of Hg exposure on the brainstem mediated ASR. Further, the mean ASRT for the adults was found to be within the normal range, and was slightly better (mean: 90 dB; 65-105 dB HL) than that of the children. The lower evoked ASRTs observed in some adults with hearing loss suggested loudness recruitment related to sensory hearing loss. The audiograms of a number of adults tested for this study revealed high frequency hearing losses, which may be the result of Hg exposure, noise exposure, presbyacusis or a combination of the three.
The magnitude of the ASR amplitude growth may serve as an indicator of auditory neural synchrony and summation in the spatial and temporal domains, as well as an index of loudness recruitment or hyperacusis. In this study, the growth function for the children increased in a linear fashion with increases in the intensity of the 2000 Hz activator stimulus over a 15 dB range, with no evidence of saturation in growth. In contrast, the adults exhibited a steeper growth function, and apparent saturation at the highest intensity level. The comparative steepness of the adult ASR amplitude growth curve (Figure 2) prior to saturation further suggests loudness recruitment as seen in sensory hearing loss. Previous studies have found varied results when comparing the ASR amplitude growth as a function of stimulus activator intensity in normal and sensorineural hearing impaired ears. These studies show either no significant difference in the ASR growth function between the normal and hearing impaired ears, or a steeper growth function for the normal hearing subjects. On the other hand, for a broadband noise activator stimulus, subjects with sensorineural hearing loss showed a steeper ASR amplitude growth curve than normal hearing subjects [19, 20]. In the present study, except for the saturation at the highest SL, the growth function for the adults, who presented with a mean high frequency hearing loss, was similar to that seen for a broadband noise activator in an earlier study [20].
The ASR decay or adaptation was investigated to determined whether the Hg-exposed participants evinced signs of neural damage or neuromuscular fatigue. While the results showed no statistical association between HgB level and ASRD times, the ASR recordings in some of the children and adults showed rapid adaptation or fatigue, indicating clinically abnormal decay. The mean contralateral or cross-brain ASRD time for both children and adults was approximately 8 seconds, indicating slight decay or adaptation, which is frequently seen at 2000 Hz in normal hearing and neurologically normal persons. The rapid adaptation of less than the average 8 seconds observed in some of the children and adults in this study may be related to the elevated HgB levels and possible underlying damage of neurons in the brainstem stapedius reflex arc. It may also be associated with the activator stimulus used. Moreover, some individual stapedius reflex recordings showed dysmorphic ASR responses that may suggest anomalies in the synapses of the ASR arc or the contractile properties of the stapedius muscle.
In the Hg-exposed children and adults in this study group, non-invasive ASR measurements were effective in assessing the integrity and function of the stapedius muscle, and the afferent and efferent neurons involved with the auditory brainstem reflex arc. The mean ASRT in the children of the study group was found to increase with Hg exposure level, possibly suggesting Hg-associated auditory brainstem involvement. Further, the ASRD anomalies found in some children and adults with elevated HgB levels suggested possible auditory brainstem impairment that could be related to Hg exposure. Individual susceptibility to Hg exposure must also be considered as a factor in evaluating the neuro-auditory effects of Hg exposure. For example, some individual children in the study group with high HgB levels showed no aberrant ASR responses. Other mercury intoxicated children with high HgB levels of 40 μg/L and 89 μg/L, respectively, exhibited demonstrable ASR abnormalities. However, further investigation on a larger population of Hg-exposed children and adults in the same study areas is necessary for firm conclusions regarding the effects of Hg exposure on the neuronal elements of the auditory brainstem tracts and nuclei and the skeletal muscle involved in the ASR. The acoustic stapedius reflex may serve as a useful addition to the battery of clinical tests employed in assessing the neurotoxicological effects of Hg on the auditory brainstem.
Acknowledgments
The authors thank the Administration of Universidad San Francisco de Quito and the Fundacíon Capacitar of Ecuador for support of this project. The authors thank Anthony Bruce Jacobs for excellent technical assistance, and the Mayo Medical Laboratories for laboratory support. We are grateful to the Harvard Biological Laboratories, the Harvard David Rockefeller Center for Latin American Studies, the Shriver Center/University of Massachusetts Medical School, and Dr. Jeremy Bloxham of Harvard University for support.
References
- 1.Centers for Disease Control and Prevention (CDC) Current trends acute and chronic poisoning from residential exposures to elemental mercury—Michigan, 1989 – 1990. Morb. Mort. Wkly. Rep. 1991;40:393–95. [PubMed] [Google Scholar]
- 2.National Research Council . Toxicological Effects of Methylmercury. National Academy Press; Washington, DC: 2000. Committee on the Toxicological Effects of Methylmercury. [Google Scholar]
- 3.Aschner M, Aschner JL. Mercury neurotoxicity: mechanisms of blood-brain barrier transport. Neurosci Biobehav Rev. 1990;14:169–76. doi: 10.1016/s0149-7634(05)80217-9. [DOI] [PubMed] [Google Scholar]
- 4.Falluel-Morel A, Sokolowski K, Sisti HM, Zhou X, Shors TJ, Dicicco-Bloom E. Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deficits during puberty. J Neurochem. 2007;103:1968–81. doi: 10.1111/j.1471-4159.2007.04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridges CC, Zalups RK. Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Health B Crit Rev. 2010;13:385–410. doi: 10.1080/10937401003673750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anniko M, Sarkady L. Cochlear pathology following exposure to mercury. Acta Otolaryngol. 1978;.85:213–24. doi: 10.3109/00016487809111928. [DOI] [PubMed] [Google Scholar]
- 7.Rothwell JA, Boyd PJ. Amalgam dental fillings and hearing loss. Int J Audiol. 2008;47:770–76. doi: 10.1080/14992020802311224. [DOI] [PubMed] [Google Scholar]
- 8.Chang YC, Yeh CY, Wang JD. Subclinical neurotoxicity of mercury vapor revealed by a multimodality evoked potential study of chloralkali workers. Am J Ind Med. 1995;27:271–79. doi: 10.1002/ajim.4700270211. [DOI] [PubMed] [Google Scholar]
- 9.Discalzi GL, Fabbro D, Meliga F, Mocellini A, Capellaro F. Effects of occupational exposure to mercury and lead on brainstem auditory evoked potentials. Int J Psychophysiol. 1993;14:21–25. doi: 10.1016/0167-8760(93)90080-9. [DOI] [PubMed] [Google Scholar]
- 10.Murata K, Weihe P, Renzoni A, Debe F, Vasconcelos R, Zino F, Araki S, Jørgensen PJ, White RF, Grandjean P. Delayed evoked potentials in children exposed to methylmercury from seafood. Neurotoxicol. Teratol. 1999a;19:417–28. doi: 10.1016/s0892-0362(99)00011-2. [DOI] [PubMed] [Google Scholar]
- 11.Counter SA. Neurophysiological anomalies in brainstem responses of mercury-exposed children of Andean gold miners. J. Occup. Environ. Med. 2003;45:87–95. doi: 10.1097/00043764-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Counter SA, Buchanan LH, Laurell G, Ortega F. Blood mercury and auditory neuro-sensory responses in children and adults in the Nambija gold mining area of Ecuador. Neurotoxicology. 1998;19:185–96. [PubMed] [Google Scholar]
- 13.Counter SA, Buchanan LH, Ortega F. Mercury levels in urine and hair of children in an Andean gold-mining settlement. Int. J. Occup. Environ. Health. 2005;11:132–37. doi: 10.1179/oeh.2005.11.2.132. [DOI] [PubMed] [Google Scholar]
- 14.Borg E. On the neuronal organization of the acoustic middle ear reflex. A physiological and anatomical study. Brain Res. 1973;49:101–23. doi: 10.1016/0006-8993(73)90404-6. [DOI] [PubMed] [Google Scholar]
- 15.Counter SA. Brainstem mediation of the stapedius muscle reflex in hydrancephaly. Acta Oto-Laryngol. 2007;127:498–504. doi: 10.1080/00016480600951376. [DOI] [PubMed] [Google Scholar]
- 16.Nixon DE, Moyer TP. Routine clinical determination of lead, arsenic, cadmium, and thallium in urine and whole blood by inductively coupled plasma mass spectrometry. Spectrochimica Acta Part B. 1996;51:13–25. [Google Scholar]
- 17.Schober SE, Sinks TH, Jones RI, Bolger PM, et al. Blood mercury levels in U.S. children and women of childbearing age, 1999-2000. J. Am. Med. Assoc. 2003;289:1667–74. doi: 10.1001/jama.289.13.1667. [DOI] [PubMed] [Google Scholar]
- 18.Seifert B, Becker K, Helm D, Krause C, Schultz C, Seiwert M. The German environmental survey 1990/1992 GerES II): Reference concentrations of selected environmental pollutants in blood, urine, hair, house dust, drinking water and indoor air. J Expos Anal Environ Epidemiol. 2000;10:552–65. doi: 10.1038/sj.jea.7500111. [DOI] [PubMed] [Google Scholar]
- 19.Hall JW. Acoustic reflex amplitude: II. Effects of age-related auditory dysfunction. Audiol. 1982;21:386–99. doi: 10.3109/00206098209072753. [DOI] [PubMed] [Google Scholar]
- 20.Silman S, Popelka G, Gelfand S. Effect of sensorineural hearing loss on acoustic stapedius reflex growth functions. J Acous Soc Am. 1978;64:1406–11. doi: 10.1121/1.382107. [DOI] [PubMed] [Google Scholar]